Abstract
Neurostimulation carries high therapeutic potential, accompanied by an excellent safety profile. In this review, we argue that an arena in which these tools could provide breakthrough benefits is traumatic brain injury (TBI). TBI is a major health problem worldwide, with the majority of cases identified as mild TBI (mTBI). MTBI is of concern because it is a modifiable risk factor for dementia. A major challenge in studying mTBI is its inherent heterogeneity across a large feature space (e.g., etiology, age of injury, sex, treatment, initial health status, etc.). Parallel lines of research in human and rodent mTBI can be collated to take advantage of the full suite of neuroscience tools, from neuroimaging (electroencephalography: EEG; functional magnetic resonance imaging: fMRI; diffusion tensor imaging: DTI) to biochemical assays. Despite these attractive components and the need for effective treatments, there are at least two major challenges to implementation. First, there is insufficient understanding of how neurostimulation alters neural mechanisms. Second, there is insufficient understanding of how mTBI alters neural function. The goal of this review is to assemble interrelated but disparate areas of research to identify important gaps in knowledge impeding the implementation of neurostimulation.
1. Introduction
This is a narrative literature review of the current research status regarding rodent and human repetitive transcranial magnetic stimulation (rTMS) and other stimulation techniques in the context of cognitive rehabilitation from traumatic brain injury (TBI). The goal is to synthesize across interrelated but separate literatures to clarify which clinically relevant translational goals are approaching implementation and to highlight gaps in progress. This review focuses on research involving rTMS and related neuromodulation techniques, including transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and deep brain stimulation (DBS), because these techniques exhibit treatment potential for cognitive deficits associated with mild TBI (mTBI) and there are sizeable literatures describing these effects. Thus, this review does not cover psychiatric effects or motor effects, it does not extend to moderate or severe TBI or epileptic patients, and it does not include all forms of neurostimulation.
It is important to reduce the silos between human and rodent researchers. Rodent models inform neurobiological mechanisms, which are essential for any intervention. Rodents’ accelerated lifespans and reproduction rates permit testing treatments in hundreds of mice per year. In turn, this streamlines and accelerates clinical trials in humans. The research will be further advanced by a systematic hypothesis-driven approach to experimental design regarding stimulation parameters. This review emphasizes the difficulty in synthesis and comparisons between protocols when there is little consistency in paradigms [1]. This issue can be remediated by integration across fields and with emphasis on mechanisms—even in the face of a heterogeneous condition like mTBI. This discussion will hopefully accelerate progress in addressing mTBI via collaborations between researchers studying different models who are incorporating neurostimulation and neuromodulation approaches.
2. Mild TBI (mTBI) and Repeat mTBI (rmTBI) Are Serious Public Health Problems
The question of when, where, and how neurostimulation can treat TBI recovery arises from the daunting prevalence of TBI. Worldwide there are an estimated 69 million TBIs each year. Following severe TBI, outcomes are variable, with mortality rates ranging from 24% to 35% [2]. Survivors experience a range of outcomes from permanent vegetative states or severe disability on the low end to good recovery on the high end. Critically, outcomes depended on factors including injury type (i.e., subdural hematoma and diffuse axonal injury), age at injury, duration of hospital stay, presence of persistent coma, or high intracranial pressures) [2,3,4]. Depending on the clinical population, the proportion of favorable outcomes (Glasgow Outcome Extended >4) ranged from 21 to 74%. However, the vast majority of TBIs (>80%) are classified as mild in both civilian [5] and active duty populations [6]. MTBI is not typically associated with gross anatomical damage, and the majority of patients return to work well within a year (GOS-E > 5; [7]). Behavioral symptoms of mTBI typically resolve over days to months [8] and without evidence of permanent damage [9]. However, an estimated 5–15% of mTBI cases exhibit enduring cognitive deficits even years post-injury [10,11]. Patterns of deficit are idiosyncratic because injuries are heterogeneous [12]. Notably, this is separate from those individuals whose physical symptoms persist, a condition termed post-concussive syndrome, which has its own attendant literature for diagnosis and treatment (reviewed in [13,14,15,16,17]). Critically, rates of TBI are rising worldwide, thereby increasing the international burden of TBI [18].
In the United States, some 5.5% or 40,000 emergency department visits for TBI evaluation involved individuals reporting a rmTBI within 1 year [19]. Repeat mTBI (rmTBI) is associated with worse long-term physical and cognitive outcomes [20] and is a key antecedent to chronic traumatic encephalopathy (CTE; [21]. Furthermore, annually, an estimated 3,000,000 individuals worldwide experience rmTBI. These mTBI and rmTBI patients are seldom treated for more than a few months. Importantly, it is now acknowledged that a mid-life mTBI is a modifiable risk factor for dementia [22,23]. Unfortunately, there is little that can be done to treat mTBI apart from addressing physical symptoms, prescribing moderate rest, and slow return to activities; in more severe cases of TBI, there can be longer-term rehabilitation provided by allied health fields [24]. There are significant gaps in the understanding of long-term physical, behavioral, cognitive, mental health, and health cost implications attributable to mTBI, which hampers progress in treatment development. Uncovering these mechanisms and harnessing the potential for neuromodulation may allow us to treat those patients with long-term disability and, perhaps, reduce recovery times for those patients who otherwise take months to return to their pre-injury cognitive state.
3. Example Given: Persistent Cognitive Deficits
A small literature characterizes lasting effects even after concussion, which is an mTBI that has no associated findings from neuroimaging (e.g., computed tomography: CT or magnetic resonance imaging: MRI). One cognitive domain revealing deficits is working memory (WM), the ability to hold a small amount of information over a brief period of time. For example, remembering the name of a new acquaintance to introduce them to another person. This may be because WM engages large swaths of the brain to encode, maintain/manipulate, and retrieve information. Immediately following pediatric and adolescent rmTBI, it is clear that cognitive processing slows [25] and WM performance falls [26,27,28,29]. Perhaps surprisingly, recent findings from the Transforming Research and Clinical Knowledge (TRACK-TBI) longitudinal study reveal that 53% of mTBI patients bear physical or cognitive traces for at least one year post-injury [30]. Psychiatric and behavioral health are also affected. Data from the Adolescent Brain Cognitive Development Study reveal that lasting emotional (anxiety) and behavioral (aggression, social, thought, conduct disorders) significantly increase for girls with rmTBI, whereas a past mTBI increases anxiety and attention deficits [31]. In contrast, for boys, a history of mTBI (hmTBI) or rmTBI heightened aggression [31]. Yet, little research is devoted to disparate health outcomes after mTBI or rmTBI across the lifespan.
Because executive function, including WM, is essential for successful academic performance, it is surprising that undergraduates with a hmTBI (mean ~4 years post-injury) or rmTBI exhibit WM impairment at the group level. Performance deficits emerge in a WM change detection task requiring retention of three-color patches for 500 msec [32]. These WM deficits extend across WM tasks, stimuli, and retrieval demands [32,33,34]. Other deficits in hmTBI include impaired performance in attentional tasks such as multiple object tracking [35] and visual cueing [36]. Looking at the heterogeneity in the hmTBI population indicates that group effects are driven by ~30% of hmTBI participants who perform >2 standard deviations from the control mean. Inherent to mTBI is heterogeneity [37], with multiple variables influencing long-term outcomes, including pre-TBI health and socioeconomic status [38]. Indeed, most people fully recover, but not everyone. It is important to identify the characteristics that are most predictive of poor recovery trajectories.
4. Linking mTBI Sequelae to Dementia Risk
As noted, rehabilitation is important because TBI is associated with an increased risk of dementia [23]. HmTBI, especially rmTBI or moderate/severe TBI, increases the likelihood of dementia [39,40]. Older Veterans with hmTBI, regardless of when experienced, also show increased dementia risk [41]. Recent findings link acute and subacute mTBI recovery with another risk factor, the apolipoprotein epsilon 4 (APOE e4) genetic polymorphism. These data revealed that APOE e4 carriers with mTBI had even lower cerebral oxygen saturation [42] and irregular neural slow wave activity [43] and lower perfusion [44] than non-carriers. In short, in mTBI, recovery is complicated by interactions with other risk factors that are only beginning to be understood.
Research at the cellular and molecular levels is characterizing how mTBI increases dementia risk. Acutely, pathological amyloid and hyperphosphorylated tau (pTau) can develop and can persist for years post-injury [45]. Proper clearance of pathological proteins is critical for maintaining brain health and preventing dementia [46,47]. MTBI may also induce microglia to express a pro-inflammatory phenotype, which may impair debris clearance and is seen in neurodegenerative diseases [48]. Thus, microglia function may mediate the progression from TBI to degeneration [49]. Similarly, hyperreactive astrocytes have been reported following injury [50] and in degenerative diseases [51,52].
At the neuronal level, axonal injury from shearing forces and swelling following TBI may contribute to degenerative physiology [53]. White matter atrophy can occur in chronic post-injury pathology [54] and may be related to pTau [55]. Recent reports identify white matter loss as a key contributor to cognitive decline in healthy aging [56] and neurodegeneration [57]. As glial cells support many aspects of neural communication and health, any long-lasting, unhealthy post-injury alterations would reduce neural health and plasticity. Indeed, mTBIs and advancing age reduce the brain’s regenerative capabilities [49,58,59,60].
CTE is a degenerative disease linked to rmTBI, especially in high-contact sports athletes [61]. CTE is characterized by loss of the cognitive control required for WM and attention, and neurobiologically, with cell loss and large accumulations of pTau in neurofibrillary and astrocytic tangles [21,62,63,64]. These changes are likely due to axonal injury and exacerbated by impaired ability to repair damage and clear debris [65], resulting in aggressive neurodegeneration. However, even without CTE, the downstream pathological response to rmTBI is related to other degenerative diseases and could be a contributing factor to future disease development. Researchers must uncover reliable biomarkers to determine if this mechanism can be modulated by neurostimulation [66]. Biochemical research in animal models will improve our overall understanding of this transition and focus the search for predictive biomarkers.
5. Rodent Models Elucidate Mechanism and Inform Treatment
On the other side of health science biology, rodents with rmTBI exhibit parallel cognitive deficits akin to WM deficits observed in humans. Models of rmTBI in mice [67,68] and rats [69,70,71] produce cognitive deficits. In rodents, one mTBI is typically not sufficient to impair behavior, but rats with rmTBI demonstrate WM deficits in novel object recognition [72,73] and novel context mismatch [74], as do humans.
Microglia and inflammation. Rodent models clarify inflammatory signaling and microglia activation states post-TBI, both of which are linked to neuronal loss [75]. In mice, several days after the control cortical impact paradigm, microglia activation shifts to pro-inflammatory phenotyping, including the production of superoxides [76]. As mentioned, microglia dysfunction is linked to CTE following injuries, so research in animal models should focus on countering overactivation and subsequent downstream consequences. For example, inhibiting the production of superoxides reduced oxidative damage [76]. Similarly, the pro-inflammatory signal podoplanin increased in mice following injury, with some in vitro evidence that knocking down podoplanin reduces pro-inflammatory microglia states [77]. As microglia dysfunction is a favored underlying component of Alzheimer’s disease (AD) in rodents and humans, research in rodent models may further inform effective preventative therapeutic strategies.
