Increased Inhibition May Contribute to Maintaining Normal Network Function in the Ventral Hippocampus of a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Hippocampal Slices
2.2. Electrophysiological Recordings
2.3. Western Blotting
2.4. Statistics
3. Results
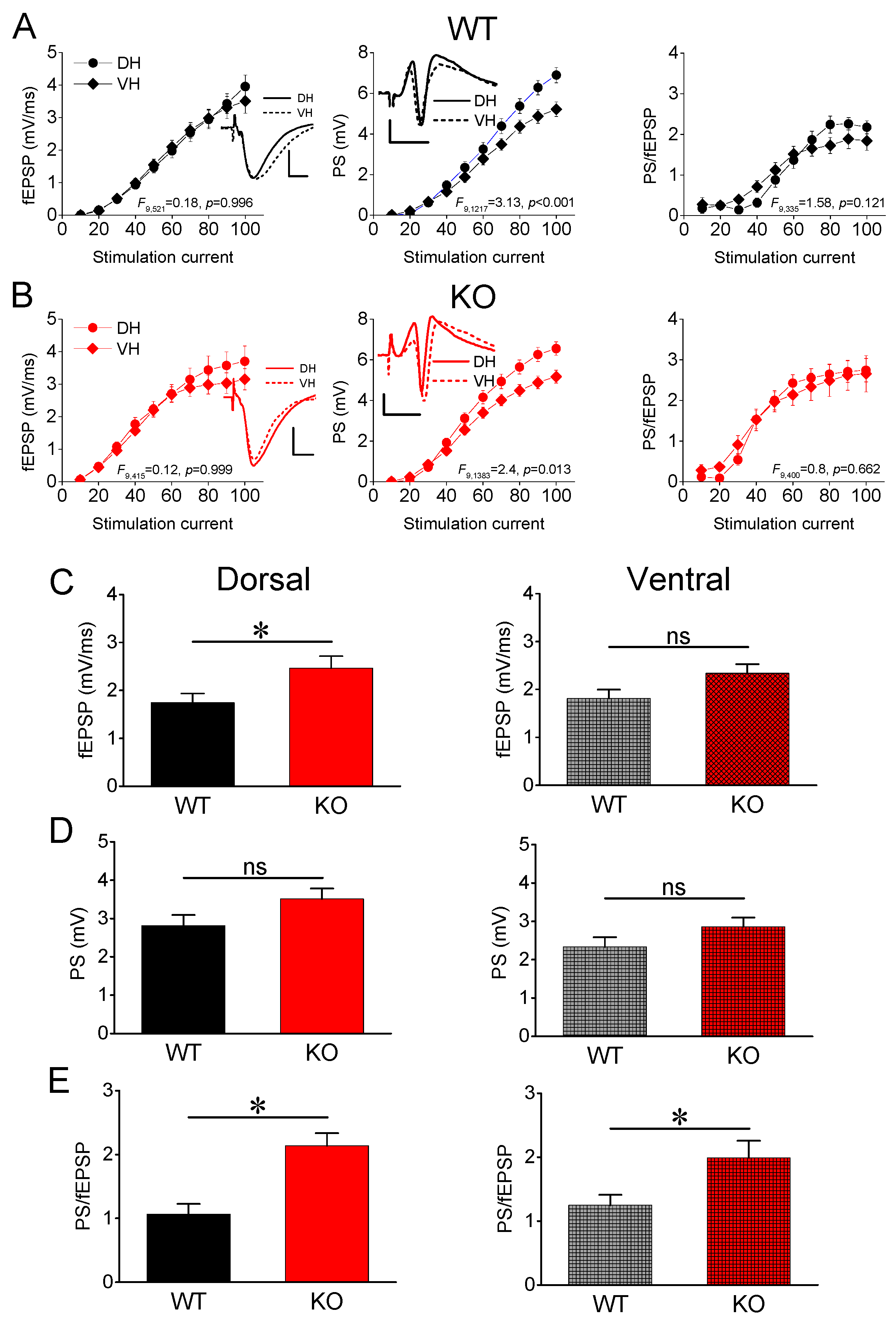
3.1. Synaptic Transmission and Neuronal Excitability
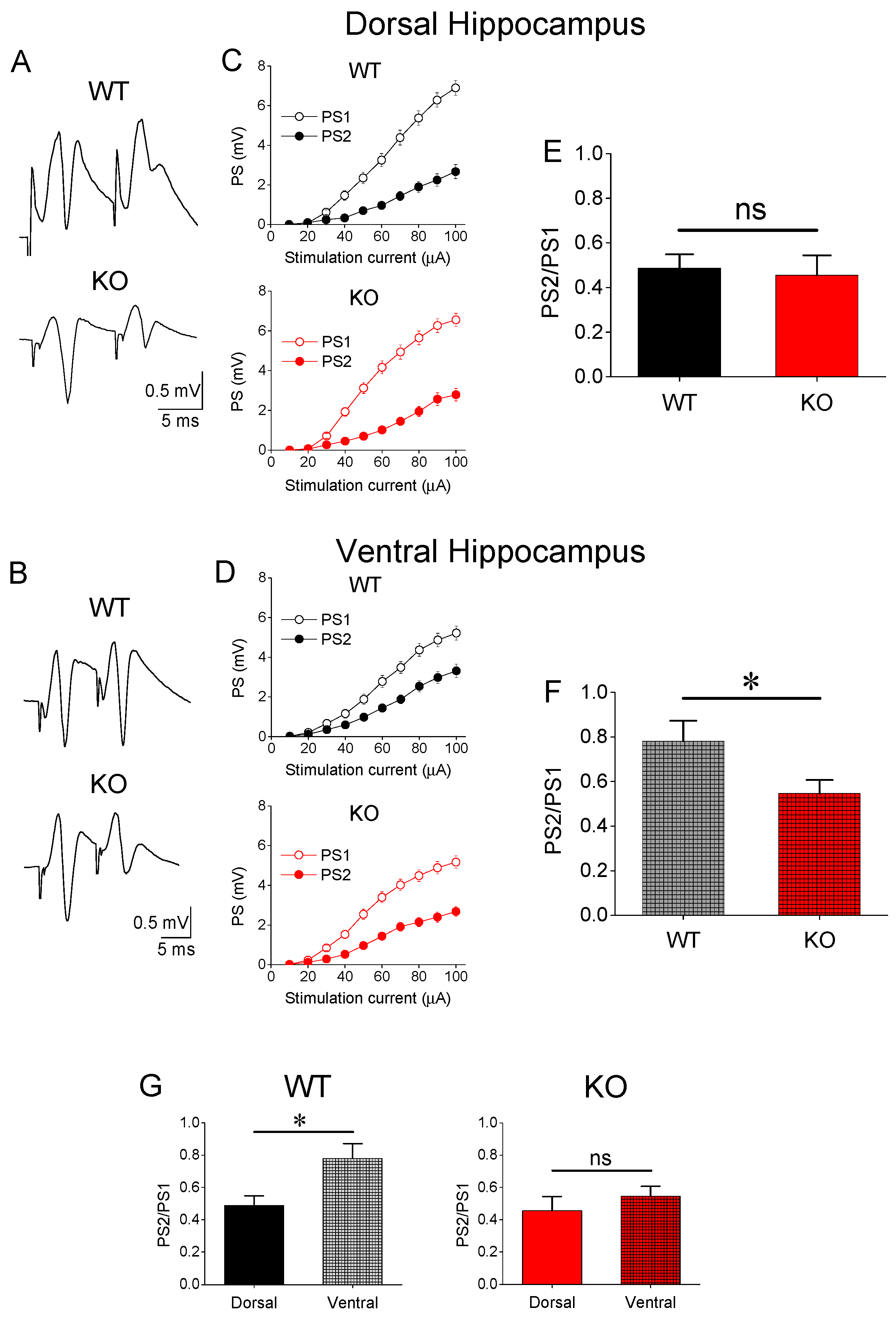
3.2. Paired-Pulse Inhibition (PPI)
3.3. Expression of α1 GABAA Receptors
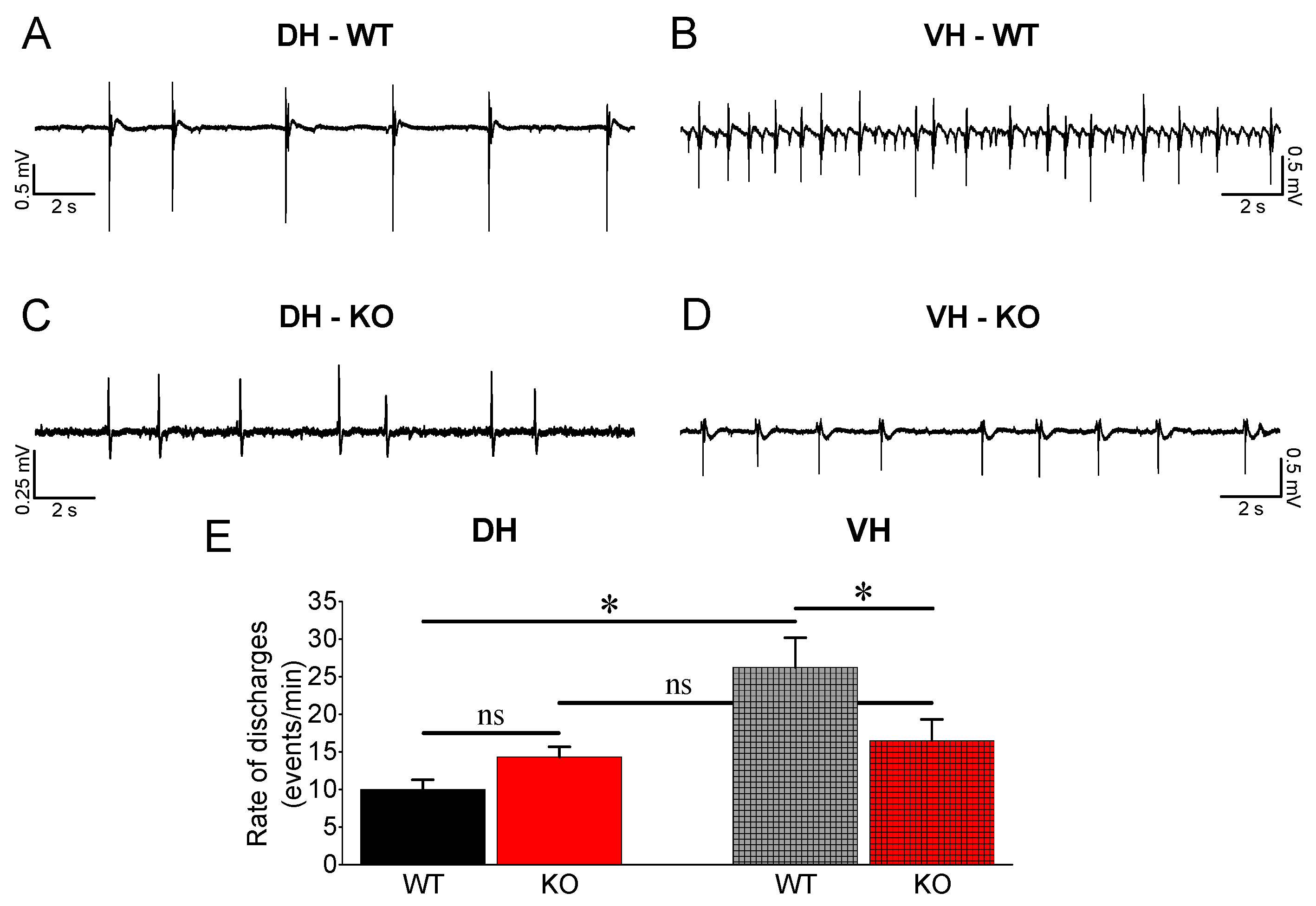
3.4. Epileptiform Activity
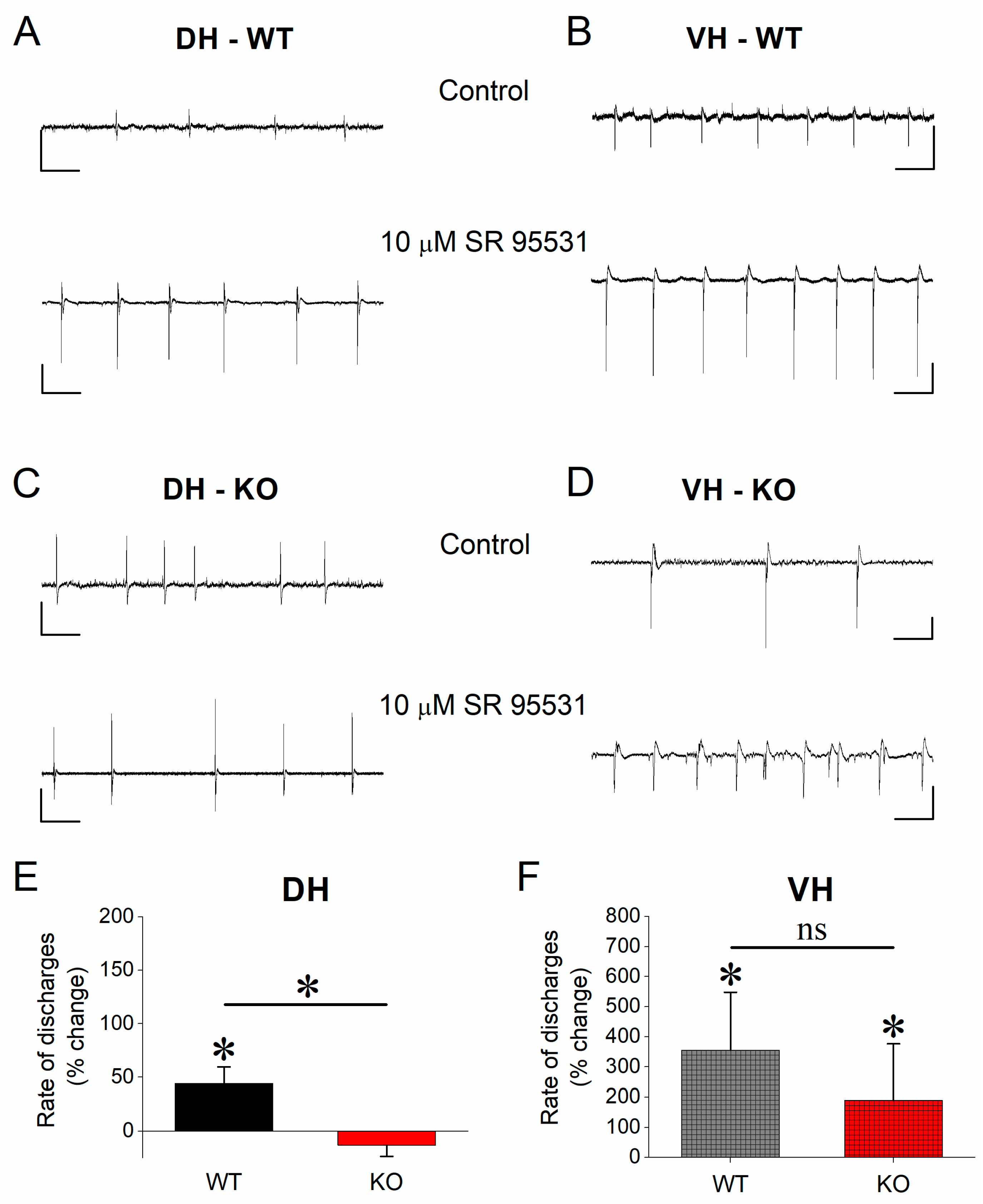
3.5. Effect of SR 95531 on Epileptiform Population Discharges
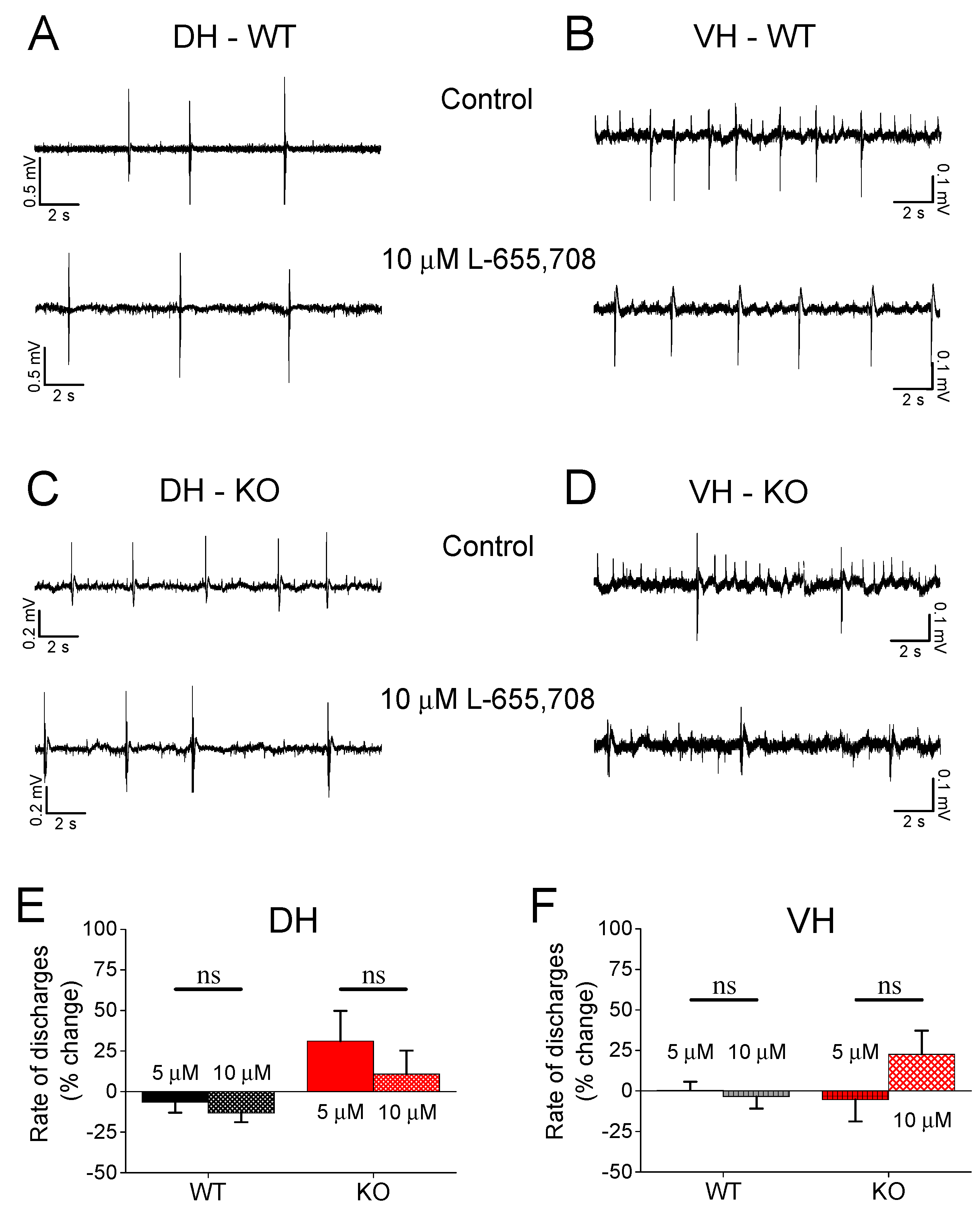
3.6. Effect of L-655,708 on Epileptiform Population Discharges
3.7. Normal Protein Expression of α5 GABAA Receptors in KO Dorsal and Ventral Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kooy, R.F.; D’Hooge, R.; Reyniers, E.; Bakker, C.E.; Nagels, G.; De Boulle, K.; Storm, K.; Clincke, G.; De Deyn, P.P.; Oostra, B.A.; et al. Transgenic mouse model for the fragile X syndrome. Am. J. Med. Genet. 1996, 64, 241–245. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef]
- Kidd, S.A.; Lachiewicz, A.; Barbouth, D.; Blitz, R.K.; Delahunty, C.; McBrien, D.; Visootsak, J.; Berry-Kravis, E. Fragile X syndrome: A review of associated medical problems. Pediatrics 2014, 134, 995–1005. [Google Scholar] [CrossRef]
- Kaufmann, W.E.; Kidd, S.A.; Andrews, H.F.; Budimirovic, D.B.; Esler, A.; Haas-Givler, B.; Stackhouse, T.; Riley, C.; Peacock, G.; Sherman, S.L.; et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 2017, 139, S194–S206. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Mesibov, G.B.; Hatton, D.D.; Clark, R.D.; Roberts, J.E.; Mayhew, L. Autistic behavior in young boys with fragile X syndrome. J. Autism Dev. Disord. 1998, 28, 499–508. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Jackson, A.W., 3rd; Levitas, A.; Rimland, B.; Braden, M. An analysis of autism in fifty males with the fragile X syndrome. Am. J. Med. Genet. 1986, 23, 359–374. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Booker, S.A.; Kind, P.C. Mechanisms regulating input-output function and plasticity of neurons in the absence of FMRP. Brain Res. Bull. 2021, 175, 69–80. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Nelson, S.B.; Valakh, V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698. [Google Scholar] [CrossRef]
- Selby, L.; Zhang, C.; Sun, Q.Q. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci. Lett. 2007, 412, 227–232. [Google Scholar] [CrossRef]
- D’Hulst, C.; De Geest, N.; Reeve, S.P.; Van Dam, D.; De Deyn, P.P.; Hassan, B.A.; Kooy, R.F. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006, 1121, 238–245. [Google Scholar] [CrossRef]
- D’Hulst, C.; Heulens, I.; Brouwer, J.R.; Willemsen, R.; De Geest, N.; Reeve, S.P.; De Deyn, P.P.; Hassan, B.A.; Kooy, R.F. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res. 2009, 1253, 176–183. [Google Scholar] [CrossRef]
