Mu-Opioid Receptor 1 and C-Reactive Protein Single Nucleotide Polymorphisms as Biomarkers of Pain Intensity and Opioid Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Anesthesia and Postoperative Analgesia
2.3. Genotyping Data
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Estimation of the Relationship between Selected Sociodemographic and Clinical Parameters of Patients and OPRM1 rs1799971 SNP
3.3. Estimation of the Relationship between Selected Sociodemographic and Clinical Parameters of Patients and rs1205 CRP Polymorphism Types
3.4. Investigation of the Relationship between PACU Stay and Post-Extubation Pain Assessment, the Flow of the Drug, [ug/h] and Flow of the Drug, [µg/kg/h]
3.5. Investigation of the Relationship between the Stay in the PACU and the Use of Coanalgesics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niv, D.; Devor, M. Chronic Pain as a Disease in Its Own Right. Pain Pract. 2004, 4, 179–181. [Google Scholar] [CrossRef]
- Zieliński, J.; Morawska-Kochman, M.; Zatoński, T. Pain Assessment and Management in Children in the Postoperative Period: A Review of the Most Commonly Used Postoperative Pain Assessment Tools, New Diagnostic Methods and the Latest Guidelines for Postoperative Pain Therapy in Children. Adv. Clin. Exp. Med. 2020, 29, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.S. Management of Postoperative Pain in Children. Arch. Dis. Child.—Educ. Pract. 2007, 92, ep14–ep19. [Google Scholar] [CrossRef]
- Astuto, M.; Rosano, G.; Rizzo, G.; Disma, N.; Di Cataldo, A. Methodologies for the Treatment of Acute and Chronic Nononcologic Pain in Children. Minerva Anestesiol. 2007, 73, 459–465. [Google Scholar] [PubMed]
