Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics
Abstract
1. Introduction
2. Materials and Methods
3. Results
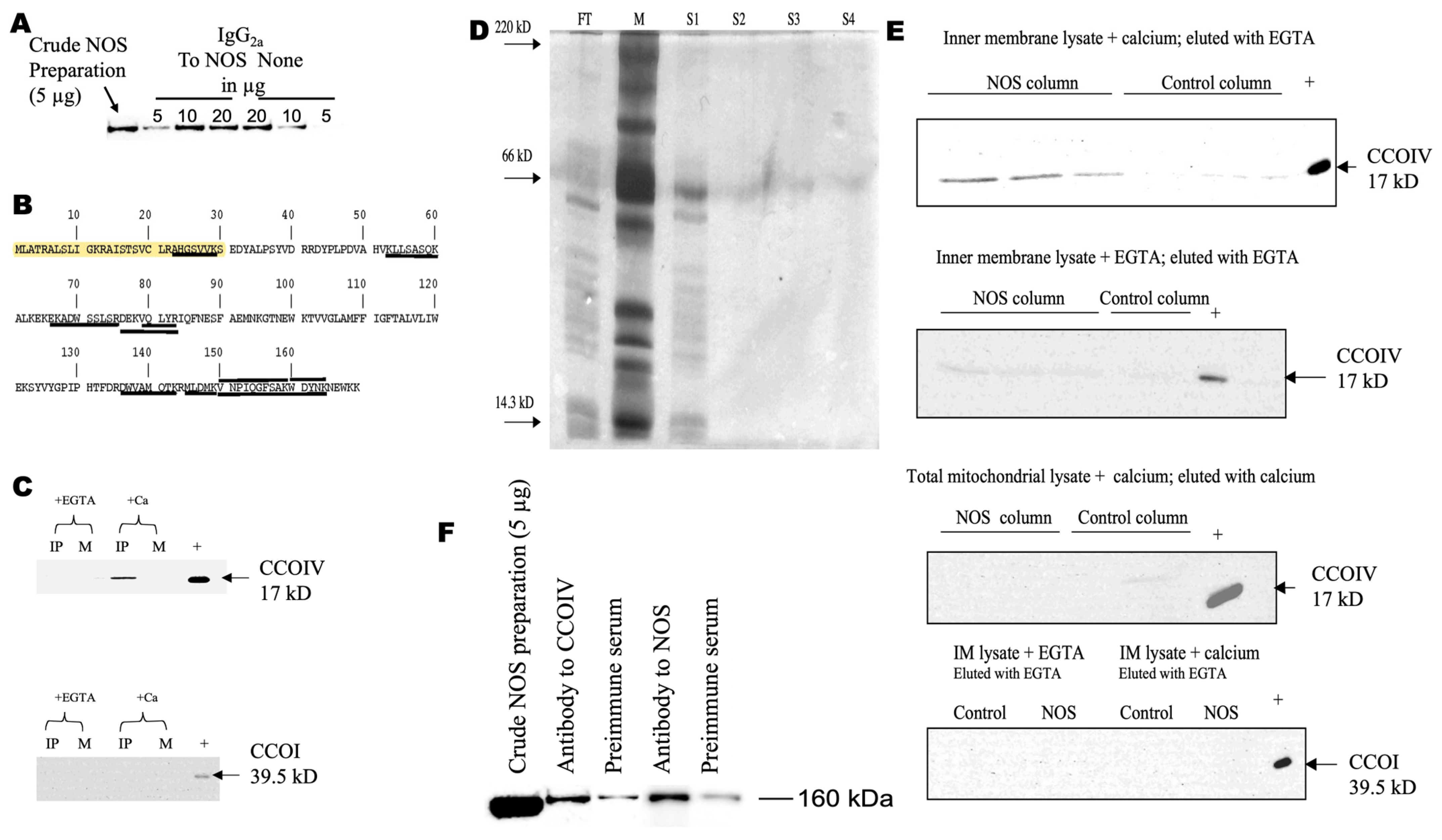
3.1. Identification of CCO Subunit IV as an Interacting Protein of NOS
3.2. Calcium Ions Are Required for NOS–CCOIV Protein–Protein Interaction
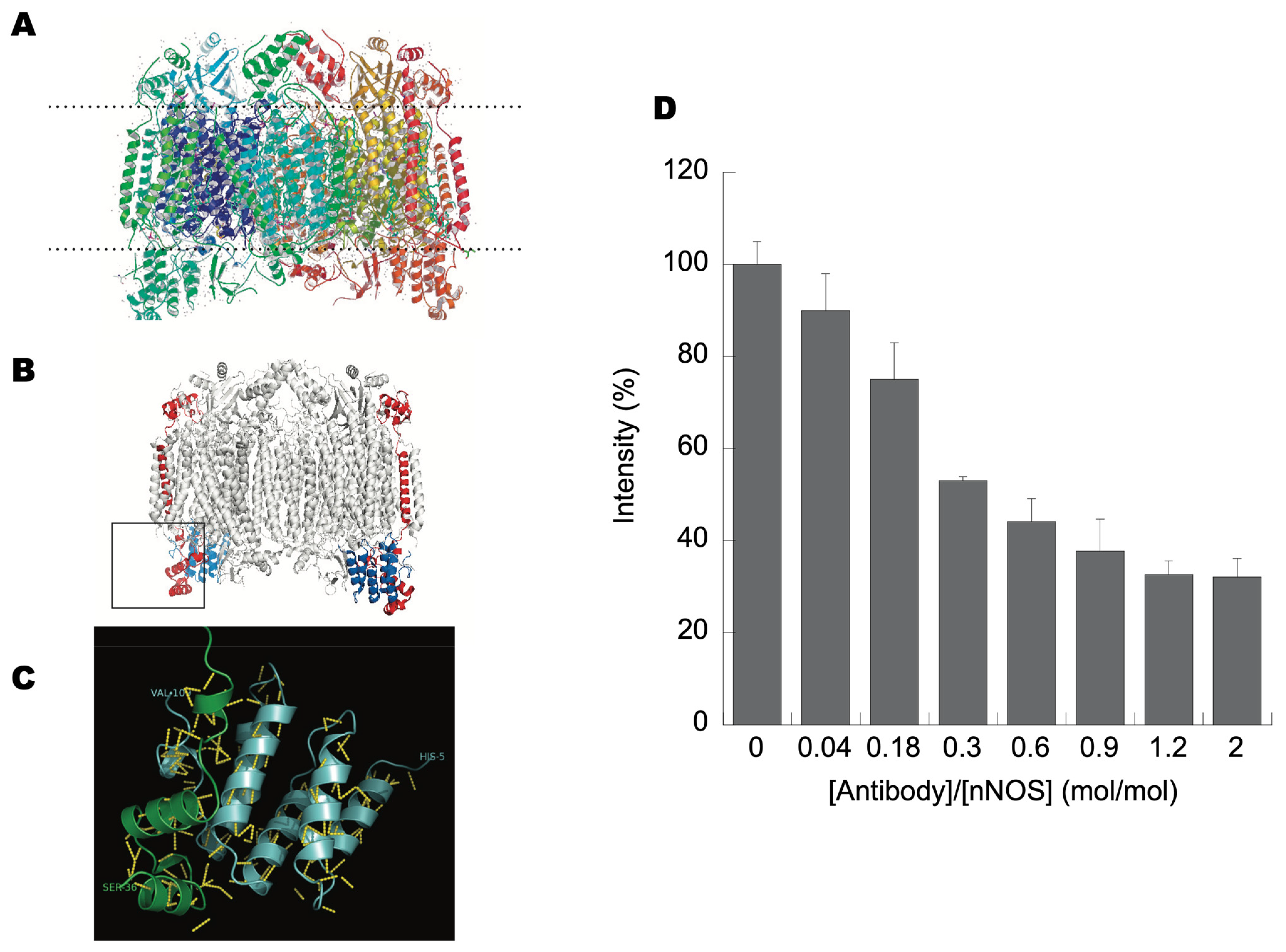
3.3. NOS Binds Directly to the Matrix Side of CCOIV
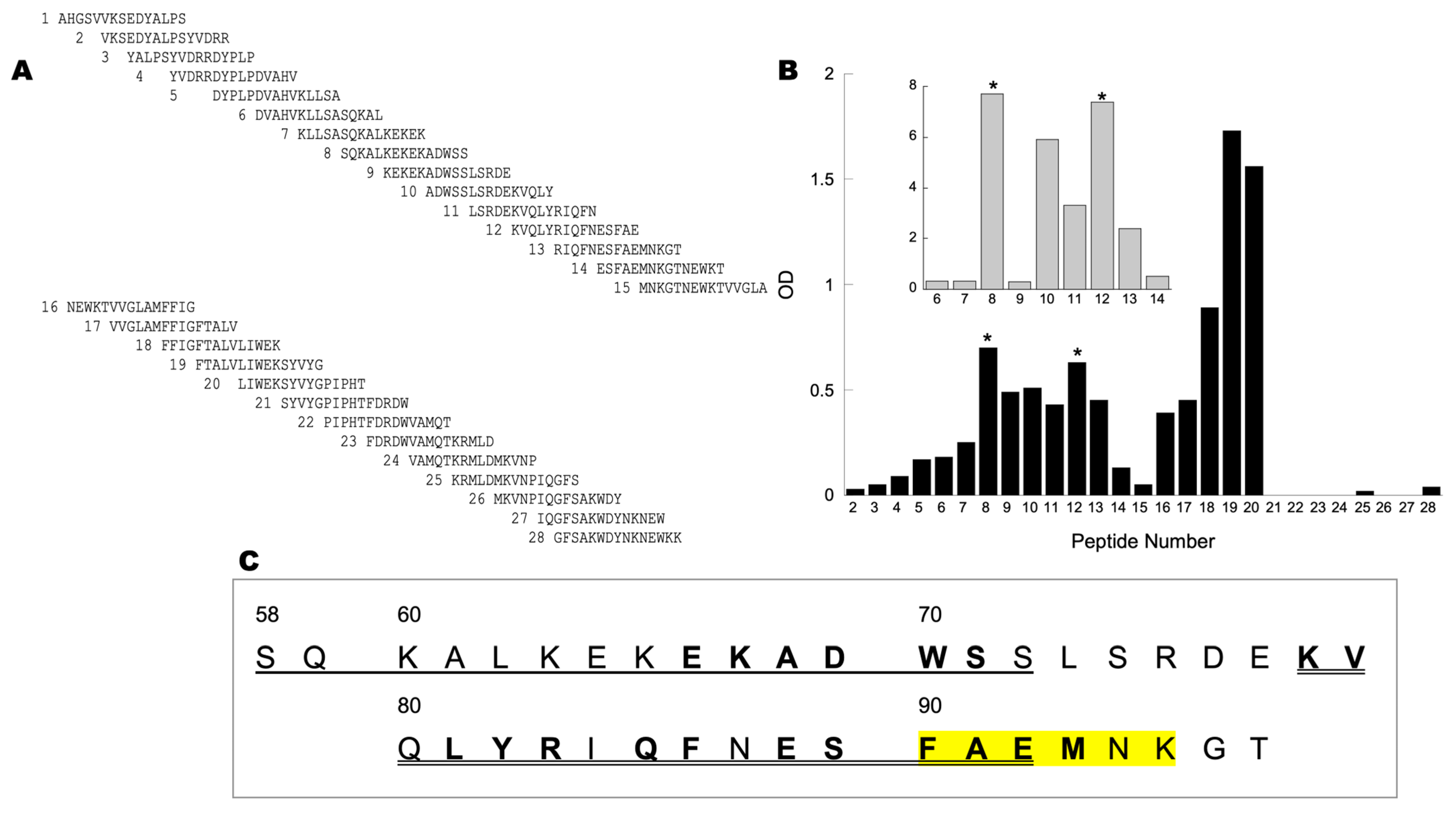
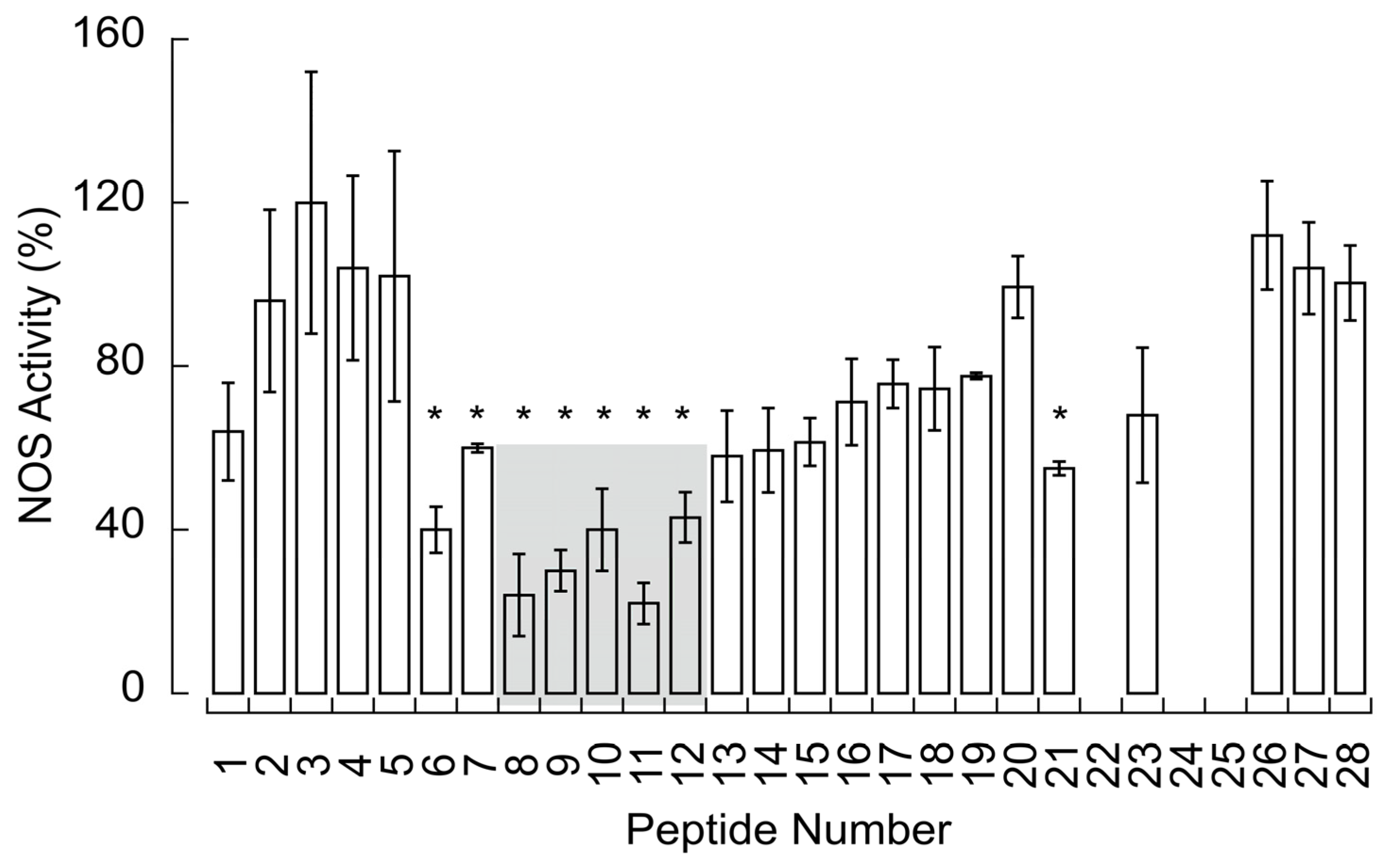
3.4. N-Terminus End of NOS Binds CCOIV
3.5. NO• Production Is Decreased by CCOIV Interaction but Still Decreases ATP-Linked Oxygen Uptake by Isolated Brain Mitochondria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal 2009, 2, re2. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Nitric oxide, a novel neuronal messenger. Neuron 1992, 8, 3–11. [Google Scholar] [CrossRef]
- Ignarro, L.J. Endothelium-derived nitric oxide: Actions and properties. FASEB J. 1989, 3, 31–36. [Google Scholar] [CrossRef]
- Lancaster, J.J. Nitric Oxide: Principles and Actions; Academic Press: London, UK, 1996. [Google Scholar]
- Moncada, S.; Radomski, M.W.; Palmer, R.M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 1988, 37, 2495–2501. [Google Scholar] [CrossRef]
- Ogura, T.; Yokoyama, T.; Fujisawa, H.; Kurashima, Y.; Esumi, H. Structural diversity of neuronal nitric oxide synthase mRNA in the nervous system. Biochem. Biophys. Res. Commun. 1993, 193, 1014–1022. [Google Scholar] [CrossRef]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef]
- Harris, M.B.; Ju, H.; Venema, V.J.; Liang, H.; Zou, R.; Michell, B.J.; Chen, Z.P.; Kemp, B.E.; Venema, R.C. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J. Biol. Chem. 2001, 276, 16587–16591. [Google Scholar] [CrossRef]
- Giulivi, C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem. J. 1998, 332 Pt 3, 673–679. [Google Scholar] [CrossRef]
- Giulivi, C.; Poderoso, J.J.; Boveris, A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998, 273, 11038–11043. [Google Scholar] [CrossRef]
- Tatoyan, A.; Giulivi, C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J. Biol. Chem. 1998, 273, 11044–11048. [Google Scholar] [CrossRef]
- Giulivi, C. Characterization and function of mitochondrial nitric-oxide synthase. Free Radic. Biol. Med. 2003, 34, 397–408. [Google Scholar] [CrossRef]
- Elfering, S.L.; Sarkela, T.M.; Giulivi, C. Biochemistry of mitochondrial nitric-oxide synthase. J. Biol. Chem. 2002, 277, 38079–38086. [Google Scholar] [CrossRef]
- Gao, S.; Chen, J.; Brodsky, S.V.; Huang, H.; Adler, S.; Lee, J.H.; Dhadwal, N.; Cohen-Gould, L.; Gross, S.S.; Goligorsky, M.S. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: A pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J. Biol. Chem. 2004, 279, 15968–15974. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 2001, 1504, 46–57. [Google Scholar] [CrossRef] [PubMed]
- De Palma, C.; Falcone, S.; Pisoni, S.; Cipolat, S.; Panzeri, C.; Pambianco, S.; Pisconti, A.; Allevi, R.; Bassi, M.T.; Cossu, G.; et al. Nitric oxide inhibition of Drp1-mediated mitochondrial fission is critical for myogenic differentiation. Cell Death Differ. 2010, 17, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Sarkela, T.; Berthiaume, J.; Elfering, S. Modulation of mitochondrial respiration by endogenous nitric oxide. FASEB J. 1999, 13, A1554. [Google Scholar]