Sequencing. Techniques such as RNA sequencing can provide a means for understanding the biological components of mTBI and cellular loss. This technique involves transcriptome-wide analysis of gene expression and has provided novel insights into the biochemical milieu of mTBI in addition to other domains of neuroscience. Immediate changes in gene expression following mTBI include many pathways involving cellular death, inflammation, and astrocytosis, whereas long-term alterations include genes related to metabolism and neurodegenerative processes [78]. In rmTBI mice, memory deficits persist 3 months following injury with congruent transcriptomic changes in memory pathways, including long-term potentiation (LTP) [79]. It is difficult to design medications to target all the differentially expressed genes as an intervention, but these approaches expand the number of possible treatment targets. Unfortunately, rodent researchers must be cognizant of differences in metabolism and overall brain structures between species [80] when extrapolating towards human mechanisms. Additionally, most behavioral or biochemical measurements occur at one point in time, typically at the end of the study. However, we can use electroencephalography (EEG) to safely and noninvasively monitor neural activity longitudinally in both rodents and humans. Moreover, unlike pharmacology, neurostimulation has the potential to modulate multiple treatment targets across levels of scale (e.g., metabolism, neurotransmission, inflammation).
6. Electroencephalography in Humans and Rodents
Studies using EEG can track disease time course and treatment effects in animal models and can be readily translated into human research. EEG is a method for recording changes in electric fields generated from within the brain with excellent temporal resolution. When there is a separation of charge between two regions, say between cell bodies in a pyramidal layer and their dendrites in the molecular layer, this leads to the generation of a dipole moment and an electric field. Changes in electric fields can be driven by action potentials, local movements of ions down an axon, and also by more local events such as postsynaptic excitatory and inhibitory potentials. As the nervous system is well organized, with layers of pyramidal neurons and collections of synapses, the dipoles generated by thousands of cells sum to generate the electric fields recorded on the EEG. Patterned oscillatory activity captured in the EEG reflects coordinated neuronal activity [81]. Importantly, EEG is practical. EEG systems are affordable and easily deployable. They are safe and can be applied at any time during the course of recovery (e.g., hours to years after injury), used during the performance of cognitive tasks, and, as the temporal resolution is sub-second, neural activity can be attributed to different aspects of learning and recovery.
7. Electroencephalography in hmTBI
Any TBI inducing a detectable gross anatomical change or acute sequela is not mild. Thus, imaging (MRI or positron emission tomography PET scans) is appropriate for moderate/severe TBI in animals and humans alike but is generally blind to mTBI [9]. Fortunately, EEG provides a widely available tool sensitive to neural changes after TBI, including in oscillatory activity, event-related potentials (ERPs), connectivity, and other analyses. In EEG, there are multiple frequency bands exhibiting characteristic oscillatory activity. WM involves theta band activity (4–8 Hz human, 4–12 Hz rat) [82,83]. In both species, mTBI suppresses theta power [84,85]. EEG power spectra demonstrate restoration in theta power over the first 6 months of mTBI recovery [86]. Similarly, theta phase synchrony, a measure of oscillatory coherence between two electrodes, recovers during several months post-injury in humans [87]. Theta measures fall with mTBI and return slowly over time.
EEG data collected during repetitions of the same stimulus can be averaged to calculate event-related potentials (ERPs). With regard to ERP findings after mTBI, rmTBI ERP amplitudes are lower during WM [88,89,90], semantic processing [91], and memory encoding [92]. However, a key feature that enhances clinical relevance is that ERP amplitude improves over time, providing a measure to track the degree of recovery [89]. EEG connectivity analyses can reveal disconnection post-TBI due to cortical or white matter damage. For example, there is abnormal coherence during a WM task but not during rest after mTBI [93]. Indeed, resting state EEG data are sufficiently sensitive to predict the classification of acute mTBI status [94] and hmTBI status in retired professional football players [95]. Pairing EEG with other imaging modalities, such as diffusion tensor imaging (DTI), permits detailing of lasting white matter damage due to TBI and can be evaluated in both animal models as well as clinically. For example, one combined DTI-EEG study in blast-mTBI patients linked the EEG-derived atypical phase synchrony between frontal lobes with altered fractional anisotropy in white matter tracts derived from the DTI [96]. Because EEG is available, affordable, and versatile, it provides an incredibly important tool for researchers and clinicians interested in documenting recovery and describing mechanisms after an mTBI.
A fundamental challenge in studying mTBI in patients is the heterogeneity of injury (i.e., impact site, severity) and the challenge of capturing longitudinal data. For example, it is not always possible to test a patient within the first 3–6 h post-injury. Patients may not reliably return for follow-up evaluations, particularly for research. Rodent models, however, allow for the investigator to model a range of injury types and severities, including, for example, the controlled cortical impact injury, which can create a focal injury, the lateral fluid percussion, a mixed diffuse/focal injury, and the weight drop and CHIMERA models more diffuse injuries [97]. Each of these injuries can be scaled for severity (e.g., mild, moderate, or severe), and both chronic constriction injury (CCI) and fluid-percussion injury (FPI) can be applied over different regions of the brain. There is also a model of ballistic penetrating injury [97]. Thus, the research community has the ability to model much of the heterogeneity observed in humans, and individual laboratories often choose to model a specific injury type, allowing for higher reproducibility with smaller sample sizes. After injury, rodent researchers may then study the nature of such injury using clinically relevant tools such as EEG, MRI, or PET paired with distinct laboratory techniques such as biochemistry. Rodent models provide the opportunity to identify mechanisms and targets for therapy and novel, innovative strategies for treatment. For example, we make the case that electrophysiology can be used to identify a mechanism related to cognitive dysfunction and a target for treatment. What is critical, however, is for researchers to consider optimal neuromodulation parameters for treatment. These include identifying ideal targets, types of stimulation (e.g., rTMS, DBS), stimulation frequency, and duration of stimulation, amongst others.
8. Neurostimulation Techniques (Table 1)
Unlike activating/inactivating a receptor or scavenging reactive oxygen species (ROS), it is unclear that a pharmacological intervention can modulate oscillations in general, let alone in a specific frequency band. Neuromodulation, however, can modulate neural activity in ways that result in changes in theta, delta, and gamma oscillations (reviewed in [98]). There are multiple techniques for stimulating neural activity non-invasively, such as tDCS, tACS, and rTMS, where investigators can place a combination of anode(s) and cathode(s) on the scalp (tDCS, tACS) or use magnetic stimulation to drive neural activity in specific cortical regions (rTMS). These stimulation paradigms are flexible as they can target a variety of cortical regions, can be varied in frequency and amplitude, and, critically, are non-invasive. Noninvasive brain stimulation likely interacts with myriad receptors, neurotransmitter systems, and ion channels, thus creating the potential for a variety of neural mechanisms depending on the task. There is FDA approval for rTMS applied to the left dorsolateral prefrontal cortex as a target for depression [99], and although many researchers chose this region for mTBI, some neuromodulation publications target different sites to address other issues, including verbal retrieval [100] and chronic pain [101]. There are also well-characterized invasive techniques for neuromodulation, including DBS. Although DBS requires a surgical implantation of electrodes and an internal pulse generator, the lead can be placed directly in a target region, including subcortical regions that are typically inaccessible using extracranial stimulation. DBS, like other approaches, has the flexibility to alternate the amplitude and frequency (Hz) of stimulation. However, an added benefit of DBS is that stimulation can be applied continuously or intermittently. Each of these stimulation techniques will be considered below for their potential to modulate theta oscillations and outcomes in the context of mTBI.


Table 1.
Popular methods of neurostimulation in humans and rodent models. The optimal parameters for improving cognitive performance in people with mTBI are still under investigation. To date, the most common target is DLPFC, but there is some variability across other key parameters. Thus, we include references to the biological systems that are targeted to help with the conceptualization of the underlying mechanism for each approach. Up and down arrows refer to increases or decreases due to neurostimulation.
Table 1.
Popular methods of neurostimulation in humans and rodent models. The optimal parameters for improving cognitive performance in people with mTBI are still under investigation. To date, the most common target is DLPFC, but there is some variability across other key parameters. Thus, we include references to the biological systems that are targeted to help with the conceptualization of the underlying mechanism for each approach. Up and down arrows refer to increases or decreases due to neurostimulation.
| Stim. | Clinical Applications | Method | Limitations | Advantages | Biological System Target |
|---|---|---|---|---|---|
| Repetitive Transcranial Magnetic Stimulation (rTMS) | - ↑ post-encoding memory (mice) - ↓ depression - FDA-approved for chronic pain, major depressive disorder, and obsessive compulsive disorder | - Applies alternating magnetic fields generated by coil placed on scalp | - Rodents: under anesthesia/restraint - Human: office visit required; coil type dictates depth - Little systematicity across investigations | - Noninvasive - Focal - Minimal side effects - FDA approval for some | - ↓ apoptosis - ↑ neural survival - ↑ cholinergic and neurotrophic factor signaling in mice (important for cognition and TBI recovery) - ↑ metabolism - alters neural excitability |
| Transcranial Direct Current Stimulation (tDCS) and Transcranial Alternating Current (tACS) | - ↑ task performance - ↑ WM - ↑ spatial WM (rats) - ↑ ChAT potentially reduces transition from injury → degeneration | - Electrical current through two scalp electrodes - Current/field modified for depth/intensity | - Cognition benefits are temporary - Specific optimized timeframe - Not focal - Requires frequency-specific tailoring to individual | - Noninvasive - Affordable - Potentially self-administrable - Safe - Can target deeper structures (e.g., hippocampus) | - ↑ theta synchrony, phase synchrony, phase–amplitude coupling, and theta-gamma cross-frequency coupling - ↑ plasticity and BDNF release in the hippocampus and frontal cortex (rodent) - ↑ ChAT in hippocampus (rodent) -Alters resting potential |
| Deep Brain Stimulation (DBS) | - Rehabilitate from cognitive and neurological disturbances - May slow cognitive decline in AD model rodents - FDA-approved: PD, OCD, epilepsy, dystonia | - Surgical implantation of embedded electrodes in brain | - Risks associated with brain surgery - Invasive procedure | - Highly Focal - Low maintenance - Independently operating - Can be adapted for closed-loop | - ↑ metabolism |
9. rTMS and tDCS Benefits in Rodents after TBI
rTMS. A decade of theoretical pieces [102,103,104,105] and review papers [103,106,107,108,109,110] enthuse the potential of rTMS to treat the long-term cognitive and psychiatric consequences of TBI with a more tempered set of outcomes. rTMS applies alternating magnetic fields generated from a coil placed on the scalp to non-invasively induce a neural response with minimal side effects [111]. High-frequency stimulation (>5 Hz) is thought to induce an excitable response and increase cerebral blood flow, whereas low-frequency stimulation (<1 Hz) typically induces inhibitory effects [112]. In clinical trials, researchers vary stimulation intensity in terms of percent motor threshold (MT). MT is determined in individuals by stimulating the hand area of the primary motor cortex and looking for the lowest intensity pulse that elicits muscle twitches half of the time. Although there is little systematic investigation on the effects of stimulation intensity, rodent research has shown that intensity can influence biochemical response [113]. Overall, there is evidence that rTMS is a potential treatment modality to remediate cognition, but more research needs to fully uncover the optimal parameters and interventions to stop degeneration.