- Taylor, E.M.; Boyer, K.; Campbell, F.A. Pain in Hospitalized Children: A Prospective Cross-Sectional Survey of Pain Prevalence, Intensity, Assessment and Management in a Canadian Pediatric Teaching Hospital. Pain Res. Manag. 2008, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.; Carlson, B.B.; Bunch, J.; Jackson, R.S.; Burton, D. Impact of Unoperated Adolescent Idiopathic Scoliosis in Adulthood: A 10-Year Analysis. Spine Deform. 2020, 8, 1009–1016. [Google Scholar] [CrossRef]
- Bas, T.; Franco, N.; Bas, P.; Bas, J.L. Pain and Disability Following Fusion for Idiopathic Adolescent Scoliosis: Prevalence and Associated Factors. Evid.-Based Spine-Care J. 2012, 3, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Théroux, J.; Le May, S.; Fortin, C.; Labelle, H. Prevalence and Management of Back Pain in Adolescent Idiopathic Scoliosis Patients: A Retrospective Study. Pain Res. Manag. 2015, 20, 153–157. [Google Scholar] [CrossRef]
- Bąbel, P.; Pieniążek, L.; Zarotyński, D. The Effect of the Type of Pain on the Accuracy of Memory of Pain and Affect. Eur. J. Pain 2015, 19, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Poorly Controlled Postoperative Pain: Prevalence, Consequences, and Prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef]
- Rabbitts, J.A.; Fisher, E.; Rosenbloom, B.N.; Palermo, T.M. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J. Pain 2017, 18, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, T.M.; Agarwal, R.; Monitto, C.L. Error Traps in Acute Pain Management in Children. Paediatr. Anaesth. 2022, 32, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Cettler, M.; Zielińska, M.; Rosada-Kurasińska, J.; Kubica-Cielińska, A.; Jarosz, K.; Bartkowska-Śniatkowska, A. Guidelines for Treatment of Acute Pain in Children—The Consensus Statement of the Section of Paediatric Anaesthesiology and Intensive Therapy of the Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2022, 54, 197–218. [Google Scholar] [CrossRef]