- Sarkela, T.M.; Berthiaume, J.; Elfering, S.; Gybina, A.A.; Giulivi, C. The modulation of oxygen radical production by nitric oxide in mitochondria. J. Biol. Chem. 2001, 276, 6945–6949. [Google Scholar] [CrossRef]
- Galkin, A.; Moncada, S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J. Biol. Chem. 2007, 282, 37448–37453. [Google Scholar] [CrossRef]
- Carreras, M.C.; Franco, M.C.; Peralta, J.G.; Poderoso, J.J. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol. Asp. Med. 2004, 25, 125–139. [Google Scholar] [CrossRef]
- Ghafourifar, P.; Schenk, U.; Klein, S.D.; Richter, C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J. Biol. Chem. 1999, 274, 31185–31188. [Google Scholar] [CrossRef]
- Giulivi, C.; Kato, K.; Cooper, C.E. Nitric oxide regulation of mitochondrial oxygen consumption I: Cellular physiology. Am. J. Physiol. Cell Physiol. 2006, 291, C1225–C1231. [Google Scholar] [CrossRef]
- Persichini, T.; Mazzone, V.; Polticelli, F.; Moreno, S.; Venturini, G.; Clementi, E.; Colasanti, M. Mitochondrial type I nitric oxide synthase physically interacts with cytochrome c oxidase. Neurosci. Lett. 2005, 384, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Ewart, G.; Capaldi, R.A. Topology of subunits of the mammalian cytochrome c oxidase: Relationship to the assembly of the enzyme complex. Biochemistry 1991, 30, 3674–3681. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Giulivi, C.; Ross-Inta, C.; Omanska-Klusek, A.; Napoli, E.; Sakaguchi, D.; Barrientos, G.; Allen, P.D.; Pessah, I.N. Basal bioenergetic abnormalities in skeletal muscle from ryanodine receptor malignant hyperthermia-susceptible R163C knock-in mice. J. Biol. Chem. 2011, 286, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Song, G.; Liu, S.; Espejo, A.; Perez, C.J.; Benavides, F.; Giulivi, C. Zdhhc13-dependent Drp1 S-palmitoylation impacts brain bioenergetics, anxiety, coordination and motor skills. Sci. Rep. 2017, 7, 12796. [Google Scholar] [CrossRef]
- Ardail, D.; Gasnier, F.; Lerme, F.; Simonot, C.; Louisot, P.; Gateau-Roesch, O. Involvement of mitochondrial contact sites in the subcellular compartmentalization of phospholipid biosynthetic enzymes. J. Biol. Chem. 1993, 268, 25985–25992. [Google Scholar]
- Pedersen, P.L.; Greenawalt, J.W.; Reynafarje, B.; Hullihen, J.; Decker, G.L.; Soper, J.W.; Bustamente, E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978, 20, 411–481. [Google Scholar] [CrossRef]
- Crapo, J.D.; McCord, J.M.; Fridovich, I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978, 53, 382–393. [Google Scholar] [CrossRef]
- Freeman, B.A.; Mason, R.J.; Williams, M.C.; Crapo, J.D. Antioxidant enzyme activity in alveolar type II cells after exposure of rats to hyperoxia. Exp. Lung Res. 1986, 10, 203–222. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Zhang, Y.F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial dysfunction in autism. JAMA 2010, 304, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J. Biol. Chem. 1956, 221, 477–489. [Google Scholar] [CrossRef]