- Sabanov, V.; Braat, S.; D’Andrea, L.; Willemsen, R.; Zeidler, S.; Rooms, L.; Bagni, C.; Kooy, R.F.; Balschun, D. Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology 2017, 116, 71–81. [Google Scholar] [CrossRef]
- Adusei, D.C.; Pacey, L.K.; Chen, D.; Hampson, D.R. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 2010, 59, 167–171. [Google Scholar] [CrossRef]
- El Idrissi, A.; Ding, X.H.; Scalia, J.; Trenkner, E.; Brown, W.T.; Dobkin, C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 2005, 377, 141–146. [Google Scholar] [CrossRef]
- Davidovic, L.; Navratil, V.; Bonaccorso, C.M.; Catania, M.V.; Bardoni, B.; Dumas, M.E. A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res. 2011, 21, 2190–2202. [Google Scholar] [CrossRef]
- Braat, S.; D’Hulst, C.; Heulens, I.; De Rubeis, S.; Mientjes, E.; Nelson, D.L.; Willemsen, R.; Bagni, C.; Van Dam, D.; De Deyn, P.P.; et al. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 2015, 14, 2985–2995. [Google Scholar] [CrossRef]
- Wahlstrom-Helgren, S.; Klyachko, V.A. GABAB receptor-mediated feed-forward circuit dysfunction in the mouse model of fragile X syndrome. J. Physiol. 2015, 593, 5009–5024. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, Z.; Tong, X.; Lindemeyer, A.K.; Cetina, Y.; Huang, C.S.; Olsen, R.W.; Otis, T.S.; Houser, C.R. Decreased surface expression of the δ subunit of the GABA(A) receptor contributes to reduced tonic inhibition in dentate granule cells in a mouse model of fragile X syndrome. Exp. Neurol. 2017, 297, 168–178. [Google Scholar] [CrossRef]
- Paluszkiewicz, S.M.; Olmos-Serrano, J.L.; Corbin, J.G.; Huntsman, M.M. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J. Neurophysiol. 2011, 106, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.R.; Bartley, A.F.; Hays, S.A.; Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008, 100, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Morin-Parent, F.; Champigny, C.; Lacroix, A.; Corbin, F.; Lepage, J.F. Hyperexcitability and impaired intracortical inhibition in patients with fragile-X syndrome. Transl. Psychiatry 2019, 9, 312. [Google Scholar] [CrossRef]
- Conde, V.; Palomar, F.J.; Lama, M.J.; Martínez, R.; Carrillo, F.; Pintado, E.; Mir, P. Abnormal GABA-mediated and cerebellar inhibition in women with the fragile X premutation. J. Neurophysiol. 2013, 109, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Cea-Del Rio, C.A.; Nunez-Parra, A.; Freedman, S.M.; Kushner, J.K.; Alexander, A.L.; Restrepo, D.; Huntsman, M.M. Disrupted inhibitory plasticity and homeostasis in Fragile X syndrome. Neurobiol. Dis. 2020, 142, 104959. [Google Scholar] [CrossRef]
- Yang, Y.M.; Arsenault, J.; Bah, A.; Krzeminski, M.; Fekete, A.; Chao, O.Y.; Pacey, L.K.; Wang, A.; Forman-Kay, J.; Hampson, D.R.; et al. Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain. Mol. Psychiatry 2020, 25, 2017–2035. [Google Scholar] [CrossRef]
- Liu, X.; Kumar, V.; Tsai, N.P.; Auerbach, B.D. Hyperexcitability and Homeostasis in Fragile X Syndrome. Front. Mol. Neurosci. 2021, 14, 805929. [Google Scholar] [CrossRef]