- Grunau, R.E.; Holsti, L.; Peters, J.W.B. Long-Term Consequences of Pain in Human Neonates. Semin. Fetal Neonatal Med. 2006, 11, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.A.; Chou, J.; Maurer, E.L.; Kain, Z.N. Acute to Chronic Postoperative Pain in Children: Preliminary Findings. J. Pediatr. Surg. 2011, 46, 1700–1705. [Google Scholar] [CrossRef]
- Harbaugh, C.M.; Lee, J.S.; Hu, H.M.; McCabe, S.E.; Voepel-Lewis, T.; Englesbe, M.J.; Brummett, C.M.; Waljee, J.F. Persistent Opioid Use Among Pediatric Patients After Surgery. Pediatrics 2018, 141, e20172439. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Aouad, M.T.; Taha, S.K.; Nassar, A.H.; Masri, R.F.; Khoury, M.Y.; Makki, M.H.; Siddik-Sayyid, S.M. μ-Opioid Receptor Genetic Polymorphisms and Duration of Epidural Fentanyl Analgesia during Early Labor. Minerva Anestesiol. 2018, 84, 946–954. [Google Scholar] [CrossRef]
- Sia, A.T.; Landau, R. A118G Single Nucleotide Polymorphism of Human μ-Opioid Receptor Gene Influences Pain Perception and Patient-Controlled Intravenous Morphine Consumption after Intrathecal Morphine for Postcesarean Analgesia. J. Am. Soc. Anesthesiol. 2008, 109, 520–526. [Google Scholar] [CrossRef]
- Van Den Wildenberg, E.; Wiers, R.W.; Dessers, J.; Janssen, R.G.J.H.; Lambrichs, E.H.; Smeets, H.J.M.; Van Breukelen, G.J.P. A Functional Polymorphism of the μ-Opioid Receptor Gene (OPRM1) Influences Cue-Induced Craving for Alcohol in Male Heavy Drinkers. Alcohol. Clin. Exp. Res. 2007, 31, 1–10. [Google Scholar] [CrossRef]
- Carlson, C.S.; Aldred, S.F.; Lee, P.K.; Tracy, R.P.; Schwartz, S.M.; Rieder, M.; Liu, K.; Williams, O.D.; Iribarren, C.; Lewis, E.C.; et al. Polymorphisms within the C-Reactive Protein (CRP) Promoter Region Are Associated with Plasma CRP Levels. Am. J. Hum. Genet. 2005, 77, 64–77. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19 November 2023).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. CRAN 2023. Available online: https://easystats.github.io/report/ (accessed on 19 November 2023).