Biochemical changes follow rTMS in rodents with TBI, specifically in terms of neural survival and decreased apoptosis, but with mixed effects on behavior [114,115,116]. This may be because the optimal rTMS parameters are undetermined [117] or because most rodent models administer rTMS while under restraint or anesthesia [118]. Newly developed methods demonstrate how to provide consistent rTMS to mice without restraint or anesthesia [119], and this method improved post-encoding memory in mice [120]. Indeed, rTMS in unrestrained triple transgenic Alzheimer’s mice (3×TgAD) mice improved cholinergic and neurotrophic factor signaling, both of which are important for cognition and mTBI recovery [121].
A pediatric rat model of controlled cortical impact TBI applied 4 weeks of 20 Hz rTMS and improved CaMKII signaling two weeks after the stimulation ended [122]. Although this study provides some evidence that rTMS may elicit long-term synaptic plasticity-related benefits, the use of rTMS as a means to prevent degeneration in hmTBI is underexplored. As hippocampal dysfunction is a major source of memory loss after mTBI [123] and degrades early in many degenerative disorders, whether and how neurostimulation could be beneficial is yet to be determined. Although directly targeting the hippocampus in humans with rTMS is nearly impossible, stimulating highly interconnected regions such as the inferior parietal cortex may increase connectivity and memory performance in human participants [124].
tDCS. Another method, transcranial direct current stimulation (tDCS), may more flexibly target structures like the hippocampus and is a promising technique for post-injury rehabilitation. Instead of an electric field via magnetic induction, tDCS sends a small electrical current through the scalp to interface with the brain through two surface electrodes [125,126,127]. Excitatory and inhibitory stimulation may be achieved through positive (anodal) or negative (cathodal) current generation [128,129,130]. A recent meta-analysis recommended tDCS as a means to induce plasticity and brain-derived neurotrophic factor (BDNF) release in the rodent hippocampus and frontal cortex [131]. Along these lines, medial prefrontal cortical epidural direct current stimulation improved spatial working memory in healthy rats [132]. As an intervention, tDCS has improved spatial cognition in the acute phase following a lateral fluid percussion TBI [133]. Unfortunately, the benefits on cognition were temporary, and neurotrophic factor signaling improved only when treatment was administered two weeks after injury rather than one week after injury. However, the results are promising in that tDCS can modulate cognition when provided within an optimized timeframe.
Frontal tDCS applied to rats 24 h after a hippocampal injection of amyloid showed improvements in performance on the Morris Water Maze Task with high amplitude (100 uA and 200 uA) stimulation, which was accompanied by an increased expression of choline transferase (ChAT) in the hippocampus [134]. ChAT is an important marker for cholinergic function and is dysregulated early in the course of neurodegenerative diseases in humans. Thus, this may be helpful in reducing a transition from injury to degeneration. However, as the intervention was provided in a short time frame following amyloid injections, it is unclear how tDCS would fare in a scenario with longer-term damage, which is more common in human degenerative pathology. To address this, researchers applied 3 weeks of tDCS to the 3×TgAD genetic mouse model of AD, but no biochemical or behavioral benefits were found [135]. One potential confound is that a much lower amplitude was utilized in this study (50 uA), as more research needs to be conducted to find the correct stimulation patterns for different types of brain diseases. On the other hand, implanting electrodes directly into the brain allows for a more controlled targeting approach, which provides an efficacious strategy in disease states needing more frequent or constant intervention.
DBS. Deep brain stimulation via implanted electrodes is promising in its ability to rehabilitate those suffering from debilitating cognitive and neurological disturbances and has emerged as a highly efficacious treatment for Parkinson’s disease in rodents [136,137] and humans [138]. Although DBS provides a very site-specific interface, the overall excitatory or inhibitory effect may depend on the neural composition within the locus of stimulation [139]. There is some evidence that it may slow cognitive decline in rodent models of AD [140,141]. In addition, DBS in the laboratory setting has been able to improve cognitive performance in rodent models of epilepsy as well as moderate–severe TBI [142,143,144,145,146]. Although there are risks with any surgery [147], DBS is FDA-approved for treating several disorders, including Parkinson’s disease, dystonia, obsessive compulsive disorder, and epilepsy, with others on the horizon. It is important to consider the specific needs of the patient and the cost–benefit profile when considering the utility of invasive as compared to non-invasive approaches for neurostimulation.
Translatability concerns. Future directions in rodent research are abundant but should prioritize translatability. Rodent research involves precisely phenotyped injuries in genetically identical animals, creating an environment for results with large effect sizes. While animal models are, therefore, critical for fine-tuning a mechanistic understanding, as well as assessing potential safety and efficacy profiles, translation to human rehabilitation remains a challenge [148]. Therefore, researchers should aim towards systematically investigating multiple rodent genotypes and injury models when utilizing brain stimulation for rehabilitation. Questions regarding optimal timing post-injury and treatment parameters are still to be answered. Additionally, it is unclear how much change in cognition is sufficient to merit neurostimulation as an interventional strategy. Continued collaboration between scientists and clinicians will build upon the current knowledge base to tackle these questions, see Figure 1.
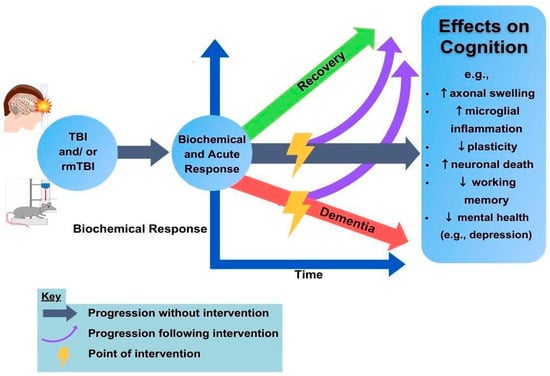
Figure 1.
TBI and neurostimulation. The human and rodent TBI populations provide a rich set of data in which to identify targets for rTMS or neuromodulatory recovery (lightning bolts). The ability to understand the unfolding biochemical response over time and the resulting consequences on cognition are aimed at improving outcome trajectories (purple arrows) to benefit recovery. Green and red arrows indicate good and poor long-term outcomes, respectively.
10. rTMS and tDCS Benefits in Humans after TBI
A major challenge in building synthesis for treatment approaches across noninvasive brain stimulation tools is that the only consistency across labs is inconsistency. Studies are often underpowered and variable in terms of parameters, tasks, and populations. In this section, common threads are extracted from the field by focusing on recent reviews and meta-analyses to pinpoint a signal amidst considerable noise. It is important to note that stimulation to improve cognition in TBI patients produces mixed findings. In short, some patients under some protocols show some temporary cognitive benefits [126,149,150,151]. Unfortunately, rTMS and tDCS are not panaceas, and further optimization is needed (reviewed in [107]). More specifically, effects are usually limited to an aspect of performance rather than broadly generalizable across cognition. It is important to determine the full range of effects by including multiple tasks. For example, one paper applied dorsolateral rTMS (or tDCS) and improved WM performance in TBI survivors but found no effects on processing speed, verbal learning, verbal fluency, or social cognition [152].
With regard to cognitive improvement with rTMS in TBI, more broadly, there are intriguing findings but little large-scale success and little systematicity across investigations. Several studies modify rTMS by presenting stimuli in theta bursts, using much briefer sessions (>5 min) than the typical rTMS (20–40 min), but having long-lasting benefits primarily in treating depression [153,154,155]. Theta burst stimulation (TBS) is thought to more closely mimic natural neural rhythmic activity and may be excitatory or inhibitory, depending on the pattern [154]. Case studies revealed improved attention in one hemispatial neglect patient after continuous theta burst TMS (cTBS), which is associated with induction of long-term depression [156,157], and a different patient with upper motor neuron damage showed improved reaching after three months of rTMS [158]. The application of intermittent theta burst stimulation (iTBS) targeting the DLPFC in patients 1 month post-mTBI provided no cognitive benefit [159] but did help control participants [160]. ITBS is associated with the induction of long-term potentiation [153,157]. Thus, further advances in pulse sequencing may be associated with a full palette of mechanisms that can be selected for tailored effects.
Meta-analyses evaluating the effect of FDA-approved rTMS for depression confirm efficacy, e.g., [161,162,163,164], but effects do not appear to extend to provide generalizable cognitive benefits in TBI patients (reviewed in [165]). One paper found the expected relief from depression, but cognitive tests revealed only a moderate benefit to visuospatial memory (Brief Visuospatial Memory Test) and a minimal benefit on selective attention (Stroop color–word task, Trails Making Tests A, B) [140]. The mechanism of relief is attributed to stronger resting state connectivity [162]. Similarly, Veterans with depression (n = 321), or depression and hmTBI (n = 337), showed relief from depression after 30-rTMS sessions [166]. Their interpretation was that hmTBI did not interfere with the benefit of rTMS for depression, but neither did rTMS provide additional benefit. These data provide reassurance that rTMS is safe for use in people with depression and hmTBI, two commonly comorbid conditions in Veterans. A second meta-analysis found that those with TBI showed that rTMS reduced depression and chronic pain but did not improve cognition [167]. To date, evidence is lacking that rTMS using the FDA-approved depression protocol provides any benefit to cognitive performance (reviewed in [165]).
Such inconsistent benefits of rTMS in TBI may reflect the push to rapidly develop and deploy technologies without understanding the underlying mechanism(s) of cognitive impairment. Better targets, for example, strengthening theta oscillations post-TBI, may produce better outcomes, or some other putative mechanism may be superior. However, it is also possible that there is no optimal target for theta rTMS. In summary, to effectively translate neuromodulation modalities to rehabilitate TBI, it is critical to move from inconsistency to interdisciplinary synthesis and hypothesis testing. Next, we explore such a combined effort to take a deeper dive into theta oscillations, which are at the forefront of TBI research and rehabilitation efforts.
11. The Role of Theta Oscillations in Plasticity and Learning
The question is clear: In the context of TBI-induced cognitive deficits, what is a reasonable therapeutic target? One potential target is slow wave theta oscillation. Theta oscillations are attractive because they are prominent across the brain. Theta oscillations are present in hippocampal local field potentials (LFP) [168], and they modulate hippocampal LTP [169,170], which underlies learning and memory [171,172]. LTP is more robust when high-frequency stimulation coincides with the theta peak [173,174,175], and LTP is diminished if theta is attenuated [176]. Indeed, theta oscillations coordinate local and distal neural networks. For example, there are coherent theta oscillations between the hippocampus and prefrontal cortex [177,178] and cross-frequency interactions such as phase–amplitude coupling between theta and gamma. Specifically, as theta power increases, so does in-phase gamma power in both rodents [179,180,181] and humans [182,183,184]. Disrupted coupling worsens cognitive performance [185,186]; reviewed in [187,188].
Coherent rhythms are also critical for spatial navigation as hippocampal place cell activity is coupled to oscillatory activity, termed phase precession [189,190]. Specifically, as animals enter a hippocampal place field, action potentials initially occur late in the oscillation, but as the animals approach an edge, action potentials progressively occur earlier [189,190,191]. Critically, similar to rodents, in humans, intracranial recordings of local field potentials from epileptic patients revealed that hippocampal theta oscillations are prevalent during spatial navigation (virtual and real-world) [192,193,194,195], successful object recognition tasks [196,197,198,199], and successful recall [200]. In summary, theta oscillations play a prominent role in coordinating neural activity associated with learning and memory.