- Berry-Kravis, E. Epilepsy in fragile X syndrome. Dev. Med. Child Neurol. 2002, 44, 724–728. [Google Scholar] [CrossRef]
- Incorpora, G.; Sorge, G.; Sorge, A.; Pavone, L. Epilepsy in fragile X syndrome. Brain Dev. 2002, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Kluger, G.; Böhm, I.; Laub, M.C.; Waldenmaier, C. Epilepsy and fragile X gene mutations. Pediatr. Neurol. 1996, 15, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Filipink, R.A.; Frye, R.E.; Golla, S.; Morris, S.M.; Andrews, H.; Choo, T.H.; Kaufmann, W.E. Seizures in Fragile X Syndrome: Associations and Longitudinal Analysis of a Large Clinic-Based Cohort. Front. Pediatr. 2021, 9, 736255. [Google Scholar] [CrossRef]
- Sabaratnam, M.; Vroegop, P.G.; Gangadharan, S.K. Epilepsy and EEG findings in 18 males with fragile X syndrome. Seizure 2001, 10, 60–63. [Google Scholar] [CrossRef]
- Wisniewski, K.E.; French, J.H.; Fernando, S.; Brown, W.T.; Jenkins, E.C.; Friedman, E.; Hill, A.L.; Miezejeski, C.M. Fragile X syndrome: Associated neurological abnormalities and developmental disabilities. Ann. Neurol. 1985, 18, 665–669. [Google Scholar] [CrossRef]
- Anagnostou, E.; Taylor, M.J. Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Mol. Autism 2011, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Fetit, R.; Hillary, R.F.; Price, D.J.; Lawrie, S.M. The neuropathology of autism: A systematic review of post-mortem studies of autism and related disorders. Neurosci. Biobehav. Rev. 2021, 129, 35–62. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Gong, H.; Liu, T.; Li, X.; Fan, X. Implication of Hippocampal Neurogenesis in Autism Spectrum Disorder: Pathogenesis and Therapeutic Implications. Curr. Neuropharmacol. 2023, 21, 2266–2282. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Ordemann, G.J.; Apgar, C.J.; Chitwood, R.A.; Brager, D.H. Altered A-type potassium channel function impairs dendritic spike initiation and temporoammonic long-term potentiation in Fragile X syndrome. J. Neurosci. 2021, 41, 5947–5962. [Google Scholar] [CrossRef]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Ducourneau, E.G.; Parkes, S.L.; Ferreira, G. Pathway specific interventions reveal the multiple roles of ventral hippocampus projections in cognitive functions. Rev. Neurosci. 2023, 34, 825–838. [Google Scholar] [CrossRef]
- Risold, P.Y.; Swanson, L.W. Structural evidence for functional domains in the rat hippocampus. Science 1996, 272, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Van Groen, T.; Lopes da Silva, F.H. Septotemporal distribution of entorhinal projections to the hippocampus in the cat: Electrophysiological evidence. J. Comp. Neurol. 1985, 238, 1–9. [Google Scholar] [CrossRef]
- Pikkarainen, M.; Ronkko, S.; Savander, V.; Insausti, R.; Pitkanen, A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999, 403, 229–260. [Google Scholar] [CrossRef]