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois. R Package, Version 2.1.6. 2021. Available online: https://CRAN.R-project.org/package=psych (accessed on 19 November 2023).
- Sjoberg, D.; Whiting, K.; Curry, M.; Lavery, J.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package, version 1.3.1; 2019. Available online: https://CRAN.R-project.org/package=readxl (accessed on 19 November 2023).
- Zaed, I.; Bossi, B.; Ganau, M.; Tinterri, B.; Giordano, M.; Chibbaro, S. Current State of Benefits of Enhanced Recovery After Surgery (ERAS) in Spinal Surgeries: A Systematic Review of the Literature. Neurochirurgie 2022, 68, 61–68. [Google Scholar] [CrossRef]
- Zacha, S.; Szwed, A.; Miegoń, J.; Skonieczna-Żydecka, K.; Andrzejewska, A.; Modrzejewska, E.; Horecki, M.; Jarosz, K.; Biernawska, J. Novel Interdisciplinary Enhanced Recovery after Surgery Protocol Implementation in Paediatric Orthopaedics. J. Pers. Med. 2023, 13, 1417. [Google Scholar] [CrossRef]
- Gadiya, A.D.; Koch, J.E.J.; Patel, M.S.; Shafafy, M.; Grevitt, M.P.; Quraishi, N.A. Enhanced Recovery after Surgery (ERAS) in Adolescent Idiopathic Scoliosis (AIS): A Meta-Analysis and Systematic Review. Spine Deform. 2021, 9, 893–904. [Google Scholar] [CrossRef]
- Bąbel, P. The Effect of Positive Affect on the Memory of Pain. Pain Manag. Nurs. 2017, 18, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, M.M.; Garibay, E.R.; Jenkins, B.N.; Kain, Z.N.; Fortier, M.A. Postoperative Pain: Factors and Tools to Improve Pain Management in Children. Pain Manag. 2019, 9, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-Y.; Yang, L.-C.; Lu, H.-F.; Ko, J.-Y.; Wang, C.-H.; Lin, S.-H.; Lee, T.-H.; Concejero, A.; Hsu, C.-J. Association of Μ-opioid Receptor Gene Polymorphism (A118G) with Variations in Morphine Consumption for Analgesia after Total Knee Arthroplasty. Acta Anaesthesiol. Scand. 2006, 50, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Park, J.