12. TBI Disrupts Theta Oscillations in Humans and Rodents
Critical to TBI, whether talking about oscillations at the synapse level (LTP) or through coordinating distal neural activity (phase coherence), theta disruption impairs plasticity and learning. When neural activity in the medial septum is depressed pharmacologically, rats perform poorly on the radial arm [201], T [202], and Morris water mazes [203]. Low theta phase coherence is related to cognitive dysfunction in rats [204,205] and humans [206]. Phase precession is disrupted in aging rats [207,208] and in epileptic rats [209]. Hippocampal interneurons involved in theta oscillations are vulnerable to cell death after TBI [210,211,212]. There is also evidence that TBI alone can change activity in inter- and CA1 hippocampal neurons [213,214,215]. We found that theta power and theta coherence are disrupted for days to weeks after mild [85] or moderate LFP [144,216] inductions of TBI. Importantly, stimulating the medial septum to strengthen theta oscillations improved cognitive performance in rats with TBI [145,146] and epilepsy [142,143]. Thus, theta oscillations are important for cognition, but TBI disrupts them, and strengthening theta improves cognition. Therefore, disrupted theta oscillations are a potential mechanism that can explain changes in cognitive function following mTBI. Interventions that restore theta oscillations, power, coherence, and phase–amplitude coupling may effectively treat patients with mTBI, even months to years post-injury.
13. Theta as a Potential Target of Neurostimulation in Neurotypical Humans
Neuromodulatory approaches, most commonly tDCS, can improve task performance. TDCS involves the application of an electrical current through two scalp electrodes with current flowing between them [126,217]. The strength and breadth of the current field can be modified by modeling current flow [218] and tailoring current appropriately for pathological conditions [219] and to reach deeper targets [220,221]. It is appealing for translational use because it is affordable, could be self-administered at home, and has a strong safety profile.
In humans, some evidence shows that neuromodulation alters theta activity. For example, cognitive training paired with several neuromodulation approaches (e.g., tDCS, tACS) is beneficial to healthy and clinical populations [126,217,222,223,224,225,226,227]. This combined approach can elicit task transfer [228,229], the most highly sought outcome.
To identify the underlying mechanism of improvement, it is helpful to pair neuroimaging with neuromodulation. Combined EEG and tDCS studies reveal that improved WM is due to enhanced theta attributes, including phase synchrony, phase–amplitude coupling, and theta-gamma cross-frequency coupling [230,231,232]. Transcranial alternating current (tACS) also strengthens theta synchrony [233] but requires frequency-specific tailoring to the individual [234]. Other forms of neuromodulation, including transcranial random noise stimulation (tRNS) and vagus nerve stimulation (VNS), are beginning to be used to address the physical and psychiatric symptoms of TBI [235]. It is worth noting that there are concerns regarding neuromodulation-induced changes that might harm cognition [236] because the current flow is diffuse and, therefore, can influence many regions. Determining how neuromodulation can realign theta and other neural patterns holds promise in TBI. Importantly, there are other possible mechanisms, as there are many effects of neurostimulation on the brain. For instance, changes in other frequency bands, such as reduced delta activity, after tDCS in moderate TBI patients were associated with superior cognitive outcomes [237]. More work is needed to identify the relevant measurements associated with behavioral improvement.
14. Cautionary Tale: Getting Ahead of the Data
The sheer prevalence of mTBI/rmTBI and encouraging findings from non-invasive brain stimulation approaches put pressure on bench-to-beside translation. Currently, in the United States, several TMS devices and protocols are federally approved to treat treatment-resistant depression, obsessive compulsive disorder, anxious depression, anxiety, and migraine. Beyond these approved protocols, a growing hoard of fee-for-service clinics offers off-label rTMS services for conditions as diverse as pediatric mental health and autism, insomnia, dementia, chronic pain, tinnitus, dementia, and stroke recovery. There is also a vibrant do-it-yourself brain-hacking community providing online instructions for building and applying your own device. Individual clinicians are moving more quickly than the research consensus or federal regulatory agencies, but the collective view remains cautiously optimistic that neurostimulation techniques will bear fruit [238].
In the field of TBI, there are clear opportunities as well as threats pertaining to the translation of neurostimulation techniques, whether non-invasive rTMS and tDCS or invasive DBS. For example, there was a potentially groundbreaking case study where central thalamic DBS rescued a patient from a persistent minimally conscious state [239]. However, in a subsequent study of 14 patients, DBS did not improve outcomes [240]. Perhaps what differentiated the two studies was neuroanatomy. In the patients with improved outcomes, their neuroanatomy was largely intact following injury, whereas patients with larger lesions did not improve. It is critical to consider which circuits are damaged by TBI and to what extent. Are there remaining connections or cortical regions available to stimulate non-invasively? Or is the only viable target subcortical, such as the medial septum or nucleus accumbens? Can intermittent stimulation lead to persistent benefits, or is continuous stimulation required to maintain a cognitive benefit? Invasive neurosurgery for someone with a subtle but significant mTBI-related attention disorder may not make sense, whereas non-invasive stimulation may significantly improve outcomes. Patients with more severe dysfunction may require continuous stimulation, similar to patients with Parkinson’s disease or epilepsy, and therefore might respond better to an implantable stimulator, regardless of whether the stimulation is from an extradural or depth electrode. If we, as scientists and clinicians, take a haphazard approach and ignore details such as the target behavior and the severity of the underlying injury, we risk rejecting technologies that may benefit a subset of patients or advance a study based on a case study into a patient population who will be unlikely to benefit.
Another opportunity involves researching and optimizing stimulation parameters for those with mood disorders experiencing cognitive deficits due to mTBI and an adjacent study testing patients with moderate/severe TBI, e.g., [241,242,243,244,245]. Although the focus of this review was solely on cognition, hmTBI is highly comorbid with anxiety or depressive disorders, especially within the Veteran community [246]. Unfortunately, results from rTMS studies are mixed, with some showing some evidence of benefit on some measures [243] and others showing little or no improvement [247]. One possible explanation could be differences in protocols. Whereas these studies stimulated over the left dorsolateral prefrontal cortex, they used a different number of pulses and pulse amplitudes. A highly researched topic following mTBI is neuromodulation to alleviate post-concussive headache, and again, varying stimulation techniques have been deployed in these studies [101,248,249,250]. Because mTBI is heterogeneous, the addition of a mood disorder or other condition creates a difficult empirical question, as intervention technique [113,251] and timing [252] can be important contributing factors to experimental outcomes.
15. Conclusions
Our goal in reviewing TBI response to neurostimulation was to highlight limitations attributable to heterogeneous approaches and to raise the possibility of a more efficient, efficacious path. Similarly, previous reports have noted a wide variety of stimulation parameters have hindered the ability to draw comparisons between protocols and outcomes [1]. To accelerate the research and federal consensus on the mechanisms and proper research parameters for each condition, more interdisciplinary research that is mechanistically focused is needed. Specifically, this mechanical focus should systematically assess treatment parameters to optimize the rehabilitation potential for those experiencing cognitive deficits following an mTBI. Such collaborative projects between basic and clinical science will facilitate crossing scales and uncovering patterns that can improve clinical care decisions for clinicians, parents, coaches, and the broader community. While human trials in both cognition and neuroscience can provide a knowledge base on clinical efficacy, basic science in rodents is needed to create a deeper understanding of the mechanism to achieve efficacious treatment parameters.
As an example, we described how, in both humans and rats, theta oscillations are critical to plasticity and learning and that attenuated theta (i.e., power, synchrony) correlates with impaired function. Therefore, aligning, restoring, or strengthening theta rhythms shows promise in rodents and humans, and we should follow up on these successes with renewed vigor. Moreover, it is critical to integrate translational tools, such as EEG, MRI, or PET, that can cross scales from rodents to large animal models to humans. Not only are these tools diagnostic, perhaps helping to identify those patients who would most benefit from therapy, but they are also theragnostic, as only EEG, for example, can determine whether modulation has influenced oscillatory activity. Despite the innate difficulties of collaborative, interdisciplinary work and the known difficulty in predicting human response based on rodent findings [253], more research is needed in this effort. After two decades of exploratory research using neurostimulation, a stronger theoretical and mechanistic framework is needed to alleviate the consequences of TBI.
Author Contributions
Conceptualization, M.W.M., G.G.G. and M.E.B.; writing—original and revised draft preparation, M.W.M. and M.E.B.; writing—review and editing, M.W.M., G.G.G. and M.E.B.; visualization, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by VA Biomedical Laboratory Research and Development 5IK2BX004105 to M.W.M., NIMH R15MH122935 to M.E.B., NINDS R01 NS084026 and NINDS U54 NS127758, and VA grant I50BX005878 to G.G.G. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziesel, D.; Nowakowska, M.; Scheruebel, S.; Kornmueller, K.; Schäfer, U.; Schindl, R.; Baumgartner, C.; Üçal, M.; Rienmüller, T. Electrical stimulation methods and protocols for the treatment of traumatic brain injury: A critical review of preclinical research. J. NeuroEngineering Rehabil. 2023, 20, 51. [Google Scholar] [CrossRef]
- Mostert, C.Q.B.; Singh, R.D.; Gerritsen, M.; Kompanje, E.J.O.; Ribbers, G.M.; Peul, W.C.; van Dijck, J. Long-term outcome after severe traumatic brain injury: A systematic literature review. Acta Neurochir. 2022, 164, 599–613. [Google Scholar] [CrossRef]
- Huovinen, A.; Marinkovic, I.; Isokuortti, H.; Korvenoja, A.; Maki, K.; Nybo, T.; Raj, R.; Melkas, S. Return to work after mild traumatic brain injury: Association with positive CT and MRI findings. Acta Neurochir. 2022, 164, 1707–1717. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Defense Medical Surveillance System (DMSS); Theater Medical Data Store (TMDS); Armed Forces Health Surveillance Division. DOD Numbers for Traumatic Brain Injury Worldwide—Totals. Available online: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers (accessed on 9 July 2023).