- van Strien, N.M.; Cappaert, N.L.; Witter, M.P. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci. 2009, 10, 272–282. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C. Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Front. Biosci. (Landmark Ed.) 2018, 23, 109–145. [Google Scholar] [CrossRef]
- Petrides, T.; Georgopoulos, P.; Kostopoulos, G.; Papatheodoropoulos, C. The GABAA receptor-mediated recurrent inhibition in ventral compared with dorsal CA1 hippocampal region is weaker, decays faster and lasts less. Exp. Brain Res. 2007, 177, 370–383. [Google Scholar] [CrossRef]
- Maggio, N.; Segal, M. Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Milior, G.; Castro, M.A.; Sciarria, L.P.; Garofalo, S.; Branchi, I.; Ragozzino, D.; Limatola, C.; Maggi, L. Electrophysiological Properties of CA1 Pyramidal Neurons along the Longitudinal Axis of the Mouse Hippocampus. Sci. Rep. 2016, 6, 38242. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C.; Asprodini, E.; Nikita, I.; Koutsona, C.; Kostopoulos, G. Weaker synaptic inhibition in CA1 region of ventral compared to dorsal rat hippocampal slices. Brain Res. 2002, 948, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Netsyk, O.; Hammoud, H.; Korol, S.V.; Jin, Z.; Tafreshiha, A.S.; Birnir, B. Tonic GABA-activated synaptic and extrasynaptic currents in dentate gyrus granule cells and CA3 pyramidal neurons along the mouse hippocampal dorsoventral axis. Hippocampus 2020, 30, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.D.; Spencer, S.S.; Mattson, R.H.; Williamson, P.D.; Novelly, R.A. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery 1984, 15, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Babb, T.L.; Brown, W.J.; Pretorius, J.; Davenport, C.; Lieb, J.P.; Crandall, P.H. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia 1984, 25, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Quigg, M.; Bertram, E.H.; Jackson, T. Longitudinal distribution of hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsy Res. 1997, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.D.; Jefferys, J.G.; Miles, R. Analysis of the propagation of disinhibition-induced after-discharges along the guinea-pig hippocampal slice in vitro. J. Physiol. 1993, 472, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Derchansky, M.; Shahar, E.; Wennberg, R.A.; Samoilova, M.; Jahromi, S.S.; Abdelmalik, P.A.; Zhang, L.; Carlen, P.L. Model of frequent, recurrent, and spontaneous seizures in the intact mouse hippocampus. Hippocampus 2004, 14, 935–947. [Google Scholar] [CrossRef]
- Gilbert, M.; Racine, R.J.; Smith, G.K. Epileptiform burst responses in ventral vs dorsal hippocampal slices. Brain Res. 1985, 361, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, A.C.; Taylor, D.M.; Wilson, W.A. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Res. 1986, 378, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Xie, C.W.; Lewis, D.V.; Wilson, W.A.; Mitchell, C.L.; Hong, J.S. Opioid-induced epileptiform bursting in hippocampal slices: Higher susceptibility in ventral than dorsal hippocampus. J. Pharmacol. Exp. Ther. 1990, 253, 545–551. [Google Scholar]