-Y.; Myung, S.-K.; Ahn, H.Y.; Fukuda, K.; Liao, Q. OPRM1 A118G Gene Variant and Postoperative Opioid Requirement. Anesthesiology 2014, 121, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Chu, H. The Relevance of the OPRM1 118A>G GeneticVariant for Opioid Requirement in PainTreatment: A Meta-Analysis. Pain Phys. 2019, 4, 331–340. [Google Scholar] [CrossRef]
- Aubrun, F.; Zahr, N.; Langeron, O.; Boccheciampe, N.; Cozic, N.; Belin, L.; Hulot, J.-S.; Khiami, F.; Riou, B. Opioid-Related Genetic Polymorphisms Do Not Influence Postoperative Opioid Requirement: A Prospective Observational Study. Eur. J. Anaesthesiol. 2018, 35, 496–504. [Google Scholar] [CrossRef]
- Matic, M.; Jongen, J.L.; Elens, L.; de Wildt, S.N.; Tibboel, D.; Sillevis Smitt, P.A.; van Schaik, R.H. Advanced Cancer Pain: The Search for Genetic Factors Correlated with Interindividual Variability in Opioid Requirement. Pharmacogenomics 2017, 18, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Z.; Zhang, J.; Hakonarson, H.; Cook-Sather, S.D. Candidate Gene Analyses for Acute Pain and Morphine Analgesia after Pediatric Day Surgery: African American versus European Caucasian Ancestry and Dose Prediction Limits. Pharmacogenom. J. 2019, 19, 570–581. [Google Scholar] [CrossRef]
- Klepstad, P.; Fladvad, T.; Skorpen, F.; Bjordal, K.; Caraceni, A.; Dale, O.; Davies, A.; Kloke, M.; Lundström, S.; Maltoni, M.; et al. Influence from Genetic Variability on Opioid Use for Cancer Pain: A European Genetic Association Study of 2294 Cancer Pain Patients. Pain 2011, 152, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Sereika, S.M.; Dai, F.; Alexander, S.; Conley, Y.; Gruen, G.; Meng, L.; Siska, P.; Tarkin, I.; Henker, R. OPRM1 and COMT Gene–Gene Interaction Is Associated With Postoperative Pain and Opioid Consumption After Orthopedic Trauma. Biol. Res. Nurs. 2017, 19, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bartošová, O.; Polanecký, O.; Perlík, F.; Adámek, S.; Slanař, O. OPRM1 and ABCB1 Polymorphisms and Their Effect on Postoperative Pain Relief With Piritramide. Physiol. Res. 2015, 64, S521–S527. [Google Scholar] [CrossRef]