- Traaseth, N.; Elfering, S.; Solien, J.; Haynes, V.; Giulivi, C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim. Biophys. Acta 2004, 1658, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Westermann, B.; Neupert, W. Analysis of protein-protein interactions in mitochondria by coimmunoprecipitation and chemical cross-linking. Methods Cell Biol. 2001, 65, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M. Conformational preferences of amino acids in globular proteins. Biochemistry 1978, 17, 4277–4285. [Google Scholar] [CrossRef]
- Deleage, G.; Roux, B. An algorithm for protein secondary structure prediction based on class prediction. Protein Eng. 1987, 1, 289–294. [Google Scholar] [CrossRef]
- Alderton, W.K.; Boyhan, A.; Lowe, P.N. Nitroarginine and tetrahydrobiopterin binding to the haem domain of neuronal nitric oxide synthase using a scintillation proximity assay. Biochem. J. 1998, 332 Pt 1, 195–201. [Google Scholar] [CrossRef]
- Firestein, B.L.; Bredt, D.S. Interaction of neuronal nitric-oxide synthase and phosphofructokinase-M. J. Biol. Chem. 1999, 274, 10545–10550. [Google Scholar] [CrossRef]
- Solien, J.; Haynes, V.; Giulivi, C. Differential requirements of calcium for oxoglutarate dehydrogenase and mitochondrial nitric-oxide synthase under hypoxia: Impact on the regulation of mitochondrial oxygen consumption. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 111–117. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Modulation of mitochondrial Ca2+ by nitric oxide in cultured bovine vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2005, 289, C836–C845. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Denton, R.M. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem. J. 1988, 252, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; McCormack, J.G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; McCormack, J.G. The role of calcium in the regulation of mitochondrial metabolism. Biochem. Soc. Trans. 1980, 8, 266–268. [Google Scholar] [CrossRef]
- Denton, R.M.; McCormack, J.G.; Edgell, N.J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J. 1980, 190, 107–117. [Google Scholar] [CrossRef]
- Gostimskaya, I.S.; Grivennikova, V.G.; Zharova, T.V.; Bakeeva, L.E.; Vinogradov, A.D. In situ assay of the intramitochondrial enzymes: Use of alamethicin for permeabilization of mitochondria. Anal. Biochem. 2003, 313, 46–52. [Google Scholar] [CrossRef]
- Mathew, M.K.; Nagaraj, R.; Balaram, P. Membrane channel-forming polypeptides. Aqueous phase aggregation and membrane-modifying activity of synthetic fluorescent alamethicin fragments. J. Biol. Chem. 1982, 257, 2170–2176. [Google Scholar] [CrossRef]
- Sheng, M.; Sala, C. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 2001, 24, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hillier, B.J.; Christopherson, K.S.; Prehoda, K.E.; Bredt, D.S.; Lim, W.A. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 1999, 284, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Huttemann, M.; Kadenbach, B.; Grossman, L.I. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 2001, 267, 111–123. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.O.; Hunt, C.A.; Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 1992, 9, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Brenman, J.E.; Christopherson, K.S.; Craven, S.E.; McGee, A.W.; Bredt, D.S. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J. Neurosci. 1996, 16, 7407–7415. [Google Scholar] [CrossRef] [PubMed]
