Gait Domains May Be Used as an Auxiliary Diagnostic Index for Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Demographic and Clinical Data Collection
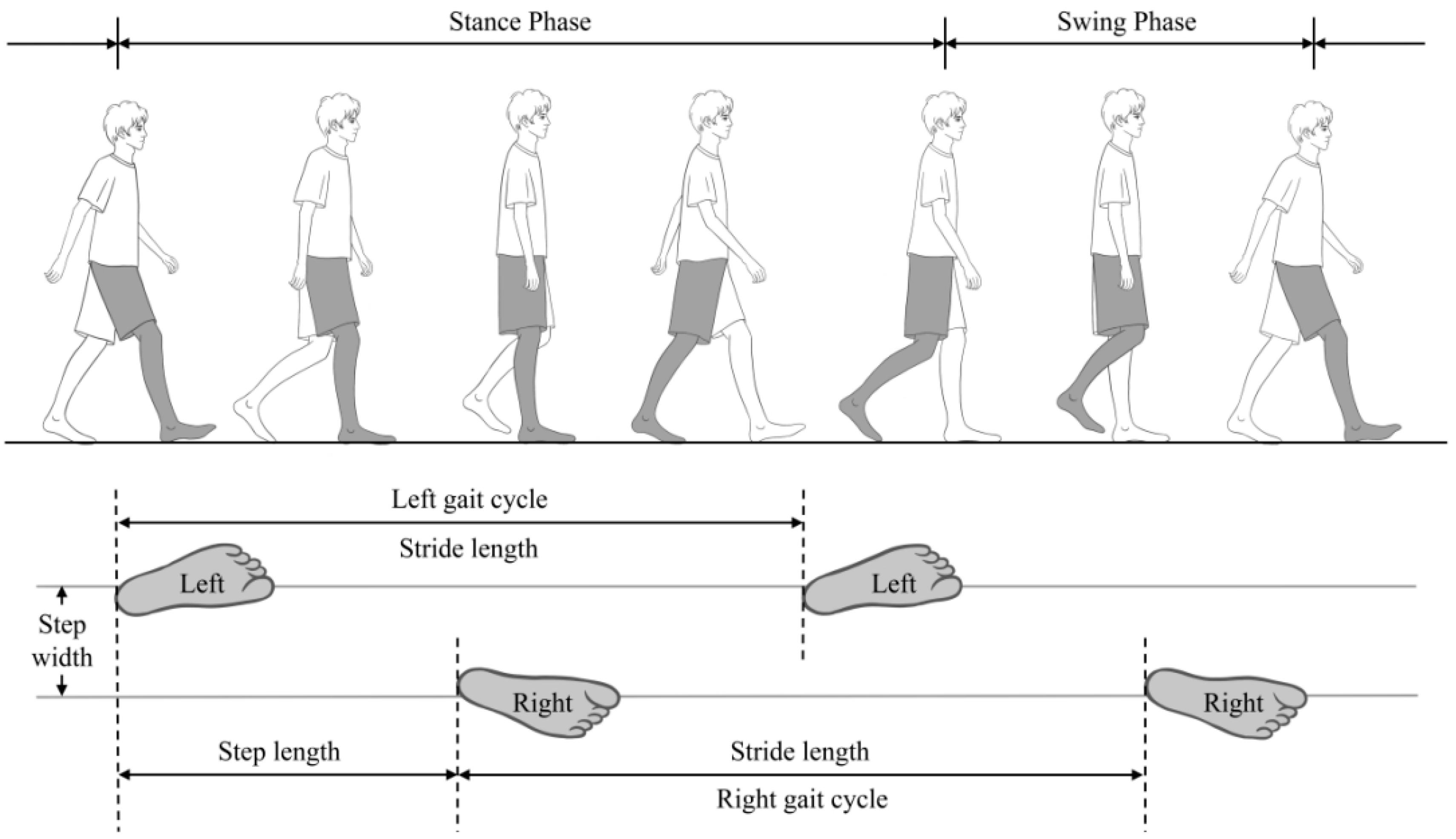
2.3. Gait Assessment
2.4. Statistical Analyses
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Gait Parameters
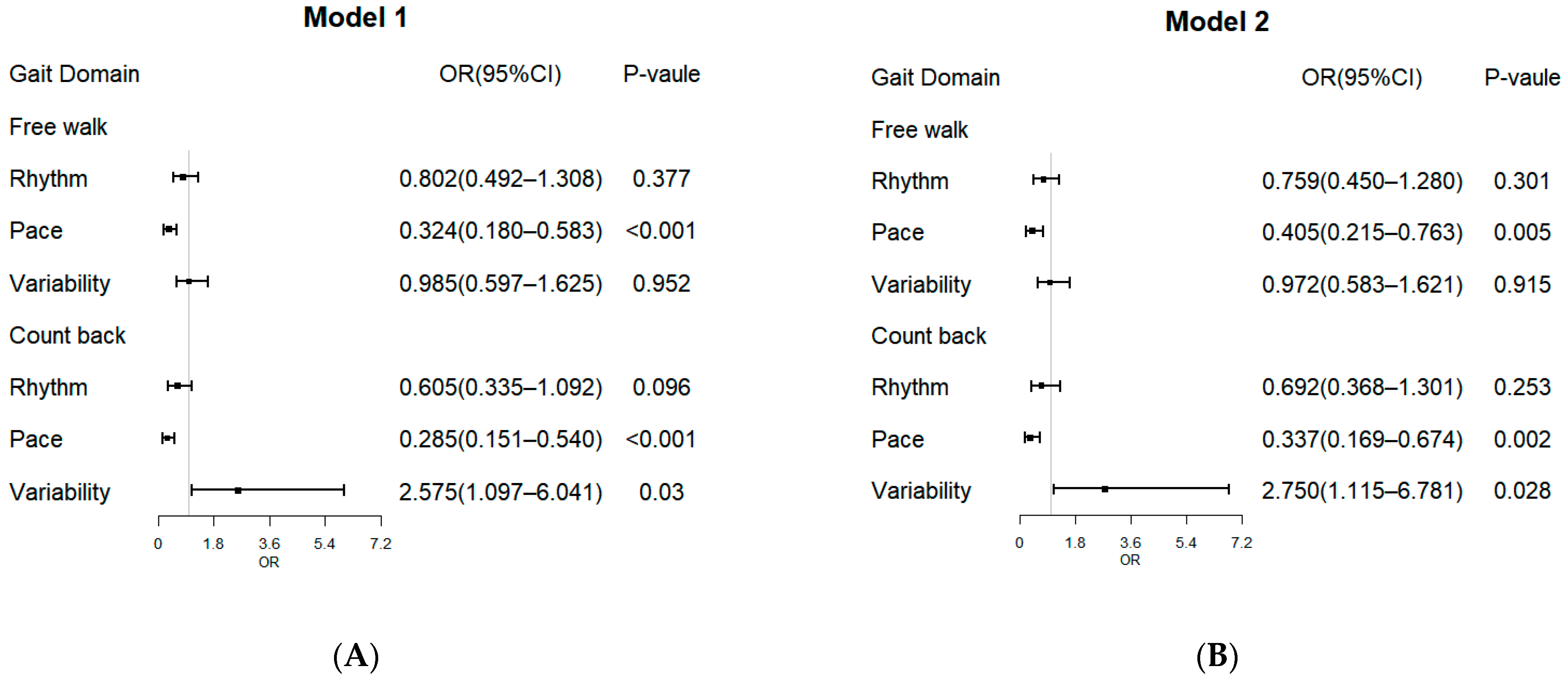
3.3. Gait Domains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural Products in Alzheimer’s Disease Therapy: Would Old Therapeutic Approaches Fix the Broken Promise of Modern Medicines? Molecules 2019, 24, 1519. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers. Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.R.; Verghese, J. Visual-Somatosensory Integration and Quantitative Gait Performance in Aging. Front. Aging Neurosci. 2018, 10, 377. [Google Scholar] [CrossRef]
- Oh-Park, M.; Holtzer, R.; Xue, X.; Verghese, J. Conventional and robust quantitative gait norms in community-dwelling older adults. J. Am. Geriatr. Soc. 2010, 58, 1512–1518. [Google Scholar] [CrossRef]
- Rosano, C.; Studenski, S.A.; Aizenstein, H.J.; Boudreau, R.M.; Longstreth, W.T., Jr.; Newman, A.B. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing 2012, 41, 58–64. [Google Scholar] [CrossRef]
- Janeh, O.; Fründt, O.; Schönwald, B.; Gulberti, A.; Buhmann, C.; Gerloff, C.; Steinicke, F.; Pötter-Nerger, M. Gait Training in Virtual Reality: Short-Term Effects of Different Virtual Manipulation Techniques in Parkinson’s Disease. Cells 2019, 8, 419. [Google Scholar] [CrossRef]
- Ricciardi, L.; Ricciardi, D.; Lena, F.; Plotnik, M.; Petracca, M.; Barricella, S.; Bentivoglio, A.R.; Modugno, N.; Bernabei, R.; Fasano, A. Working on asymmetry in Parkinson’s disease: Randomized, controlled pilot study. Neurol. Sci. 2015, 36, 1337–1343. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Johansen-Berg, H.; Kischka, U.; Zarei, M.; Cockburn, J.; Dawes, H. Prefrontal Cortex Activation While Walking Under Dual-Task Conditions in Stroke: A Multimodal Imaging Study. Neurorehabil. Neural. Repair. 2016, 30, 591–599. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Bergman, H.; Phillips, N.A.; Wong, C.H.; Sourial, N.; Chertkow, H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009, 9, 41. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Launay, C.; Herrmann, F.R.; Annweiler, C. Gait variability at fast-pace walking speed: A biomarker of mild cognitive impairment? J. Nutr. Health Aging 2013, 17, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Vasudev, A.; Burhan, A.M.; Ionson, E.; Montero-Odasso, M. Does Dual-Task Gait Differ in those with Late-Life Depression versus Mild Cognitive Impairment? Am. J. Geriatr. Psychiatry 2019, 27, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Auvinet, B.; Touzard, C.; Montestruc, F.; Delafond, A.; Goeb, V. Gait disorders in the elderly and dual task gait analysis: A new approach for identifying motor phenotypes. J. Neuroeng. Rehabil. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.P.; Huang, J.; Fan, X.M.; Tian, F. Application of Human-Computer Interaction Technology in Ancillary Diagnosis of Nervous System Diseases: Current Situation and Prospect. Med. J. Peking Union Med. Coll. Hosp. 2021, 12, 608–613. [Google Scholar]
- Rosenfeld, J.V.; Wong, Y.T. Neurobionics and the brain-computer interface: Current applications and future horizons. Med. J. Aust. 2017, 206, 363–368. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- van Schooten, K.S.; Pijnappels, M.; Rispens, S.M.; Elders, P.J.; Lips, P.; van Dieen, J.H. Ambulatory fall-risk assessment: Amount and quality of daily-life gait predict falls in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 608–615. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chung, P.C.; Wang, W.H.; Pai, M.C.; Wang, C.Y.; Lin, C.W.; Wang, J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014, 18, 1822–1830. [Google Scholar] [CrossRef]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative spatiotemporal gait parameters in older adults. Gait. Posture 2011, 34, 111–118. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R.; Xue, X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 929–935. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Coleman, S.; Yarnall, A.; Burn, D.; Rochester, L. Cognition and gait show a selective pattern of association dominated by phenotype in incident Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, G.; Gouelle, A.; Adamson, M.B.; Pieterman, M.; Jog, M. Forward and backward walking in Parkinson disease: A factor analysis. Gait. Posture 2019, 74, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ayers, E.I.; Tow, A.C.; Holtzer, R.; Verghese, J. Walking while talking and falls in aging. Gerontology 2014, 60, 108–113. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers. Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Ripoll, L.H. Clinical psychopharmacology of borderline personality disorder: An update on the available evidence in light of the Diagnostic and Statistical Manual of Mental Disorders—5. Curr. Opin. Psychiatry 2012, 25, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Hong, X.; Li, H. The mini-mental state examination in the Chinese residents population aged 55 years and over in the urban and rural areas of Beijing. Chin. J. Neurol. 1999, 32, 149–153. [Google Scholar]
- Phan, S.V.; Osae, S.; Morgan, J.C.; Inyang, M.; Fagan, S.C. Neuropsychiatric Symptoms in Dementia: Considerations for Pharmacotherapy in the USA. Drugs R D 2019, 19, 93–115. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Y.; Tao, S.; Huang, S.; Zhang, C.; Lv, Z. Wearable Sensor-Based Daily Life Walking Assessment of Gait for Distinguishing Individuals with Amnestic Mild Cognitive Impairment. Front. Aging Neurosci. 2019, 11, 285. [Google Scholar] [CrossRef]
- Phanpho, C.; Rao, S.; Moffat, M. Immediate effect of visual, auditory and combined feedback on foot strike pattern. Gait Posture 2019, 74, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, X.W.; Cai, H.Y.; Lv, Z.P.; Hu, C.Y.; Xie, H.Q. Gait based biometric personal authentication by using MEMS inertial sensors. J. Ambient. Intell. Humaniz. Comput. 2018, 9, 1705–1712. [Google Scholar] [CrossRef]
- Gao, Q.; Lv, Z.; Zhang, X.; Hou, Y.; Liu, H.; Gao, W.; Chang, M.; Tao, S. Validation of the JiBuEn® System in Measuring Gait Parameters. In Human Interaction, Emerging Technologies and Future Applications IV; IHIET-AI 2021, Advances in Intelligent Systems and Computing; Ahram, T., Taiar, R., Groff, F., Eds.; Springer: Cham, Switzerland, 2021; Volume 1378, pp. 526–531. [Google Scholar]