- Perry, T.E.; Muehlschlegel, J.D.; Liu, K.-Y.; Fox, A.A.; Collard, C.D.; Body, S.C.; Shernan, S.K.; CABG Genomics Investigators. C-Reactive Protein Gene Variants Are Associated with Postoperative C-Reactive Protein Levels after Coronary Artery Bypass Surgery. BMC Med. Genet. 2009, 10, 38. [Google Scholar] [CrossRef]
- Bergomi, P.; Scudeller, L.; Pintaldi, S.; Dal Molin, A. Efficacy of Non-Pharmacological Methods of Pain Management in Children Undergoing Venipuncture in a Pediatric Outpatient Clinic: A Randomized Controlled Trial of Audiovisual Distraction and External Cold and Vibration. J. Pediatr. Nurs. 2018, 42, e66–e72. [Google Scholar] [CrossRef]
- Sullivan, M.D. Opioid Overprescribing or Underprescribing After Surgery? Mayo Clin. Proc. 2021, 96, 1108–1110. [Google Scholar] [CrossRef]
- Grösch, S.; Niederberger, E.; Lötsch, J.; Skarke, C.; Geisslinger, G. A Rapid Screening Method for a Single Nucleotide Polymorphism (SNP) in the Human MOR Gene: Short Report. Br. J. Clin. Pharmacol. 2001, 52, 711–714. [Google Scholar] [CrossRef]
- Diatchenko, L.; Nackley, A.G.; Slade, G.D.; Bhalang, K.; Belfer, I.; Max, M.B.; Goldman, D.; Maixner, W. Catechol- O -Methyltransferase Gene Polymorphisms Are Associated with Multiple Pain-Evoking Stimuli. Pain 2006, 125, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sia, A.T.; Sng, B.L.; Lim, E.C.; Law, H.; Tan, E.C. The Influence of ATP-Binding Cassette Sub-Family B Member -1 (ABCB1) Genetic Polymorphisms on Acute and Chronic Pain after Intrathecal Morphine for Caesarean Section: A Prospective Cohort Study. Int. J. Obstet. Anesth. 2010, 19, 254–260. [Google Scholar] [CrossRef] [PubMed]
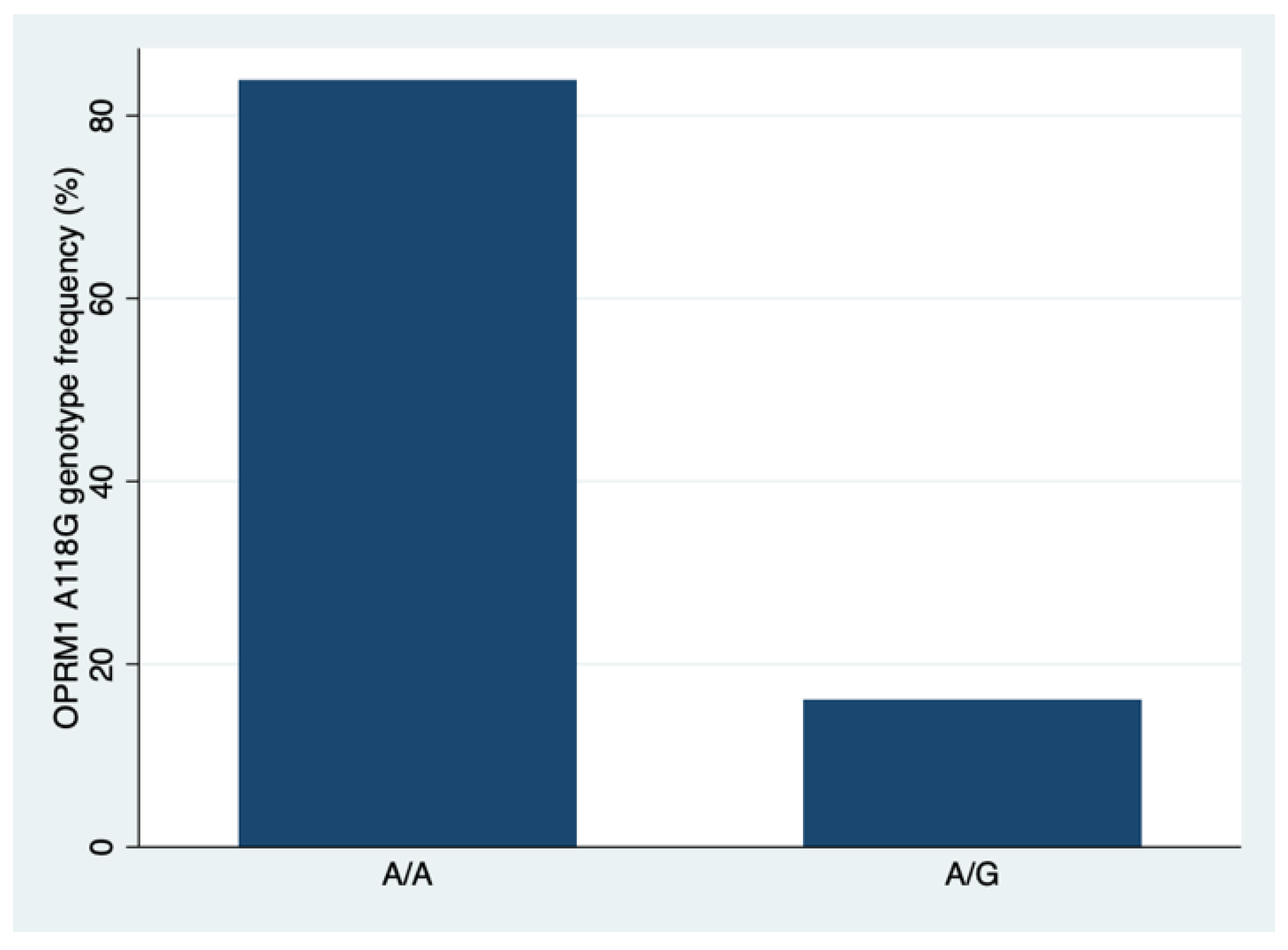