- Jaffrey, S.R.; Snyder, S.H. PIN: An associated protein inhibitor of neuronal nitric oxide synthase. Science 1996, 274, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; LaNoue, K.F.; Cheung, J.Y.; Scaduto, R.C., Jr. Regulation of citric acid cycle by calcium. J. Biol. Chem. 1989, 264, 13430–13439. [Google Scholar] [CrossRef]
- Allen, L.A.; Zhao, X.J.; Caughey, W.; Poyton, R.O. Isoforms of yeast cytochrome c oxidase subunit V affect the binuclear reaction center and alter the kinetics of interaction with the isoforms of yeast cytochrome c. J. Biol. Chem. 1995, 270, 110–118. [Google Scholar] [CrossRef]
- Waterland, R.A.; Basu, A.; Chance, B.; Poyton, R.O. The isoforms of yeast cytochrome c oxidase subunit V alter the in vivo kinetic properties of the holoenzyme. J. Biol. Chem. 1991, 266, 4180–4186. [Google Scholar] [CrossRef]
- Kadenbach, B.; Arnold, S. A second mechanism of respiratory control. FEBS Lett. 1999, 447, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Huttemann, M.; Arnold, S.; Lee, I.; Bender, E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol. Med. 2000, 29, 211–221. [Google Scholar] [CrossRef]
- Napiwotzki, J.; Kadenbach, B. Extramitochondrial ATP/ADP-ratios regulate cytochrome c oxidase activity via binding to the cytosolic domain of subunit IV. Biol. Chem. 1998, 379, 335–339. [Google Scholar] [CrossRef]
- Bender, E.; Kadenbach, B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000, 466, 130–134. [Google Scholar] [CrossRef]
- Hodge, M.R.; Kim, G.; Singh, K.; Cumsky, M.G. Inverse regulation of the yeast COX5 genes by oxygen and heme. Mol. Cell Biol. 1989, 9, 1958–1964. [Google Scholar] [CrossRef]
- Poyton, R.O.; Burke, P.V. Oxygen regulated transcription of cytochrome c and cytochrome c oxidase genes in yeast. Biochim. Biophys. Acta 1992, 1101, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; Trueblood, C.E.; Wright, R.M.; Farrell, L.E. Expression and function of cytochrome c oxidase subunit isologues. Modulators of cellular energy production? Ann. N. Y. Acad. Sci. 1988, 550, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, C.E.; Poyton, R.O. Identification of REO1, a gene involved in negative regulation of COX5b and ANB1 in aerobically grown Saccharomyces cerevisiae. Genetics 1988, 120, 671–680. [Google Scholar] [CrossRef]
- Trueblood, C.E.; Wright, R.M.; Poyton, R.O. Differential regulation of the two genes encoding Saccharomyces cerevisiae cytochrome c oxidase subunit V by heme and the HAP2 and REO1 genes. Mol. Cell Biol. 1988, 8, 4537–4540. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Mukhaleva, E.; Azarkina, N.V.; Konstantinov, A.A. Cytochrome c oxidase inhibition by calcium at physiological ionic composition of the medium: Implications for physiological significance of the effect. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Giulivi, C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am. J. Physiol. Cell Physiol. 2007, 292, C1993–C2003. [Google Scholar] [CrossRef]