- Qu, X. Age-related cognitive task effects on gait characteristics: Do different working memory components make a difference? J. Neuroeng. Rehabil. 2014, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Vetter, T.R. Logistic Regression in Medical Research. Anesth. Analg. 2021, 132, 365–366. [Google Scholar] [CrossRef]
- Cullen, S.; Borrie, M.; Carroll, S.; Sarquis-Adamson, Y.; Pieruccini-Faria, F.; McKay, S.; Montero-Odasso, M. Are Cognitive Subtypes Associated with Dual-Task Gait Performance in a Clinical Setting? J. Alzheimers. Dis. 2019, 71, S57–S64. [Google Scholar] [CrossRef]
- Machado, A.; Ferreira, D.; Grothe, M.J.; Eyjolfsdottir, H.; Almqvist, P.M.; Cavallin, L.; Lind, G.; Linderoth, B.; Seiger, Å.; Teipel, S.; et al. Alzheimer’s Disease Neuroimaging Initiative. The cholinergic system in subtypes of Alzheimer’s disease: An in vivo longitudinal MRI study. Alzheimers. Res. Ther. 2020, 12, 51. [Google Scholar] [CrossRef]
- Lundin-Olsson, L.; Nyberg, L.; Gustafson, Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet 1997, 349, 617. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Galna, B.; Donaghy, P.; Thomas, A.; Rochester, L. Do Alzheimer’s and Lewy body disease have discrete pathological signatures of gait? Alzheimers Dement. 2019, 15, 1367–1377. [Google Scholar] [CrossRef]
- Allan, L.M.; Ballard, C.G.; Burn, D.J.; Kenny, R.A. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J. Am. Geriatr. Soc. 2005, 53, 1681–1687. [Google Scholar] [CrossRef]
| AD (n = 41) | HC (n = 41) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean ± SD | 68.2 ± 8.1 | 62.1 ± 8.3 | <0.001 |
| Sex, female, n (%) | 26 (63.4%) | 20 (48.8%) | 0.182 |
| BMI (kg/m2), mean ± SD | 23.2 ± 2.8 | 23.5 ± 3.3 | 0.655 |
| Education levels, n (%) | 0.010 | ||
| Illiteracy | 12 (29.3%) | 4 (9.8%) | |
| Primary school | 14 (34.1%) | 27 (65.8%) | |
| Middle school and higher | 15 (36.6%) | 10 (24.4%) | |
| MMSE score, median (IQR) | 13.0 (7.0–19.0) | 25.0 (25.0–28.0) | <0.001 |
| Medications, n (%) | <0.001 | ||
| Aricept | 38 (92.7%) | 0 (0.0%) | |
| Memantine | 25 (61.0%) | 0 (0.0%) | |
| SSRI (sertraline) | 5 (12.2%) | 0 (0.0%) | |
| Antipsychotics | |||
| Olanzapine | 5 (12.2%) | 0 (0.0%) | |
| Risperidone | 2 (4.9%) | 0 (0.0%) | |
| Quetiapine | 1 (2.4%) | 0 (0.0%) | |
| Cozapine | 1 (2.4%) | 0 (0.0%) | |
| Gait parameters | |||
| Free walk | |||