- Wei, S.; Wei, Y.; Gong, Y.; Chen, Y.; Cui, J.; Li, L.; Yan, H.; Yu, Y.; Lin, X.; Li, G.; et al. Metabolomics as a valid analytical technique in environmental exposure research: Application and progress. Metabolomics 2022, 18, 35. [Google Scholar] [CrossRef]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Primers 2016, 2, 16084. [Google Scholar] [CrossRef]
- Bigler, E.D. Volumetric MRI Findings in Mild Traumatic Brain Injury (mTBI) and Neuropsychological Outcome. Neuropsychol. Rev. 2023, 33, 5–41. [Google Scholar] [CrossRef]
- Rutherford, W.H.; Merrett, J.D.; McDonald, J.R. Symptoms at one year following concussion from minor head injuries. Injury 1979, 10, 225–230. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Levin, H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11. [Google Scholar] [CrossRef]
- Chadwick, L.; Peckham, S.B.; Yeates, K.O. Adult Cognitive Outcomes Following Childhood Mild Traumatic Brain Injury: A Scoping Review. J. Head Trauma Rehabil. 2022, 37, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Haider, M.N.; Noble, J.M.; Rieger, B.; Flanagan, S.; McPherson, J.I.; Shubin-Stein, K.; Saleem, G.T.; Corsaro, L.; Willer, B. Clinical Assessment of Concussion and Persistent Post-Concussive Symptoms for Neurologists. Curr. Neurol. Neurosci. Rep. 2021, 21, 70. [Google Scholar] [CrossRef]
- Leddy, J.J.; Haider, M.N.; Noble, J.M.; Rieger, B.; Flanagan, S.; McPherson, J.I.; Shubin-Stein, K.; Saleem, G.T.; Corsaro, L.; Willer, B. Management of Concussion and Persistent Post-Concussive Symptoms for Neurologists. Curr. Neurol. Neurosci. Rep. 2021, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.M.; Ciobica, A.; Luca, A.C.; Radu, I.; Dobrin, R.P.; et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics 2022, 12, 740. [Google Scholar] [CrossRef]
- Minen, M.T.; Boubour, A.; Walia, H.; Barr, W. Post-Concussive Syndrome: A Focus on Post-Traumatic Headache and Related Cognitive, Psychiatric, and Sleep Issues. Curr. Neurol. Neurosci. Rep. 2016, 16, 100. [Google Scholar] [CrossRef]
- Yumul, J.N.; Crowe, L.; Catroppa, C.; Anderson, V.; McKinlay, A. Post-concussive Signs and Symptoms in Preschool Children: A Systematic Review. Neuropsychol. Rev. 2022, 32, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Injury, G.B.D.T.B.; Spinal Cord Injury, C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Lasry, O.; Liu, E.Y.; Powell, G.A.; Ruel-Laliberte, J.; Marcoux, J.; Buckeridge, D.L. Epidemiology of recurrent traumatic brain injury in the general population: A systematic review. Neurology 2017, 89, 2198–2209. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Marshall, S.W.; Bailes, J.; McCrea, M.; Cantu, R.C.; Randolph, C.; Jordan, B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005, 57, 719–726. [Google Scholar] [CrossRef]
- McKee, A.C.; Abdolmohammadi, B.; Stein, T.D. The neuropathology of chronic traumatic encephalopathy. Handb. Clin. Neurol. 2018, 158, 297–307. [Google Scholar] [CrossRef]
- Graham, A.; Livingston, G.; Purnell, L.; Huntley, J. Mild Traumatic Brain Injuries and Future Risk of Developing Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2022, 87, 969–979. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Stephens, J.A.; Williamson, K.-N.; Berryhill, M.E. Cognitive Rehabilitation after Traumatic Brain Injury: A Reference for Occupational Therapists. OTJR Occup. Particip. Health 2015, 35, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.N.; Bouchard, H.C.; VA Mid-Atlantic MIRECC Workgroup; Welsh-Bohmer, K.A.; Morey, R.A. Assessment of Neuropsychological Function in Veterans with Blast-Related Mild Traumatic Brain Injury and Subconcussive Blast Exposure. Front. Psychol. 2021, 12, 686330. [Google Scholar] [CrossRef]
- Montenigro, P.H.; Alosco, M.L.; Martin, B.M.; Daneshvar, D.H.; Mez, J.; Chaisson, C.E.; Nowinski, C.J.; Au, R.; McKee, A.C.; Cantu, R.C.; et al. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J. Neurotrauma 2017, 34, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef]
- Hylin, M.J.; Orsi, S.A.; Rozas, N.S.; Hill, J.L.; Zhao, J.; Redell, J.B.; Moore, A.N.; Dash, P.K. Repeated mild closed head injury impairs short-term visuospatial memory and complex learning. J. Neurotrauma 2013, 30, 716–726. [Google Scholar] [CrossRef]
- Broadway, J.M.; Rieger, R.E.; Campbell, R.A.; Quinn, D.K.; Mayer, A.R.; Yeo, R.A.; Wilson, J.K.; Gill, D.; Fratzke, V.; Cavanagh, J.F. Executive function predictors of delayed memory deficits after mild traumatic brain injury. Cortex 2019, 120, 240–248. [Google Scholar] [CrossRef]
- Nelson, L.D.; Temkin, N.R.; Dikmen, S.; Barber, J.; Giacino, J.T.; Yuh, E.; Levin, H.S.; McCrea, M.A.; Stein, M.B.; Mukherjee, P.; et al. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol. 2019, 76, 1049–1059. [Google Scholar] [CrossRef]
- Veliz, P.T.; Berryhill, M.E. Gender Differences in Adolescents’ Affective Symptoms and Behavioral Disorders after Mild Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2023, 38, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Arciniega, H.; Kilgore-Gomez, A.; Harris, A.; Peterson, D.J.; McBride, J.; Fox, E.; Berryhill, M.E. Visual working memory deficits in undergraduates with a history of mild traumatic brain injury. Atten. Percept. Psychophys. 2019, 81, 2597–2603. [Google Scholar] [CrossRef]
- Arciniega, H.; Kilgore-Gomez, A.; McNerney, M.W.; Lane, S.; Berryhill, M.E. Loss of consciousness, but not etiology, predicts better working memory performance years after concussion. J. Clin. Transl. Res. 2020, 5, 169–177. [Google Scholar] [CrossRef]
- Arciniega, H.; Shires, J.; Furlong, S.; Kilgore-Gomez, A.; Cerreta, A.; Murray, N.G.; Berryhill, M.E. Impaired visual working memory and reduced connectivity in undergraduates with a history of mild traumatic brain injury. Sci. Rep. 2021, 11, 2789. [Google Scholar] [CrossRef]
- Alnawmasi, M.M.; Khuu, S.K. Deficits in multiple object-tracking and visual attention following mild traumatic brain injury. Sci. Rep. 2022, 12, 13727. [Google Scholar] [CrossRef] [PubMed]
- Alnawmasi, M.M.; Walz, J.A.; Khuu, S.K. Deficits in visuospatial attentional cueing following mild traumatic brain injury. Neuropsychologia 2022, 177, 108422. [Google Scholar] [CrossRef]
- Emery, C.A.; Barlow, K.M.; Brooks, B.L.; Max, J.E.; Villavicencio-Requis, A.; Gnanakumar, V.; Robertson, H.L.; Schneider, K.; Yeates, K.O. A Systematic Review of Psychiatric, Psychological, and Behavioural Outcomes following Mild Traumatic Brain Injury in Children and Adolescents. Can. J. Psychiatry 2016, 61, 259–269. [Google Scholar] [CrossRef]
- von Steinbuechel, N.; Hahm, S.; Muehlan, H.; Arango-Lasprilla, J.C.; Bockhop, F.; Covic, A.; Schmidt, S.; Steyerberg, E.W.; Maas, A.I.R.; Menon, D.; et al. Impact of Sociodemographic, Premorbid, and Injury-Related Factors on Patient-Reported Outcome Trajectories after Traumatic Brain Injury (TBI). J. Clin. Med. 2023, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, A.; Nordstrom, P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med. 2018, 15, e1002496. [Google Scholar] [CrossRef]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic Brain Injury and Risk of Alzheimer’s Disease and Related Dementias in the Population. J. Alzheimers Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef]
- Barnes, D.E.; Kaup, A.; Kirby, K.A.; Byers, A.L.; Diaz-Arrastia, R.; Yaffe, K. Traumatic brain injury and risk of dementia in older veterans. Neurology 2014, 83, 312–319. [Google Scholar] [CrossRef]
- Wu, Z.; Xiong, S.; Sun, X.; Shi, Q.; Dan, W.; Zhan, Y.; Xie, Y.; Jiang, L. Effects of Apolipoprotein E Polymorphism on Cerebral Oxygen Saturation after Traumatic Brain Injury. Front. Neurol. 2020, 11, 539627. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liang, X.; Wu, Z.; Sun, X.; Shi, Q.; Zhan, Y.; Dan, W.; Zheng, D.; Xia, Y.; Deng, B.; et al. APOE gene polymorphism alters cerebral oxygen saturation and quantitative EEG in early-stage traumatic brain injury. Clin. Neurophysiol. 2022, 136, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, Q.; Sun, X.; Shi, Q.; Dan, W.; Zhan, Y.; Deng, B.; Xia, Y.; Xie, Y.; Jiang, L. Effects of apolipoprotein E polymorphism on cerebral oxygen saturation, cerebral perfusion, and early prognosis after traumatic brain injury. Ann. Clin. Transl. Neurol. 2023, 10, 1002–1011. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E.; Moir, R.D.; Wagner, S.L. Clearance of Alzheimer’s Abeta peptide: The many roads to perdition. Neuron 2004, 43, 605–608. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed]
- Mannix, R.C.; Whalen, M.J. Traumatic brain injury, microglia, and Beta amyloid. Int. J. Alzheimers Dis. 2012, 2012, 608732. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef]
- Norris, C.M.; Kadish, I.; Blalock, E.M.; Chen, K.C.; Thibault, V.; Porter, N.M.; Landfield, P.W.; Kraner, S.D. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci. 2005, 25, 4649–4658. [Google Scholar] [CrossRef]
- Abdul, H.M.; Sama, M.A.; Furman, J.L.; Mathis, D.M.; Beckett, T.L.; Weidner, A.M.; Patel, E.S.; Baig, I.; Murphy, M.P.; LeVine, H., 3rd; et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009, 29, 12957–12969. [Google Scholar] [CrossRef]
- Collins-Praino, L.E. Chapter 38—Traumatic axonal injury as a key driver of the relationship between traumatic brain injury, cognitive dysfunction, and dementia. In Cellular, Molecular, Physiological, and Behavioral Aspects of Traumatic Brain Injury; Rajendram, R., Preedy, V.R., Martin, C.R., Eds.; Acdemic Press: Cambridge, MA, USA, 2022; pp. 475–486. [Google Scholar]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Havlicek, D.F.; Furhang, R.; Nikulina, E.; Smith-Salzberg, B.; Lawless, S.; Severin, S.A.; Mallaboeva, S.; Nayab, F.; Seifert, A.C.; Crary, J.F.; et al. A single closed head injury in male adult mice induces chronic, progressive white matter atrophy and increased phospho-tau expressing oligodendrocytes. Exp. Neurol. 2023, 359, 114241. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Foltz, A.G.; Atkins, M.; Kedir, B.; Moran-Losada, P.; Guldner, I.H.; Munson, C.; Kern, F.; Pálovics, R.; Lu, N.; et al. A spatiotemporal map of the aging mouse brain reveals white matter tracts as vulnerable foci. bioRxiv 2022. bioRxiv:2022.09.18.508419. [Google Scholar] [CrossRef]
- McAleese, K.E.; Walker, L.; Graham, S.; Moya, E.L.J.; Johnson, M.; Erskine, D.; Colloby, S.J.; Dey, M.; Martin-Ruiz, C.; Taylor, J.P.; et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017, 134, 459–473. [Google Scholar] [CrossRef]