- Strutynska, N.; Goshovska, Y.; Mys, L.; Strutynskyi, R.; Luchkova, A.; Fedichkina, R.; Okhai, I.; Korkach, Y.; Sagach, V. Glutathione restores the mitochondrial redox status and improves the function of the cardiovascular system in old rats. Front. Physiol. 2022, 13, 1093388. [Google Scholar] [CrossRef]
- Fellet, A.L.; Balaszczuk, A.M.; Arranz, C.; Lopez-Costa, J.J.; Boveris, A.; Bustamante, J. Autonomic regulation of pacemaker activity: Role of heart nitric oxide synthases. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1246–H1254. [Google Scholar] [CrossRef][Green Version]
- Zanella, B.; Giordano, E.; Muscari, C.; Zini, M.; Guarnieri, C. Nitric oxide synthase activity in rat cardiac mitochondria. Basic. Res. Cardiol. 2004, 99, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, E.; Lopez-Bernardo, E.; Cadenas, S. Functional evidence for nitric oxide production by skeletal-muscle mitochondria from lipopolysaccharide-treated mice. Mitochondrion 2012, 12, 126–131. [Google Scholar] [CrossRef]
- Navarro, A.; Sanchez-Pino, M.J.; Gomez, C.; Bandez, M.J.; Cadenas, E.; Boveris, A. Dietary thioproline decreases spontaneous food intake and increases survival and neurological function in mice. Antioxid. Redox Signal 2007, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Riobo, N.A.; Melani, M.; Sanjuan, N.; Fiszman, M.L.; Gravielle, M.C.; Carreras, M.C.; Cadenas, E.; Poderoso, J.J. The modulation of mitochondrial nitric-oxide synthase activity in rat brain development. J. Biol. Chem. 2002, 277, 42447–42455. [Google Scholar] [CrossRef]
- Lopez, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; Leon, J.; Acuna-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Seidlmayer, L.K.; Blatter, L.A. Mitochondria-mediated cardioprotection by trimetazidine in rabbit heart failure. J. Mol. Cell Cardiol. 2013, 59, 41–54. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J. Physiol. 2009, 587, 851–872. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Ji, X.; Lipsius, S.L.; Blatter, L.A. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2004, 286, C406–C415. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Giulivi, C. Critical overview of mitochondrial nitric-oxide synthase. Front. Biosci. 2006, 11, 2725–2738. [Google Scholar] [CrossRef][Green Version]
- Ghafourifar, P.; Cadenas, E. Mitochondrial nitric oxide synthase. Trends Pharmacol. Sci. 2005, 26, 190–195. [Google Scholar] [CrossRef]
- Zaobornyj, T.; Ghafourifar, P. Strategic localization of heart mitochondrial NOS: A review of the evidence. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1283–H1293. [Google Scholar] [CrossRef]
- Lopez-Figueroa, M.O.; Caamano, C.; Morano, M.I.; Ronn, L.C.; Akil, H.; Watson, S.J. Direct evidence of nitric oxide presence within mitochondria. Biochem. Biophys. Res. Commun. 2000, 272, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ghafourifar, P.; Richter, C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997, 418, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Carreras, M.C.; Peralta, J.G.; Converso, D.P.; Finocchietto, P.V.; Rebagliati, I.; Zaninovich, A.A.; Poderoso, J.J. Modulation of liver mitochondrial NOS is implicated in thyroid-dependent regulation of O2 uptake. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2282–H2288. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Gao, S.; Li, H.; Goligorsky, M.S. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2130–H2139. [Google Scholar] [CrossRef]