| Characteristic | N | OPRM1 | p 3 0.001 | |
|---|---|---|---|---|
| AA, n = 26 (83.9%) 1 | AG, n = 5 (16.1%) 1 | |||
| Age, years | 31 | 15.0 (13.0, 16.0) | 13.0 (11.0, 14.0) | 0.302 |
| Age group: | 31 | 0.224 4 | ||
| up to 9 yrs. | 3.0 (11.5%) 2 | 1.0 (20.0%) 2 | ||
| 10–14 yrs. | 7.0 (26.9%) 2 | 3.0 (60.0%) 2 | ||
| 15–19 yrs. | 16.0 (61.5%) 2 | 1.0 (20.0%) 2 | ||
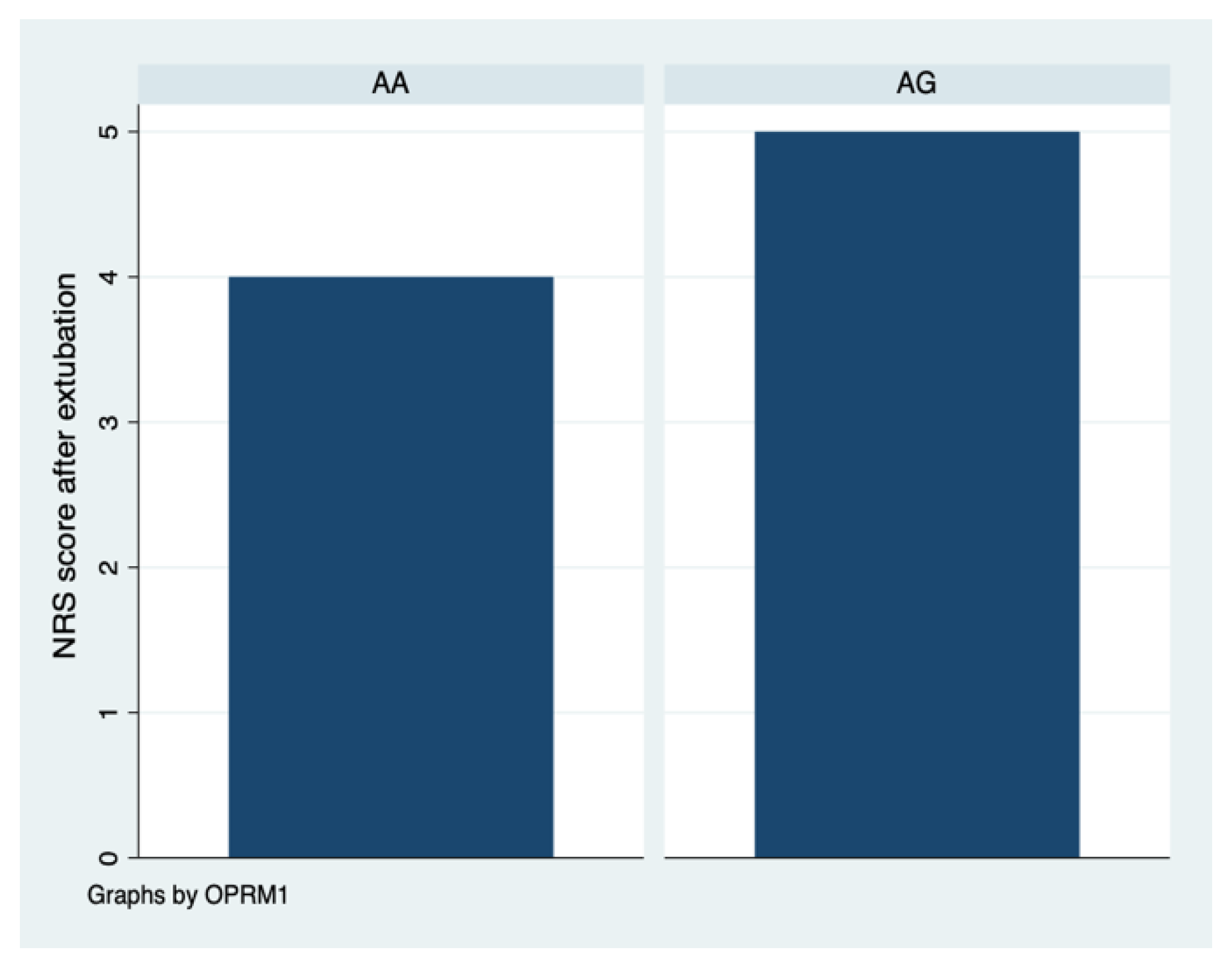
| Pain assessment after extubation | 31 | 4.0 (2.0, 7.0) | 5.0 (4.0, 6.0) | 0.684 |
| Flow of the drug, [µg/h] | 31 | 4.2 (1.6) 5 | 4.1 (2.0) 5 | 0.970 6 |
| Flow of the drug, [µg/kg/h] | 31 | 0.09 (0.04) 5 | 0.09 (0.01) 5 | 0.790 6 |
| Stay in the PACU, hours | 31 | 2.2 (1.9, 2.5) | 2.1 (2.0, 2.3) | 0.610 |
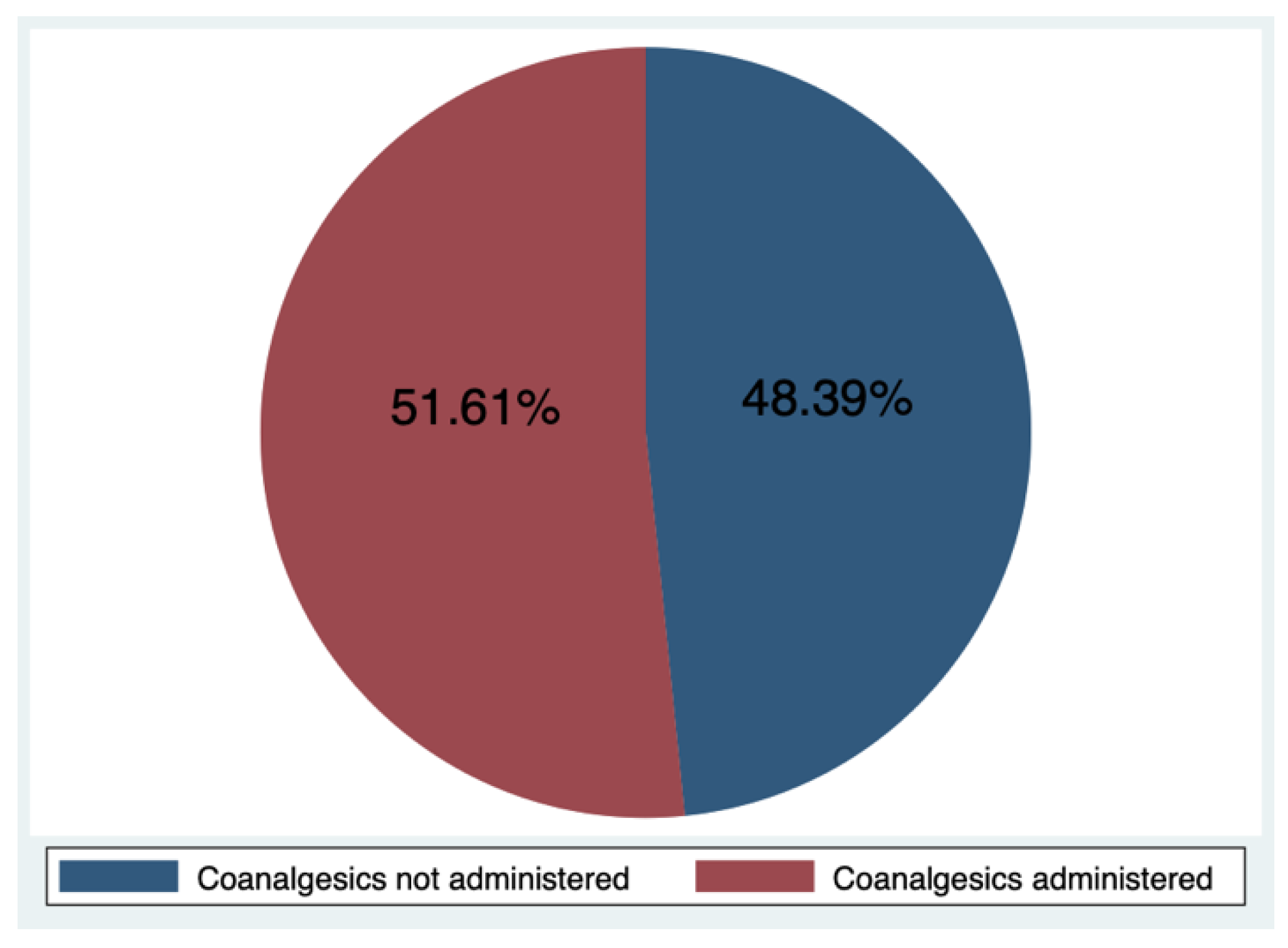
| Use of coanalgesics: | 31 | 0.333 | ||
| no | 14.0 (53.8%) | 1.0 (20.0%) | ||
| Yes | 12.0 (46.2%) | 4.0 (80.0%) | ||
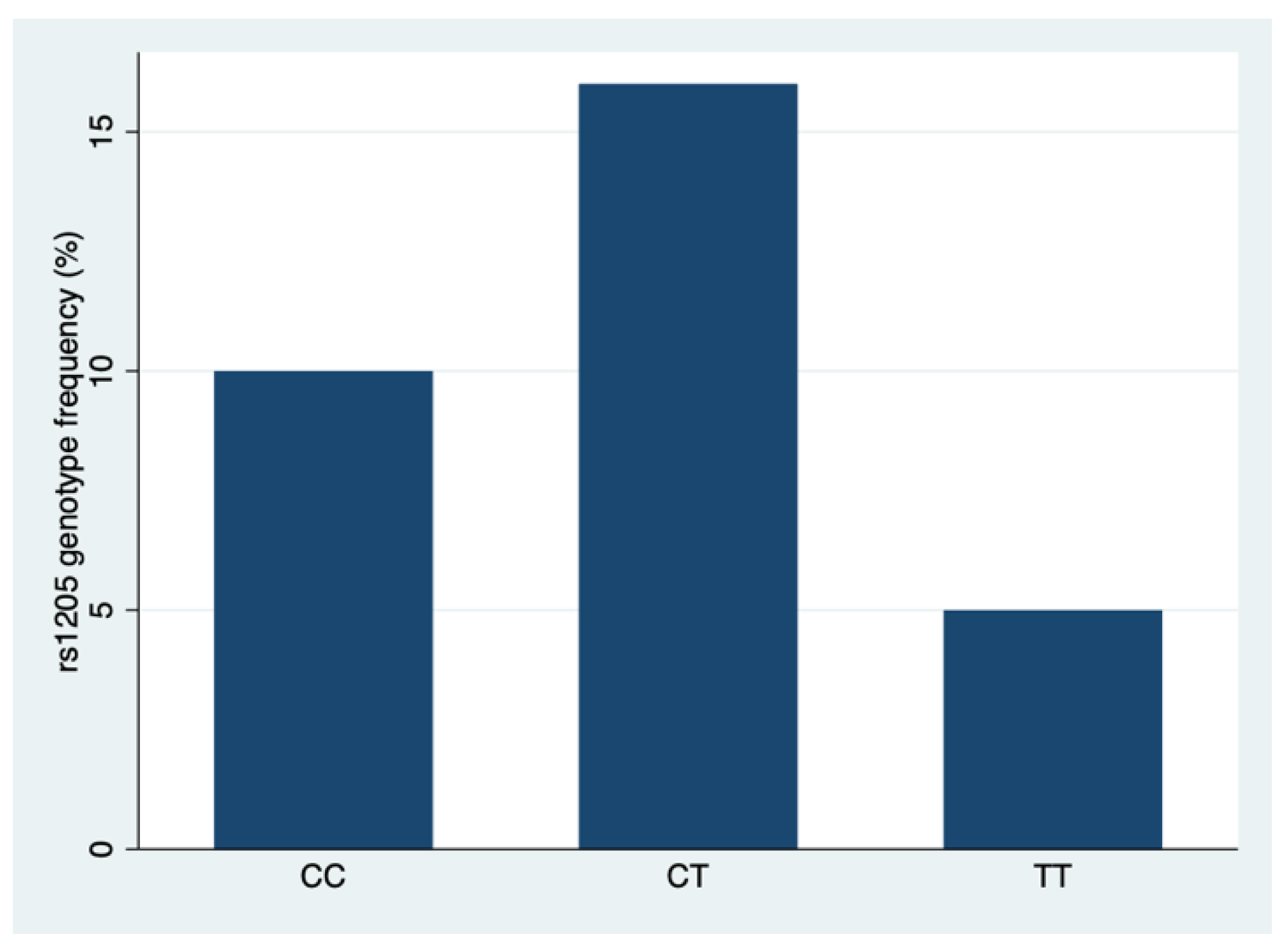
| Characteristic | N | rs1205 CRP | p 4 0.001 | ||
|---|---|---|---|---|---|
| CC, n = 10 (32.3%) 1 | CT, n = 16 (51.6%) 1 | TT, n = 5 (16.1%) 1 | |||
| Age, years | 31 | 15.0 (12.2, 15.8) | 13.5 (11.8, 15.0) | 16.0 (16.0, 16.0) | 0.021 |
| Age group: | 31 | 0.121 5 | |||
| up to 9 yrs | 2.0 (20.0%) 2 | 2.0 (12.5%) 2 | 0.0 (0.0%) 2 | ||
| 10–14 yrs | 2.0 (20.0%) 2 | 8.0 (50.0%) 2 | 0.0 (0.0%) 2 | ||
| 15–19 yrs | 6.0 (60.0%) 2 | 6.0 (37.5%) 2 | 5.0 (100.0%) 2 | ||
| Pain assessment after extubation | 31 | 4.2 (2.8) 3 | 5.3 (2.6) 3 | 4.8 (2.2) 3 | 0.630 6 |
| Flow of the drug, [µg/h] | 31 | 4.03 (1.88) 3 | 4.29 (1.65) 3 | 4.0 (1.25) 3 | 0.900 6 |
| Flow of the drug, [µg/kg/h] | 31 | 0.09 (0.04) 3 | 0.09 (0.03) 3 | 0.07 (0.03) 3 | 0.470 6 |
| Stay in the PACU, hours | 31 | 2.2 (1.9, 2.7) | 2.0 (1.8, 2.3) | 2.4 (2.4, 2.5) | 0.189 |
| Use of coanalgesics: | 31 | 1.000 | |||
| no | 5.0 (50.0%) | 8.0 (50.0%) | 2.0 (40.0%) | ||
| yes | 5.0 (50.0%) | 8.0 (50.0%) | 3.0 (60.0%) | ||