- Papatheodoropoulos, C.; Moschovos, C.; Kostopoulos, G. Greater contribution of N-methyl-D-aspartic acid receptors in ventral compared to dorsal hippocampal slices in the expression and long-term maintenance of epileptiform activity. Neuroscience 2005, 135, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoropoulos, C. NMDA receptor-dependent high-frequency network oscillations (100-300 Hz) in rat hippocampal slices. Neurosci. Lett. 2007, 414, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Moschovos, C.; Kostopoulos, G.; Papatheodoropoulos, C. Endogenous adenosine induces NMDA receptor-independent persistent epileptiform discharges in dorsal and ventral hippocampus via activation of A2 receptors. Epilepsy Res. 2012, 100, 157–167. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C. Higher intrinsic network excitability in ventral compared with the dorsal hippocampus is controlled less effectively by GABAB receptors. BMC Neurosci. 2015, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Mikroulis, A.V.; Psarropoulou, C. Endogenous ACh effects on NMDA-induced interictal-like discharges along the septotemporal hippocampal axis of adult rats and their modulation by an early life generalized seizure. Epilepsia 2012, 53, 879–887. [Google Scholar] [CrossRef]
- Kouvaros, S.; Papatheodoropoulos, C. Theta burst stimulation-induced LTP: Differences and similarities between the dorsal and ventral CA1 hippocampal synapses. Hippocampus 2016, 26, 1542–1559. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C.; Kostopoulos, G. Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neurosci. Lett. 2000, 286, 57–60. [Google Scholar] [CrossRef]
- Maggio, N.; Segal, M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus 2007, 17, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.; Manahan-Vaughan, D. NMDA receptor-dependent synaptic plasticity in dorsal and intermediate hippocampus exhibits distinct frequency-dependent profiles. Neuropharmacology 2013, 74, 108–118. [Google Scholar] [CrossRef]
- Papaleonidopoulos, V.; Papatheodoropoulos, C. β-adrenergic receptors reduce the threshold for induction and stabilization of LTP and enhance its magnitude via multiple mechanisms in the ventral but not the dorsal hippocampus. Neurobiol. Learn. Mem. 2018, 151, 71–84. [Google Scholar] [CrossRef]
- Kouvaros, S.; Papatheodoropoulos, C. Major dorsoventral differences in the modulation of the local CA1 hippocampal network by NMDA, mGlu5, adenosine A2A and cannabinoid CB1 receptors. Neuroscience 2016, 317, 47–64. [Google Scholar] [CrossRef]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Brunig, I.; Scotti, E.; Sidler, C.; Fritschy, J.M. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 2002, 443, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Tanaka, S.; Tojo, H.; Fukumoto, S.; Imamura, S.; Takigawa, M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001, 900, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Greco, B.; Prevost, J.; Gioanni, Y. Intracerebral microinjections of dermorphin: Search for the epileptic induction thresholds. Neuroreport 1994, 5, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Haussler, U.; Bielefeld, L.; Froriep, U.P.; Wolfart, J.; Haas, C.A. Septotemporal position in the hippocampal formation determines epileptic and neurogenic activity in temporal lobe epilepsy. Cereb. Cortex 2012, 22, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Zhu, G.; Pennefather, P.; Jackson, M.F.; MacDonald, J.F.; Orser, B.A. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 2001, 59, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Bieda, M.C.; MacIver, M.B. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J. Neurophysiol. 2004, 92, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, D.M.; Ruiz, A.; Rusakov, D.M.; Scott, R.; Semyanov, A.; Walker, M.C. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: Where and why? Prog. Biophys. Mol. Biol. 2005, 87, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Caraiscos, V.B.; Elliott, E.M.; You-Ten, K.E.; Cheng, V.Y.; Belelli, D.; Newell, J.G.; Jackson, M.F.; Lambert, J.J.; Rosahl, T.W.; Wafford, K.A.; et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 3662–3667. [Google Scholar] [CrossRef] [PubMed]
- Prenosil, G.A.; Schneider Gasser, E.M.; Rudolph, U.; Keist, R.; Fritschy, J.M.; Vogt, K.E. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J. Neurophysiol. 2006, 96, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Sur, C.; Quirk, K.; Dewar, D.; Atack, J.; McKernan, R. Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric AcidA receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol. Pharmacol. 1998, 54, 928–933. [Google Scholar] [CrossRef]
- Glykys, J.; Mann, E.O.; Mody, I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 1421–1426. [Google Scholar] [CrossRef]
- Pandit, S.; Lee, G.S.; Park, J.B. Developmental changes in GABA(A) tonic inhibition are compromised by multiple mechanisms in preadolescent dentate gyrus granule cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2017, 21, 695–702. [Google Scholar] [CrossRef]
- Chuang, S.C.; Zhao, W.; Bauchwitz, R.; Yan, Q.; Bianchi, R.; Wong, R.K. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8048–8055. [Google Scholar] [CrossRef]
- Gross, C.; Yao, X.; Pong, D.L.; Jeromin, A.; Bassell, G.J. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 5693–5698. [Google Scholar] [CrossRef]
- Kalmbach, B.E.; Johnston, D.; Brager, D.H. Cell-Type Specific Channelopathies in the Prefrontal Cortex of the fmr1-/y Mouse Model of Fragile X Syndrome. eNeuro 2015, 2, ENEURO.0114-15.2015. [Google Scholar] [CrossRef]
- Luque, M.A.; Beltran-Matas, P.; Marin, M.C.; Torres, B.; Herrero, L. Excitability is increased in hippocampal CA1 pyramidal cells of Fmr1 knockout mice. PLoS ONE 2017, 12, e0185067. [Google Scholar] [CrossRef]