- Akopova, O.; Kotsiuruba, A.; Korkach, Y.; Kolchinskaya, L.; Nosar, V.; Gavenauskas, B.; Serebrovska, Z.; Mankovska, I.; Sagach, V. The Effect Of NO Donor on Calcium Uptake and Reactive Nitrogen Species Production in Mitochondria. Cell Physiol. Biochem. 2016, 39, 193–204. [Google Scholar] [CrossRef]
- Sakamuri, S.; Sperling, J.A.; Evans, W.R.; Dholakia, M.H.; Albuck, A.L.; Sure, V.N.; Satou, R.; Mostany, R.; Katakam, P.V.G. Nitric oxide synthase inhibitors negatively regulate respiration in isolated rodent cardiac and brain mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H295–H300. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.E.; Loesch, A.; Burnstock, G.; Clark, J.B. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem. Biophys. Res. Commun. 1995, 213, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Beigi, F.; Oskouei, B.N.; Zheng, M.; Cooke, C.A.; Lamirault, G.; Hare, J.M. Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide 2009, 21, 226–233. [Google Scholar] [CrossRef][Green Version]
- Batista, C.M.; Carneiro, K.; de Bittencourt-Navarrete, R.E.; Soares-Mota, M.; Cavalcante, L.A.; Mendez-Otero, R. Nitrergic dendrites in the superficial layers of the rat superior colliculus: Retinal afferents and alternatively spliced isoforms in normal and deafferented animals. J. Neurosci. Res. 2003, 71, 455–461. [Google Scholar] [CrossRef]
- Steppan, J.; Ryoo, S.; Schuleri, K.H.; Gregg, C.; Hasan, R.K.; White, A.R.; Bugaj, L.J.; Khan, M.; Santhanam, L.; Nyhan, D.; et al. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 4759–4764. [Google Scholar] [CrossRef]
- Kanai, A.J.; Pearce, L.L.; Clemens, P.R.; Birder, L.A.; VanBibber, M.M.; Choi, S.Y.; de Groat, W.C.; Peterson, J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc. Natl. Acad. Sci. USA 2001, 98, 14126–14131. [Google Scholar] [CrossRef]
- Venkatakrishnan, P.; Nakayasu, E.S.; Almeida, I.C.; Miller, R.T. Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria. J. Biol. Chem. 2009, 284, 19843–19855. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial nitric oxide synthase. Mitochondrion 2004, 3, 187–204. [Google Scholar] [CrossRef]
- Lacza, Z.; Snipes, J.A.; Zhang, J.; Horvath, E.M.; Figueroa, J.P.; Szabo, C.; Busija, D.W. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic. Biol. Med. 2003, 35, 1217–1228. [Google Scholar] [CrossRef]
- Nohl, H.; Staniek, K.; Kozlov, A.V. The existence and significance of a mitochondrial nitrite reductase. Redox Rep. 2005, 10, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K.; et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 2008, 134, 112–123. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Antonicka, H.; Lin, Z.Y.; Janer, A.; Aaltonen, M.J.; Weraarpachai, W.; Gingras, A.C.; Shoubridge, E.A. A High-Density Human Mitochondrial Proximity Interaction Network. Cell Metab. 2020, 32, 479–497.e9. [Google Scholar] [CrossRef]