| Characteristic | Rho | p |
|---|---|---|
| Post-extubation pain assessment | 0.06 | 0.732 |
| Flow of the drug, [µg/h] | −0.20 | 0.250 |
| Flow of the drug, [µg/kg/h] | −0.08 | 0.621 |
| Characteristic | N | Use of Coanalgesics | p 2 | |
|---|---|---|---|---|
| No, n = 15 1 | Yes, n = 16 1 | |||
| Stay in the PACU, hours | 31 | 2.0 (1.8, 2.5) | 2.3 (2.1, 2.5) | 0.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turczynowicz, A.; Jakubów, P.; Niedźwiecka, K.; Kondracka, J.; Pużyńska, W.; Tałałaj, M.; Guszczyn, T.; Grabala, P.; Kowalczuk, O.; Kocańda, S. Mu-Opioid Receptor 1 and C-Reactive Protein Single Nucleotide Polymorphisms as Biomarkers of Pain Intensity and Opioid Consumption. Brain Sci. 2023, 13, 1629. https://doi.org/10.3390/brainsci13121629
Turczynowicz A, Jakubów P, Niedźwiecka K, Kondracka J, Pużyńska W, Tałałaj M, Guszczyn T, Grabala P, Kowalczuk O, Kocańda S. Mu-Opioid Receptor 1 and C-Reactive Protein Single Nucleotide Polymorphisms as Biomarkers of Pain Intensity and Opioid Consumption. Brain Sciences. 2023; 13(12):1629. https://doi.org/10.3390/brainsci13121629
Chicago/Turabian StyleTurczynowicz, Aleksander, Piotr Jakubów, Karolina Niedźwiecka, Julia Kondracka, Weronika Pużyńska, Mariola Tałałaj, Tomasz Guszczyn, Paweł Grabala, Oksana Kowalczuk, and Szymon Kocańda. 2023. "Mu-Opioid Receptor 1 and C-Reactive Protein Single Nucleotide Polymorphisms as Biomarkers of Pain Intensity and Opioid Consumption" Brain Sciences 13, no. 12: 1629. https://doi.org/10.3390/brainsci13121629
APA StyleTurczynowicz, A., Jakubów, P., Niedźwiecka, K., Kondracka, J., Pużyńska, W., Tałałaj, M., Guszczyn, T., Grabala, P., Kowalczuk, O., & Kocańda, S. (2023). Mu-Opioid Receptor 1 and C-Reactive Protein Single Nucleotide Polymorphisms as Biomarkers of Pain Intensity and Opioid Consumption. Brain Sciences, 13(12), 1629. https://doi.org/10.3390/brainsci13121629