- Deng, P.Y.; Carlin, D.; Oh, Y.M.; Myrick, L.K.; Warren, S.T.; Cavalli, V.; Klyachko, V.A. Voltage-Independent SK-Channel Dysfunction Causes Neuronal Hyperexcitability in the Hippocampus of Fmr1 Knock-Out Mice. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 28–43. [Google Scholar] [CrossRef]
- Booker, S.A.; Simões de Oliveira, L.; Anstey, N.J.; Kozic, Z.; Dando, O.R.; Jackson, A.D.; Baxter, P.S.; Isom, L.L.; Sherman, D.L.; Hardingham, G.E.; et al. Input-Output Relationship of CA1 Pyramidal Neurons Reveals Intact Homeostatic Mechanisms in a Mouse Model of Fragile X Syndrome. Cell Rep. 2020, 32, 107988. [Google Scholar] [CrossRef] [PubMed]
- Asiminas, A.; Booker, S.A.; Dando, O.R.; Kozic, Z.; Arkell, D.; Inkpen, F.H.; Sumera, A.; Akyel, I.; Kind, P.C.; Wood, E.R. Experience-dependent changes in hippocampal spatial activity and hippocampal circuit function are disrupted in a rat model of Fragile X Syndrome. Mol. Autism 2022, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Paluszkiewicz, S.M.; Martin, B.S.; Huntsman, M.M. Fragile X syndrome: The GABAergic system and circuit dysfunction. Dev. Neurosci. 2011, 33, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T. Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome. Cells 2021, 10, 2610. [Google Scholar] [CrossRef]
- Deng, P.Y.; Kumar, A.; Cavalli, V.; Klyachko, V.A. FMRP regulates GABA(A) receptor channel activity to control signal integration in hippocampal granule cells. Cell Rep. 2022, 39, 110820. [Google Scholar] [CrossRef]
- Kang, J.Y.; Chadchankar, J.; Vien, T.N.; Mighdoll, M.I.; Hyde, T.M.; Mather, R.J.; Deeb, T.Z.; Pangalos, M.N.; Brandon, N.J.; Dunlop, J.; et al. Deficits in the activity of presynaptic γ-aminobutyric acid type B receptors contribute to altered neuronal excitability in fragile X syndrome. J. Biol. Chem. 2017, 292, 6621–6632. [Google Scholar] [CrossRef]
- Hong, A.; Zhang, A.; Ke, Y.; El Idrissi, A.; Shen, C.H. Downregulation of GABA(A) β subunits is transcriptionally controlled by Fmr1p. J. Mol. Neurosci. MN 2012, 46, 272–275. [Google Scholar] [CrossRef]
- Vicini, S.; Ferguson, C.; Prybylowski, K.; Kralic, J.; Morrow, A.L.; Homanics, G.E. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 3009–3016. [Google Scholar] [CrossRef]
- Curia, G.; Papouin, T.; Séguéla, P.; Avoli, M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex 2009, 19, 1515–1520. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.A.; Rubenstein, J.L.; Baraban, S.C. Bidirectional homeostatic plasticity induced by interneuron cell death and transplantation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.W.; Galanopoulou, A.S. Altered GABA signaling in early life epilepsies. Neural Plast. 2011, 2011, 527605. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Bódi, V.; Májer, T.; Kelemen, V.; Világi, I.; Szűcs, A.; Varró, P. Alterations of the Hippocampal Networks in Valproic Acid-Induced Rat Autism Model. Front. Neural Circuits 2022, 16, 772792. [Google Scholar] [CrossRef]