| Stride length (m), mean ± SD | 0.94 ± 0.19 | 1.11 ± 0.17 | <0.001 |
| Gait velocity (m/s), mean ± SD | 0.78 ± 0.20 | 0.93 ± 0.19 | 0.001 |
| Gait frequency (steps/min), mean ± SD | 98.50 ± 12.48 | 100.25 ± 10.26 | 0.489 |
| Stance phase (%), mean ± SD | 66.55 ± 3.18 | 64.57 ± 2.01 | 0.001 |
| Swing phase (%), mean ± SD | 33.45 ± 3.18 | 35.43 ± 2.02 | 0.001 |
| Stride time (s), median (IQR) | 1.19 (1.12–1.29) | 1.17 (1.11–1.30) | 0.633 |
| Swing time (s), median (IQR) | 0.78 (0.73–0.85) | 0.75 (0.70–0.85) | 0.294 |
| Stride time variability (CV), median (IQR) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 0.466 |
| Swing phase variability (CV), median (IQR) | 0.04 (0.03–0.05) | 0.03 (0.03–0.04) | 0.537 |
| Count backward | |||
| Stride length (m), mean ± SD | 0.90 ± 0.18 | 1.10 ± 0.20 | <0.001 |
| Gait velocity (m/s), mean ± SD | 0.63 ± 0.20 | 0.82 ± 0.20 | <0.001 |
| Gait frequency (steps/min), mean ± SD | 82.98 ±17.16 | 89.56 ± 14.81 | 0.068 |
| Stance phase (%), mean ± SD | 68.93 ± 4.67 | 65.66 ± 2.76 | <0.001 |
| Swing phase (%), mean ± SD | 31.07 ±4.67 | 34.34 ± 2.76 | <0.001 |
| Stride time (s), mean ± SD | 1.52 ± 0.39 | 1.38 ± 0.26 | 0.058 |
| Swing time (s), mean ± SD | 0.46 ± 0.05 | 0.47 ± 0.06 | 0.399 |
| Stride time variability (CV), median (IQR) | 0.06 (0.03–0.11) | 0.04 (0.03–0.06) | 0.023 |
| Swing phase variability (CV), median (IQR) | 0.05 (0.04–0.08) | 0.04 (0.03–0.05) | 0.004 |
| Gait Parameters | OR | 95% CI | p-Value |
|---|---|---|---|
| Free walk | |||
| Stride length (m) | 0.012 | 0.001–0.277 | 0.006 |
| Gait velocity (m/s) | 0.034 | 0.002–0.536 | 0.016 |
| Gait frequency (steps/min) | 0.980 | 0.938–1.024 | 0.375 |
| Stance phase (%) | 1.272 | 1.032–1.568 | 0.024 |
| Swing phase (%) | 0.786 | 0.638–0.969 | 0.024 |
| Stride time (s) | 6.166 | 0.368–103.435 | 0.206 |
| Swing time (s) | 8.363 | 0.399–175.135 | 0.171 |
| Stride time variability (CV) | 1.027 | 0.909–1.159 | 0.670 |
| Swing phase variability (CV) | 1.029 | 0.911–1.162 | 0.646 |
| Count backward | |||
| Stride length (m) | 0.009 | 0.000–0.199 | 0.003 |
| Gait velocity (m/s) | 0.019 | 0.001–0.333 | 0.007 |
| Gait frequency (steps/min) | 0.984 | 0.952–1.016 | 0.327 |
| Stance phase (%) | 1.244 | 1.045–1.480 | 0.014 |