- Van Houcke, J.; Marien, V.; Zandecki, C.; Seuntjens, E.; Ayana, R.; Arckens, L. Modeling Neuroregeneration and Neurorepair in an Aging Context: The Power of a Teleost Model. Front. Cell Dev. Biol. 2021, 9, 619197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Romine, J.L.; Gao, X.; Chen, J. Aging impairs dendrite morphogenesis of newborn neurons and is rescued by 7, 8-dihydroxyflavone. Aging Cell 2017, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- Omalu, B. Chronic traumatic encephalopathy. Prog. Neurol. Surg. 2014, 28, 38–49. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef]
- McKee, A.C.; Stein, T.D.; Kiernan, P.T.; Alvarez, V.E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015, 25, 350–364. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C. The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature. Semin. Neurol. 2020, 40, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, J.; Papadopoulos, Z.; McKee, A.C. Chronic Traumatic Encephalopathy: Is Latency in Symptom Onset Explained by Tau Propagation? Cold Spring Harb. Perspect. Med. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Laurer, H.L.; Bareyre, F.M.; Lee, V.M.; Trojanowski, J.Q.; Longhi, L.; Hoover, R.; Saatman, K.E.; Raghupathi, R.; Hoshino, S.; Grady, M.S.; et al. Mild head injury increasing the brain’s vulnerability to a second concussive impact. J. Neurosurg. 2001, 95, 859–870. [Google Scholar] [CrossRef]
- Mouzon, B.; Chaytow, H.; Crynen, G.; Bachmeier, C.; Stewart, J.; Mullan, M.; Stewart, W.; Crawford, F. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 2012, 29, 2761–2773. [Google Scholar] [CrossRef]
- Huh, J.W.; Widing, A.G.; Raghupathi, R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. J. Neurotrauma 2007, 24, 15–27. [Google Scholar] [CrossRef]
- Prins, M.L.; Hales, A.; Reger, M.; Giza, C.C.; Hovda, D.A. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 2010, 32, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Ondek, K.; Brevnova, O.; Jimenez-Ornelas, C.; Vergara, A.; Zwienenberg, M.; Gurkoff, G. A new model of repeat mTBI in adolescent rats. Exp. Neurol. 2020, 331, 113360. [Google Scholar] [CrossRef]
- Feng, Y.; Li, K.; Roth, E.; Chao, D.; Mecca, C.M.; Hogan, Q.H.; Pawela, C.; Kwok, W.M.; Camara, A.K.S.; Pan, B. Repetitive Mild Traumatic Brain Injury in Rats Impairs Cognition, Enhances Prefrontal Cortex Neuronal Activity, and Reduces Pre-synaptic Mitochondrial Function. Front. Cell. Neurosci. 2021, 15, 689334. [Google Scholar] [CrossRef]
- Tadepalli, S.A.; Bali, Z.K.; Bruszt, N.; Nagy, L.V.; Amrein, K.; Fazekas, B.; Buki, A.; Czeiter, E.; Hernadi, I. Long-term cognitive impairment without diffuse axonal injury following repetitive mild traumatic brain injury in rats. Behav. Brain Res. 2020, 378, 112268. [Google Scholar] [CrossRef]
- Eyolfson, E.; Yamakawa, G.R.; Griep, Y.; Collins, R.; Carr, T.; Wang, M.; Lohman, A.W.; Mychasiuk, R. Examining the Progressive Behavior and Neuropathological Outcomes Associated with Chronic Repetitive Mild Traumatic Brain Injury in Rats. Cereb. Cortex Commun. 2020, 1, tgaa002. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Li, J.; Wu, H.; Peng, Y.; Fan, L.; Chen, J.; Gu, C.; Yan, F.; Wang, L.; et al. The Polarization States of Microglia in TBI: A New Paradigm for Pharmacological Intervention. Neural Plast. 2017, 2017, 5405104. [Google Scholar] [CrossRef]
- Kumar, A.; Alvarez-Croda, D.M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Wang, H.; Zhou, M.; Deng, C.; Zhang, L.; Han, Y. Podoplanin influences the inflammatory phenotypes and mobility of microglia in traumatic brain injury. Biochem. Biophys. Res. Commun. 2020, 523, 361–367. [Google Scholar] [CrossRef]
- Catta-Preta, R.; Zdilar, I.; Jenner, B.; Doisy, E.T.; Tercovich, K.; Nord, A.S.; Gurkoff, G.G. Transcriptional Pathology Evolves over Time in Rat Hippocampus after Lateral Fluid Percussion Traumatic Brain Injury. Neurotrauma Rep. 2021, 2, 512–525. [Google Scholar] [CrossRef]
- Li, L.; Yerra, L.; Chang, B.; Mathur, V.; Nguyen, A.; Luo, J. Acute and late administration of colony stimulating factor 1 attenuates chronic cognitive impairment following mild traumatic brain injury in mice. Brain Behav. Immun. 2021, 94, 274–288. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef]
- St. Louis, E.K.; Frey, L.C. (Eds.) Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Roux, F.; Uhlhaas, P.J. Working memory and neural oscillations: Alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control mechanisms in working memory: A possible function of EEG theta oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Lewine, J.D.; Plis, S.; Ulloa, A.; Williams, C.; Spitz, M.; Foley, J.; Paulson, K.; Davis, J.; Bangera, N.; Snyder, T.; et al. Quantitative EEG Biomarkers for Mild Traumatic Brain Injury. J. Clin. Neurophysiol. 2019, 36, 298–305. [Google Scholar] [CrossRef]
- Ondek, K.; Pevzner, A.; Tercovich, K.; Schedlbauer, A.M.; Izadi, A.; Ekstrom, A.D.; Cowen, S.L.; Shahlaie, K.; Gurkoff, G.G. Recovery of Theta Frequency Oscillations in Rats Following Lateral Fluid Percussion Corresponds with a Mild Cognitive Phenotype. Front. Neurol. 2020, 11, 600171. [Google Scholar] [CrossRef]
- Montgomery, E.A.; Fenton, G.W.; McClelland, R.J.; MacFlynn, G.; Rutherford, W.H. The psychobiology of minor head injury. Psychol. Med. 1991, 21, 375–384. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Rieger, R.E.; Wilson, J.K.; Gill, D.; Fullerton, L.; Brandt, E.; Mayer, A.R. Joint analysis of frontal theta synchrony and white matter following mild traumatic brain injury. Brain Imaging Behav. 2020, 14, 2210–2223. [Google Scholar] [CrossRef] [PubMed]
- Carrier-Toutant, F.; Guay, S.; Beaulieu, C.; Leveille, E.; Turcotte-Giroux, A.; Papineau, S.D.; Brisson, B.; D’Hondt, F.; De Beaumont, L. Effects of Repeated Concussions and Sex on Early Processing of Emotional Facial Expressions as Revealed by Electrophysiology. J. Int. Neuropsychol. Soc. 2018, 24, 673–683. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Wilson, J.K.; Rieger, R.E.; Gill, D.; Broadway, J.M.; Story Remer, J.H.; Fratzke, V.; Mayer, A.R.; Quinn, D.K. ERPs predict symptomatic distress and recovery in sub-acute mild traumatic brain injury. Neuropsychologia 2019, 132, 107125. [Google Scholar] [CrossRef]
- Liu, S.; Shi, C.; Ma, X.; Zhao, B.; Chen, X.; Tao, L. Cognitive deficits and rehabilitation mechanisms in mild traumatic brain injury patients revealed by EEG connectivity markers. Clin. Neurophysiol. 2021, 132, 554–567. [Google Scholar] [CrossRef]
- Ledwidge, P.S.; Jones, C.M.; Huston, C.A.; Trenkamp, M.; Bator, B.; Laeng, J. Electrophysiology reveals cognitive-linguistic alterations after concussion. Brain Lang. 2022, 233, 105166. [Google Scholar] [CrossRef]
- Fortin, J.; Grondin, S.; Blanchet, S. Event-related potentials of episodic encoding after traumatic brain injury in older adults. Brain Res. 2021, 1766, 147504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rao, S.L.; Chandramouli, B.A.; Pillai, S.V. Reduction of functional brain connectivity in mild traumatic brain injury during working memory. J. Neurotrauma 2009, 26, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; Elbin, R.J.; Casa, D.J.; Hotz, G.A.; Neville, C.; Lopez, R.M.; Schnyer, D.M.; Yeargin, S.; Covassin, T. Validation of a Machine Learning Brain Electrical Activity-Based Index to Aid in Diagnosing Concussion among Athletes. JAMA Netw. Open 2021, 4, e2037349. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Alosco, M.L.; Armananzas, R.; Martin, B.M.; Tripodis, Y.; Stern, R.A.; Prichep, L.S. Long-Term Changes in Brain Connectivity Reflected in Quantitative Electrophysiology of Symptomatic Former National Football League Players. J. Neurotrauma 2023, 40, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sponheim, S.R.; McGuire, K.A.; Kang, S.S.; Davenport, N.D.; Aviyente, S.; Bernat, E.M.; Lim, K.O. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage 2011, 54 (Suppl. S1), S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorff, J.; Goke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Motes, M.A.; Spence, J.S.; Yeatman, K.; Jones, P.M.; Lutrell, M.; O’Hair, R.; Shakal, S.; DeLaRosa, B.L.; To, W.; Vanneste, S.; et al. High-Definition Transcranial Direct Current Stimulation to Improve Verbal Retrieval Deficits in Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 170–177. [Google Scholar] [CrossRef]
- Choi, G.S.; Kwak, S.G.; Lee, H.D.; Chang, M.C. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: A pilot study. J. Rehabil. Med. 2018, 50, 246–252. [Google Scholar] [CrossRef]
- Herrold, A.A.; Kletzel, S.L.; Harton, B.C.; Chambers, R.A.; Jordan, N.; Pape, T.L. Transcranial magnetic stimulation: Potential treatment for co-occurring alcohol, traumatic brain injury and posttraumatic stress disorders. Neural Regen. Res. 2014, 9, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.; Ellison, R.L.; Herrold, A.A.; Aaronson, A.L.; Kletzel, S.L.; Stika, M.M.; Guernon, A.; Bender Pape, T. rTMS/iTBS and Cognitive Rehabilitation for Deficits Associated with TBI and PTSD: A Theoretical Framework and Review. J. Neuropsychiatry Clin. Neurosci. 2023, 35, 28–38. [Google Scholar] [CrossRef]
- Demirtas-Tatlidede, A.; Vahabzadeh-Hagh, A.M.; Bernabeu, M.; Tormos, J.M.; Pascual-Leone, A. Noninvasive brain stimulation in traumatic brain injury. J. Head. Trauma. Rehabil. 2012, 27, 274–292. [Google Scholar] [CrossRef] [PubMed]
- Pape, T.L.; Rosenow, J.; Lewis, G. Transcranial magnetic stimulation: A possible treatment for TBI. J. Head. Trauma. Rehabil. 2006, 21, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Parr, N.J.; Vela, K. Evidence Brief: Transcranial Magnetic Stimulation (TMS) for Chronic Pain, PTSD, TBI, Opioid Addiction, and Sexual Trauma; VA Evidence-Based Synthesis Program Reports; Department of Veterans Affairs: Washington, DC, USA, 2020. [Google Scholar]
- Huntley, J.H.; Rezvani Habibabadi, R.; Vaishnavi, S.; Khoshpouri, P.; Kraut, M.A.; Yousem, D.M. Transcranial Magnetic Stimulation and its Imaging Features in Patients with Depression, Post-traumatic Stress Disorder, and Traumatic Brain Injury. Acad. Radiol. 2023, 30, 103–112. [Google Scholar] [CrossRef]
- Clayton, E.; Kinley-Cooper, S.K.; Weber, R.A.; Adkins, D.L. Brain stimulation: Neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res. 2016, 1640, 130–138. [Google Scholar] [CrossRef]
- Villamar, M.F.; Santos Portilla, A.; Fregni, F.; Zafonte, R. Noninvasive brain stimulation to modulate neuroplasticity in traumatic brain injury. Neuromodulation 2012, 15, 326–338. [Google Scholar] [CrossRef]