- Rhee, H.W.; Zou, P.; Udeshi, N.D.; Martell, J.D.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 2013, 339, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.; Zou, P.; Rhee, H.W.; Udeshi, N.D.; Cracan, V.; Svinkina, T.; Carr, S.A.; Mootha, V.K.; Ting, A.Y. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 2014, 55, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.; Lam, S.S.; Udeshi, N.D.; Svinkina, T.; Guzman, G.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. eLife 2017, 6, e24463. [Google Scholar] [CrossRef]
- Wu, W.; Wen, Y.; Chen, Y.; Ji, L.; Chao, H. A Mitochondria-Localized Iridium(III) Complex for Simultaneous Two-Photon Phosphorescence Lifetime Imaging of Downstream Products N2O3 and ONOO− of Endogenous Nitric Oxide. Anal. Chem. 2023. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Liu, J.; Zhang, H.; Huo, Y.; Lv, X.; Shi, Y.; Guo, W. A mitochondria-targetable fluorescent probe for dual-channel NO imaging assisted by intracellular cysteine and glutathione. J. Am. Chem. Soc. 2014, 136, 12520–12523. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Xiao, Y.; Zou, W.; Wang, L.; Jin, L. Targetable fluorescent probe for monitoring exogenous and endogenous NO in mitochondria of living cells. Anal. Chem. 2013, 85, 7076–7084. [Google Scholar] [CrossRef] [PubMed]
- Gellerich, F.N.; Gizatullina, Z.; Gainutdinov, T.; Muth, K.; Seppet, E.; Orynbayeva, Z.; Vielhaber, S. The control of brain mitochondrial energization by cytosolic calcium: The mitochondrial gas pedal. IUBMB Life 2013, 65, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta 2009, 1787, 1334–1341. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef]
- Glancy, B.; Willis, W.T.; Chess, D.J.; Balaban, R.S. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 2013, 52, 2793–2809. [Google Scholar] [CrossRef]
- Gellerich, F.N.; Gizatullina, Z.; Nguyen, H.P.; Trumbeckaite, S.; Vielhaber, S.; Seppet, E.; Zierz, S.; Landwehrmeyer, B.; Riess, O.; von Horsten, S.; et al. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J. Biol. Chem. 2008, 283, 30715–30724. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Folch, I.; Rueda, C.B.; Amigo, I.; del Arco, A.; Saheki, T.; Pardo, B.; Satrustegui, J. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J. Neurosci. 2013, 33, 13957–13971. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, A.; Gonzalez-Moreno, L.; Perez-Liebana, I.; Juaristi, I.; Gonzalez-Sanchez, P.; Contreras, L.; Pardo, B.; Satrustegui, J. Regulation of neuronal energy metabolism by calcium: Role of MCU and Aralar/malate-aspartate shuttle. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119468. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 1993, 262, 744–747. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef]
- Chen-Engerer, H.J.; Hartmann, J.; Karl, R.M.; Yang, J.; Feske, S.; Konnerth, A. Two types of functionally distinct Ca2+ stores in hippocampal neurons. Nat. Commun. 2019, 10, 3223. [Google Scholar] [CrossRef]
- Csordas, G.; Varnai, P.; Golenar, T.; Roy, S.; Purkins, G.; Schneider, T.G.; Balla, T.; Hajnoczky, G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 2010, 39, 121–132. [Google Scholar] [CrossRef]