- Harris, K.M.; Teyler, T.J. Evidence for late development of inhibition in area CA1 of the rat hippocampus. Brain Res. 1983, 268, 339–343. [Google Scholar] [CrossRef]
- Swann, J.W.; Brady, R.J.; Martin, D.L. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience 1989, 28, 551–561. [Google Scholar] [CrossRef]
- Hutcheon, B.; Fritschy, J.M.; Poulter, M.O. Organization of GABA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. Eur. J. Neurosci. 2004, 19, 2475–2487. [Google Scholar] [CrossRef]
- Banks, M.I.; Hardie, J.B.; Pearce, R.A. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J. Neurophysiol. 2002, 88, 3097–3107. [Google Scholar] [CrossRef]
- Chen, X.; Shu, S.; Schwartz, L.C.; Sun, C.; Kapur, J.; Bayliss, D.A. Homeostatic regulation of synaptic excitability: Tonic GABA(A) receptor currents replace I(h) in cortical pyramidal neurons of HCN1 knock-out mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 2611–2622. [Google Scholar] [CrossRef]
- Chen, Y.W.; Actor-Engel, H.; Aoki, C. α4-GABA(A) receptors of hippocampal pyramidal neurons are associated with resilience against activity-based anorexia for adolescent female mice but not for males. Mol. Cell. Neurosci. 2018, 90, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.; Houser, C.R. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J. Comp. Neurol. 1999, 412, 488–505. [Google Scholar] [CrossRef]
- Bernard, C.; Cossart, R.; Hirsch, J.C.; Esclapez, M.; Ben-Ari, Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia 2000, 41 (Suppl. S6), S90–S95. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leontiadis, L.J.; Trompoukis, G.; Felemegkas, P.; Tsotsokou, G.; Miliou, A.; Papatheodoropoulos, C. Increased Inhibition May Contribute to Maintaining Normal Network Function in the Ventral Hippocampus of a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome. Brain Sci. 2023, 13, 1598. https://doi.org/10.3390/brainsci13111598
Leontiadis LJ, Trompoukis G, Felemegkas P, Tsotsokou G, Miliou A, Papatheodoropoulos C. Increased Inhibition May Contribute to Maintaining Normal Network Function in the Ventral Hippocampus of a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome. Brain Sciences. 2023; 13(11):1598. https://doi.org/10.3390/brainsci13111598
Chicago/Turabian StyleLeontiadis, Leonidas J., George Trompoukis, Panagiotis Felemegkas, Giota Tsotsokou, Athina Miliou, and Costas Papatheodoropoulos. 2023. "Increased Inhibition May Contribute to Maintaining Normal Network Function in the Ventral Hippocampus of a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome" Brain Sciences 13, no. 11: 1598. https://doi.org/10.3390/brainsci13111598
APA StyleLeontiadis, L. J., Trompoukis, G., Felemegkas, P., Tsotsokou, G., Miliou, A., & Papatheodoropoulos, C. (2023). Increased Inhibition May Contribute to Maintaining Normal Network Function in the Ventral Hippocampus of a Fmr1-Targeted Transgenic Rat Model of Fragile X Syndrome. Brain Sciences, 13(11), 1598. https://doi.org/10.3390/brainsci13111598