- Coyle, H.L.; Ponsford, J.; Hoy, K.E. Understanding individual variability in symptoms and recovery following mTBI: A role for TMS-EEG? Neurosci. Biobehav. Rev. 2018, 92, 140–149. [Google Scholar] [CrossRef]
- Chail, A.; Saini, R.K.; Bhat, P.S.; Srivastava, K.; Chauhan, V. Transcranial magnetic stimulation: A review of its evolution and current applications. Ind. Psychiatry J. 2018, 27, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Speer, A.M.; Kimbrell, T.A.; Wassermann, E.M.; Repella, J.D.; Willis, M.W.; Herscovitch, P.; Post, R.M. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry 2000, 48, 1133–1141. [Google Scholar] [CrossRef]
- Heath, A.; Lindberg, D.R.; Makowiecki, K.; Gray, A.; Asp, A.J.; Rodger, J.; Choi, D.S.; Croarkin, P.E. Medium- and high-intensity rTMS reduces psychomotor agitation with distinct neurobiologic mechanisms. Transl. Psychiatry 2018, 8, 126. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Cho, K.H.; Kim, E.S.; Lee, M.S.; Lee, K.J. Effect of Epidural Electrical Stimulation and Repetitive Transcranial Magnetic Stimulation in Rats with Diffuse Traumatic Brain Injury. Ann. Rehabil. Med. 2015, 39, 416–424. [Google Scholar] [CrossRef]
- Lu, X.; Bao, X.; Li, J.; Zhang, G.; Guan, J.; Gao, Y.; Wu, P.; Zhu, Z.; Huo, X.; Wang, R. High-frequency repetitive transcranial magnetic stimulation for treating moderate traumatic brain injury in rats: A pilot study. Exp. Ther. Med. 2017, 13, 2247–2254. [Google Scholar] [CrossRef]
- Rajan, T.S.; Cuzzocrea, S.; Bruschetta, D.; Quartarone, A. Repetitive Transcranial Magnetic Stimulation as a Novel Therapy in Animal Models of Traumatic Brain Injury. Methods Mol. Biol. 2016, 1462, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Rodger, J.; Sherrard, R.M. Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury. Neural Regen. Res. 2015, 10, 357–359. [Google Scholar] [CrossRef]
- Gersner, R.; Kravetz, E.; Feil, J.; Pell, G.; Zangen, A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J. Neurosci. 2011, 31, 7521–7526. [Google Scholar] [CrossRef]
- Madore, M.R.; Poh, E.; Bollard, S.J.; Rivera, J.; Taylor, J.; Cheng, J.; Booth, E.; Nable, M.; Heath, A.; Yesavage, J.; et al. Moving back in the brain to drive the field forward: Targeting neurostimulation to different brain regions in animal models of depression and neurodegeneration. J. Neurosci. Methods 2021, 360, 109261. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.M.; Brewer, M.; Yesavage, J.; McNerney, M.W. Improved object recognition memory using post-encoding repetitive transcranial magnetic stimulation. Brain Stimul. 2022, 15, 78–86. [Google Scholar] [CrossRef]
- McNerney, M.W.; Heath, A.; Narayanan, S.K.; Yesavage, J. Repetitive Transcranial Magnetic Stimulation Improves Brain-Derived Neurotrophic Factor and Cholinergic Signaling in the 3xTgAD Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2022, 86, 499–507. [Google Scholar] [CrossRef]
- Lu, H.; Kobilo, T.; Robertson, C.; Tong, S.; Celnik, P.; Pelled, G. Transcranial magnetic stimulation facilitates neurorehabilitation after pediatric traumatic brain injury. Sci. Rep. 2015, 5, 14769. [Google Scholar] [CrossRef]
- Monti, J.M.; Voss, M.W.; Pence, A.; McAuley, E.; Kramer, A.F.; Cohen, N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, M.V.; Reeves, J.A.; Fioriti, C.M.; Murillo, J.; Wassermann, E.M. Reproducing the effect of hippocampal network-targeted transcranial magnetic stimulation on episodic memory. Behav. Brain Res. 2022, 419, 113707. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Martin, D.M.; Berryhill, M.E.; Dielenberg, V. Can brain stimulation enhance cognition in clinical populations? A critical review. Restor. Neurol. Neurosci. 2022, 40, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Soleimani, G.; Nitsche, M.A.; Bergmann, T.O.; Towhidkhah, F.; Violante, I.R.; Lorenz, R.; Kuplicki, R.; Tsuchiyagaito, A.; Mulyana, B.; Mayeli, A.; et al. Closing the loop between brain and electrical stimulation: Towards precision neuromodulation treatments. Transl. Psychiatry 2023, 13, 279. [Google Scholar] [CrossRef]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Romero Lauro, L.J. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef]
- Chan, M.M.Y.; Yau, S.S.Y.; Han, Y.M.Y. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci. Biobehav. Rev. 2021, 125, 392–416. [Google Scholar] [CrossRef] [PubMed]
- de Souza Custodio, J.C.; Martins, C.W.; Lugon, M.D.; Fregni, F.; Nakamura-Palacios, E.M. Epidural direct current stimulation over the left medial prefrontal cortex facilitates spatial working memory performance in rats. Brain Stimul. 2013, 6, 261–269. [Google Scholar] [CrossRef]
- Yoon, K.J.; Lee, Y.T.; Chae, S.W.; Park, C.R.; Kim, D.Y. Effects of anodal transcranial direct current stimulation (tDCS) on behavioral and spatial memory during the early stage of traumatic brain injury in the rats. J. Neurol. Sci. 2016, 362, 314–320. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Wen, H.; Zhang, Y.; Tian, X. Intensity-dependent effects of repetitive anodal transcranial direct current stimulation on learning and memory in a rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2015, 123, 168–178. [Google Scholar] [CrossRef]
- Gondard, E.; Soto-Montenegro, M.L.; Cassol, A.; Lozano, A.M.; Hamani, C. Transcranial direct current stimulation does not improve memory deficits or alter pathological hallmarks in a rodent model of Alzheimer’s disease. J. Psychiatr. Res. 2019, 114, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dorval, A.D.; Grill, W.M. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 2014, 111, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Musacchio, T.; Paulat, R.; Matthies, C.; Endres, H.; Wenger, N.; Harms, C.; Ip, C.W. Experimental deep brain stimulation in rodent models of movement disorders. Exp. Neurol. 2022, 348, 113926. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schafer, H.; Botzel, K.; Daniels, C.; Deutschlander, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef]
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Tsai, S.T.; Chen, S.Y.; Lin, S.Z.; Tseng, G.F. Rostral intralaminar thalamic deep brain stimulation ameliorates memory deficits and dendritic regression in beta-amyloid-infused rats. Brain Struct. Funct. 2020, 225, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chu, H.; Ma, Y.; Zhou, Z.; Dai, C.; Huang, X.; Fang, L.; Ao, Q.; Huang, D. The neuroprotective effect of deep brain stimulation at nucleus basalis of Meynert in transgenic mice with Alzheimer’s disease. Brain Stimul. 2019, 12, 161–174. [Google Scholar] [CrossRef]
- Izadi, A.; Pevzner, A.; Lee, D.J.; Ekstrom, A.D.; Shahlaie, K.; Gurkoff, G.G. Medial septal stimulation increases seizure threshold and improves cognition in epileptic rats. Brain Stimul. 2019, 12, 735–742. [Google Scholar] [CrossRef]
- Izadi, A.; Schedlbauer, A.; Ondek, K.; Disse, G.; Ekstrom, A.D.; Cowen, S.L.; Shahlaie, K.; Gurkoff, G.G. Early Intervention via Stimulation of the Medial Septal Nucleus Improves Cognition and Alters Markers of Epileptogenesis in Pilocarpine-Induced Epilepsy. Front. Neurol. 2021, 12, 708957. [Google Scholar] [CrossRef]
- Lee, D.J.; Gurkoff, G.G.; Izadi, A.; Berman, R.F.; Ekstrom, A.D.; Muizelaar, J.P.; Lyeth, B.G.; Shahlaie, K. Medial septal nucleus theta frequency deep brain stimulation improves spatial working memory after traumatic brain injury. J. Neurotrauma 2013, 30, 131–139. [Google Scholar] [CrossRef]
- Lee, D.J.; Gurkoff, G.G.; Izadi, A.; Seidl, S.E.; Echeverri, A.; Melnik, M.; Berman, R.F.; Ekstrom, A.D.; Muizelaar, J.P.; Lyeth, B.G.; et al. Septohippocampal Neuromodulation Improves Cognition after Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Izadi, A.; Melnik, M.; Seidl, S.; Echeverri, A.; Shahlaie, K.; Gurkoff, G.G. Stimulation of the medial septum improves performance in spatial learning following pilocarpine-induced status epilepticus. Epilepsy Res. 2017, 130, 53–63. [Google Scholar] [CrossRef]
- Schermer, M. Ethical issues in deep brain stimulation. Front. Integr. Neurosci. 2011, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R. Ten Points to Improve Reproducibility and Translation of Animal Research. Front. Behav. Neurosci. 2022, 16, 869511. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.; Carr, H.; Kelley, N.; Seneviratne, R.; Reed, C.; Parlatini, V.; Garner, M.; Solmi, M.; Rosson, S.; Cortese, S.; et al. Efficacy of neurostimulation across mental disorders: Systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 2022, 27, 2709–2719. [Google Scholar] [CrossRef]
- Patel, R.; Silla, F.; Pierce, S.; Theule, J.; Girard, T.A. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): A quantitative meta-analysis in healthy adults. Neuropsychologia 2020, 141, 107395. [Google Scholar] [CrossRef]
- Kan, R.L.D.; Padberg, F.; Giron, C.G.; Lin, T.T.Z.; Zhang, B.B.B.; Brunoni, A.R.; Kranz, G.S. Effects of repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex on symptom domains in neuropsychiatric disorders: A systematic review and cross-diagnostic meta-analysis. Lancet Psychiatry 2023, 10, 252–259. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Curcic-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Kirkovski, M.; Donaldson, P.H.; Do, M.; Speranza, B.E.; Albein-Urios, N.; Oberman, L.M.; Enticott, P.G. A systematic review of the neurobiological effects of theta-burst stimulation (TBS) as measured using functional magnetic resonance imaging (fMRI). Brain Struct. Funct. 2023, 228, 717–749. [Google Scholar] [CrossRef]
- Chung, S.W.; Hoy, K.E.; Fitzgerald, P.B. Theta-burst stimulation: A new form of TMS treatment for depression? Depress. Anxiety 2015, 32, 182–192. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Li, C.T.; Juan, C.H. A review of critical brain oscillations in depression and the efficacy of transcranial magnetic stimulation treatment. Front. Psychiatry 2023, 14, 1073984. [Google Scholar] [CrossRef] [PubMed]
- Bonni, S.; Mastropasqua, C.; Bozzali, M.; Caltagirone, C.; Koch, G. Theta burst stimulation improves visuo-spatial attention in a patient with traumatic brain injury. Neurol. Sci. 2013, 34, 2053–2056. [Google Scholar] [CrossRef]
- Jannati, A.; Oberman, L.M.; Rotenberg, A.; Pascual-Leone, A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 2023, 48, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Nakanishi, T.; Sasaki, A.; Yamaguchi, A.; Nomura, T.; Popovic, M.R.; Nakazawa, K. Cortical Re-organization After Traumatic Brain Injury Elicited Using Functional Electrical Stimulation Therapy: A Case Report. Front. Neurosci. 2021, 15, 693861. [Google Scholar] [CrossRef] [PubMed]