- Giacomello, M.; Drago, I.; Bortolozzi, M.; Scorzeto, M.; Gianelle, A.; Pizzo, P.; Pozzan, T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 2010, 38, 280–290. [Google Scholar] [CrossRef]
- Yi, M.; Weaver, D.; Hajnoczky, G. Control of mitochondrial motility and distribution by the calcium signal: A homeostatic circuit. J. Cell Biol. 2004, 167, 661–672. [Google Scholar] [CrossRef]
- Rintoul, G.L.; Filiano, A.J.; Brocard, J.B.; Kress, G.J.; Reynolds, I.J. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 2003, 23, 7881–7888. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Saotome, M.; Safiulina, D.; Szabadkai, G.; Das, S.; Fransson, A.; Aspenstrom, P.; Rizzuto, R.; Hajnoczky, G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. USA 2008, 105, 20728–20733. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schwarz, T.L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 2009, 136, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Stowers, R.S.; Megeath, L.J.; Gorska-Andrzejak, J.; Meinertzhagen, I.A.; Schwarz, T.L. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 2002, 36, 1063–1077. [Google Scholar] [CrossRef]
- Glater, E.E.; Megeath, L.J.; Stowers, R.S.; Schwarz, T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006, 173, 545–557. [Google Scholar] [CrossRef]
- Cai, Q.; Gerwin, C.; Sheng, Z.H. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J. Cell Biol. 2005, 170, 959–969. [Google Scholar] [CrossRef]
- Chen, Y.M.; Gerwin, C.; Sheng, Z.H. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J. Neurosci. 2009, 29, 9429–9438. [Google Scholar] [CrossRef]
- Macaskill, A.F.; Rinholm, J.E.; Twelvetrees, A.E.; Arancibia-Carcamo, I.L.; Muir, J.; Fransson, A.; Aspenstrom, P.; Attwell, D.; Kittler, J.T. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 2009, 61, 541–555. [Google Scholar] [CrossRef]
- Zaninello, M.; Bean, C. Highly Specialized Mechanisms for Mitochondrial Transport in Neurons: From Intracellular Mobility to Intercellular Transfer of Mitochondria. Biomolecules 2023, 13, 938. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular motors in neuronal development, intracellular transport and diseases. Curr. Opin. Neurobiol. 2004, 14, 564–573. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Tan, L.; Yu, J.T. Axonal transport defects in Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 1309–1321. [Google Scholar] [CrossRef]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009, 4, 20. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium ions in neuronal degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haynes, V.; Giulivi, C. Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics. Brain Sci. 2023, 13, 1534. https://doi.org/10.3390/brainsci13111534
Haynes V, Giulivi C. Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics. Brain Sciences. 2023; 13(11):1534. https://doi.org/10.3390/brainsci13111534
Chicago/Turabian StyleHaynes, Virginia, and Cecilia Giulivi. 2023. "Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics" Brain Sciences 13, no. 11: 1534. https://doi.org/10.3390/brainsci13111534
APA StyleHaynes, V., & Giulivi, C. (2023). Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics. Brain Sciences, 13(11), 1534. https://doi.org/10.3390/brainsci13111534