| Swing phase (%) | 0.804 | 0.676–0.957 | 0.014 |
| Stride time (s) | 2.421 | 0.435–13.480 | 0.313 |
| Swing time (s) | 0.021 | 0.000–136.087 | 0.389 |
| Stride time variability (CV) | 1.146 | 1.012–1.298 | 0.031 |
| Swing phase variability (CV) | 1.156 | 1.007–1.327 | 0.040 |
| Gait Parameters | Gait Domains | ||
|---|---|---|---|
| Rhythm Factor | Pace Factor | Variability Factor | |
| Free walk | |||
| Stride length (m) | 0.148 | 0.984 | −0.055 |
| Gait velocity (m/s) | 0.539 | 0.812 | −0.083 |
| Gait frequency (steps/min) | 0.934 | 0.114 | −0.18 |
| Stance phase (%) | −0.763 | −0.512 | 0.285 |
| Swing phase (%) | 0.763 | 0.512 | −0.285 |
| Stride time variability (CV) | −0.124 | −0.055 | 0.971 |
| Swing phase variability (CV) | −0.29 | −0.108 | 0.919 |
| Variance explained, % | 34.98 | 31.13 | 28.46 |
| Count backward | |||
| Stride length (m) | 0.179 | 0.981 | −0.027 |
| Gait velocity (m/s) | 0.632 | 0.710 | −0.190 |
| Gait frequency (steps/min) | 0.903 | 0.046 | −0.339 |
| Stance phase (%) | −0.823 | −0.466 | 0.249 |
| Swing phase (%) | 0.823 | 0.466 | −0.249 |
| Stride time variability (CV) | −0.168 | 0.003 | 0.960 |
| Swing phase variability (CV) | −0.432 | −0.218 | 0.821 |
| Variance explained, % | 40.23 | 27.86 | 26.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Q.; Zhang, Y.; Zhuang, W.; Li, W.; He, J.; Wang, Z.; Cheng, H. Gait Domains May Be Used as an Auxiliary Diagnostic Index for Alzheimer’s Disease. Brain Sci. 2023, 13, 1599. https://doi.org/10.3390/brainsci13111599
Duan Q, Zhang Y, Zhuang W, Li W, He J, Wang Z, Cheng H. Gait Domains May Be Used as an Auxiliary Diagnostic Index for Alzheimer’s Disease. Brain Sciences. 2023; 13(11):1599. https://doi.org/10.3390/brainsci13111599
Chicago/Turabian StyleDuan, Qi, Yinuo Zhang, Weihao Zhuang, Wenlong Li, Jincai He, Zhen Wang, and Haoran Cheng. 2023. "Gait Domains May Be Used as an Auxiliary Diagnostic Index for Alzheimer’s Disease" Brain Sciences 13, no. 11: 1599. https://doi.org/10.3390/brainsci13111599
APA StyleDuan, Q., Zhang, Y., Zhuang, W., Li, W., He, J., Wang, Z., & Cheng, H. (2023). Gait Domains May Be Used as an Auxiliary Diagnostic Index for Alzheimer’s Disease. Brain Sciences, 13(11), 1599. https://doi.org/10.3390/brainsci13111599