- Coyle, H.L.; Bailey, N.W.; Ponsford, J.; Hoy, K.E. Investigation of neurobiological responses to theta burst stimulation during recovery from mild traumatic brain injury (mTBI). Behav. Brain Res. 2023, 442, 114308. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Sullivan, C.M.; Cash, R.F.H.; Fitzgerald, P.B. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS-EEG and working memory performance. Hum. Brain Mapp. 2018, 39, 783–802. [Google Scholar] [CrossRef]
- Wang, J.; Nelson, B. Transcranial Magnetic Stimulation for Treatment-Resistant Depression: An Interactive Self-Learning Module. MedEdPORTAL 2018, 14, 10713. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Kandala, S.; Hacker, C.D.; Trapp, N.T.; Leuthardt, E.C.; Carter, A.R.; Brody, D.L. Individualized precision targeting of dorsal attention and default mode networks with rTMS in traumatic brain injury-associated depression. Sci. Rep. 2023, 13, 4052. [Google Scholar] [CrossRef]
- Hoy, K.E.; McQueen, S.; Elliot, D.; Herring, S.E.; Maller, J.J.; Fitzgerald, P.B. A Pilot Investigation of Repetitive Transcranial Magnetic Stimulation for Post-Traumatic Brain Injury Depression: Safety, Tolerability, and Efficacy. J. Neurotrauma 2019, 36, 2092–2098. [Google Scholar] [CrossRef]
- Rao, V.; Bechtold, K.; McCann, U.; Roy, D.; Peters, M.; Vaishnavi, S.; Yousem, D.; Mori, S.; Yan, H.; Leoutsakos, J.; et al. Low-Frequency Right Repetitive Transcranial Magnetic Stimulation for the Treatment of Depression after Traumatic Brain Injury: A Randomized Sham-Controlled Pilot Study. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 306–318. [Google Scholar] [CrossRef]
- Oberman, L.M.; Exley, S.; Philip, N.S.; Siddiqi, S.H.; Adamson, M.M.; Brody, D.L. Use of Repetitive Transcranial Magnetic Stimulation in the Treatment of Neuropsychiatric and Neurocognitive Symptoms Associated with Concussion in Military Populations. J. Head. Trauma. Rehabil. 2020, 35, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.S.; Ramanathan, D.; Gamboa, B.; Brennan, M.C.; Kozel, F.A.; Lazzeroni, L.; Madore, M.R. Repetitive Transcranial Magnetic Stimulation for Depression and Posttraumatic Stress Disorder in Veterans with Mild Traumatic Brain Injury. Neuromodulation 2023, 26, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, T.; Yu, H.; Shen, J.; Chen, Z.; Yang, X.; Huang, Z.; Yang, Y.; Feng, Y.; Zhou, X.; et al. Repetitive Transcranial Magnetic Stimulation for Neuropathic Pain and Neuropsychiatric Symptoms in Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Neural Plast. 2022, 2022, 2036736. [Google Scholar] [CrossRef] [PubMed]
- Green, J.D.; Arduini, A.A. Hippocampal electrical activity in arousal. J. Neurophysiol. 1954, 17, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Brankack, J.; Stewart, M.; Fox, S.E. Current source density analysis of the hippocampal theta rhythm: Associated sustained potentials and candidate synaptic generators. Brain Res. 1993, 615, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Hyman, J.M.; Wyble, B.P.; Goyal, V.; Rossi, C.A.; Hasselmo, M.E. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci. 2003, 23, 11725–11731. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. Learning and memory. Never fear, LTP is hear. Nature 1997, 390, 552–553. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Huerta, P.T.; Lisman, J.E. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 1993, 364, 723–725. [Google Scholar] [CrossRef]
- McCartney, H.; Johnson, A.D.; Weil, Z.M.; Givens, B. Theta reset produces optimal conditions for long-term potentiation. Hippocampus 2004, 14, 684–687. [Google Scholar] [CrossRef]
- Pavlides, C.; Greenstein, Y.J.; Grudman, M.; Winson, J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988, 439, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Maier, D.L.; Cross, B.; Doherty, J.J.; Christian, E.P. Fimbria-fornix lesions compromise the induction of long-term potentiation at the Schaffer collateral-CA1 synapse in the rat in vivo. J. Neurophysiol. 2005, 93, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Benchenane, K.; Peyrache, A.; Khamassi, M.; Tierney, P.L.; Gioanni, Y.; Battaglia, F.P.; Wiener, S.I. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron 2010, 66, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Wilson, M.A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005, 3, e402. [Google Scholar] [CrossRef] [PubMed]
- van Wingerden, M.; van der Meij, R.; Kalenscher, T.; Maris, E.; Pennartz, C.M. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning. J. Neurosci. 2014, 34, 493–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tort, A.B.; Komorowski, R.W.; Manns, J.R.; Kopell, N.J.; Eichenbaum, H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. USA 2009, 106, 20942–20947. [Google Scholar] [CrossRef]
- Tort, A.B.; Kramer, M.A.; Thorn, C.; Gibson, D.J.; Kubota, Y.; Graybiel, A.M.; Kopell, N.J. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. USA 2008, 105, 20517–20522. [Google Scholar] [CrossRef]
- Kendrick, K.M.; Zhan, Y.; Fischer, H.; Nicol, A.U.; Zhang, X.; Feng, J. Learning alters theta amplitude, theta-gamma coupling and neuronal synchronization in inferotemporal cortex. BMC Neurosci. 2011, 12, 55. [Google Scholar] [CrossRef]
- Lega, B.; Burke, J.; Jacobs, J.; Kahana, M.J. Slow-Theta-to-Gamma Phase-Amplitude Coupling in Human Hippocampus Supports the Formation of New Episodic Memories. Cereb. Cortex 2016, 26, 268–278. [Google Scholar] [CrossRef]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef]
- Xu, X.; An, L.; Mi, X.; Zhang, T. Impairment of cognitive function and synaptic plasticity associated with alteration of information flow in theta and gamma oscillations in melamine-treated rats. PLoS ONE 2013, 8, e77796. [Google Scholar] [CrossRef]
- Bazzigaluppi, P.; Beckett, T.L.; Koletar, M.M.; Lai, A.Y.; Joo, I.L.; Brown, M.E.; Carlen, P.L.; McLaurin, J.; Stefanovic, B. Early-stage attenuation of phase-amplitude coupling in the hippocampus and medial prefrontal cortex in a transgenic rat model of Alzheimer’s disease. J. Neurochem. 2018, 144, 669–679. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsaki, G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef]
- O’Keefe, J.; Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 1993, 3, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, W.E.; McNaughton, B.L.; Wilson, M.A.; Barnes, C.A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 1996, 6, 149–172. [Google Scholar] [CrossRef]
- Tsodyks, M.V.; Skaggs, W.E.; Sejnowski, T.J.; McNaughton, B.L. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: A spiking neuron model. Hippocampus 1996, 6, 271–280. [Google Scholar] [CrossRef]
- Bohbot, V.D.; Copara, M.S.; Gotman, J.; Ekstrom, A.D. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat. Commun. 2017, 8, 14415. [Google Scholar] [CrossRef] [PubMed]
- Vass, L.K.; Copara, M.S.; Seyal, M.; Shahlaie, K.; Farias, S.T.; Shen, P.Y.; Ekstrom, A.D. Oscillations Go the Distance: Low-Frequency Human Hippocampal Oscillations Code Spatial Distance in the Absence of Sensory Cues during Teleportation. Neuron 2016, 89, 1180–1186. [Google Scholar] [CrossRef]
- Ekstrom, A.D.; Caplan, J.B.; Ho, E.; Shattuck, K.; Fried, I.; Kahana, M.J. Human hippocampal theta activity during virtual navigation. Hippocampus 2005, 15, 881–889. [Google Scholar] [CrossRef]
- Watrous, A.J.; Lee, D.J.; Izadi, A.; Gurkoff, G.G.; Shahlaie, K.; Ekstrom, A.D. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 2013, 23, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Merkow, M.B.; Jacobs, J.; Kahana, M.J.; Zaghloul, K.A. Brain computer interface to enhance episodic memory in human participants. Front. Hum. Neurosci. 2014, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Sharan, A.D.; Sperling, M.R.; Ramayya, A.G.; Evans, J.J.; Healey, M.K.; Beck, E.N.; Davis, K.A.; Lucas, T.H., 2nd; Kahana, M.J. Theta and high-frequency activity mark spontaneous recall of episodic memories. J. Neurosci. 2014, 34, 11355–11365. [Google Scholar] [CrossRef] [PubMed]
- Merkow, M.B.; Burke, J.F.; Stein, J.M.; Kahana, M.J. Prestimulus theta in the human hippocampus predicts subsequent recognition but not recall. Hippocampus 2014, 24, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Rugg, M.D.; Das, S.; Stein, J.; Rizzuto, D.S.; Kahana, M.J.; Lega, B.C. Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans. Hippocampus 2017, 27, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.A.; Kragel, J.E.; Gross, R.; Lega, B.; Sperling, M.R.; Worrell, G.; Sheth, S.A.; Zaghloul, K.A.; Jobst, B.C.; Stein, J.M.; et al. Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat. Commun. 2018, 9, 4437. [Google Scholar] [CrossRef]
- Mizumori, S.J.; Barnes, C.A.; McNaughton, B.L. Behavioral correlates of theta-on and theta-off cells recorded from hippocampal formation of mature young and aged rats. Exp. Brain Res. 1990, 80, 365–373. [Google Scholar] [CrossRef]
- Givens, B.S.; Olton, D.S. Cholinergic and GABAergic modulation of medial septal area: Effect on working memory. Behav. Neurosci. 1990, 104, 849–855. [Google Scholar] [CrossRef]
- McNaughton, N.; Ruan, M.; Woodnorth, M.A. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus 2006, 16, 1102–1110. [Google Scholar] [CrossRef]
- Inostroza, M.; Brotons-Mas, J.R.; Laurent, F.; Cid, E.; de la Prida, L.M. Specific impairment of “what-where-when” episodic-like memory in experimental models of temporal lobe epilepsy. J. Neurosci. 2013, 33, 17749–17762. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Stark, K.L.; Karayiorgou, M.; Gogos, J.A.; Gordon, J.A. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 2010, 464, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J. Reduced degree of long-range phase synchrony in pathological human brain. Acta Neurobiol. Exp. 2001, 61, 309–318. [Google Scholar]
- Maurer, A.P.; McNaughton, B.L. Network and intrinsic cellular mechanisms underlying theta phase precession of hippocampal neurons. Trends Neurosci. 2007, 30, 325–333. [Google Scholar] [CrossRef]
- Huxter, J.R.; Miranda, J.A.; Dias, R. The hippocampal physiology of approaching middle-age: Early indicators of change. Hippocampus 2012, 22, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Lenck-Santini, P.P.; Holmes, G.L. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J. Neurosci. 2008, 28, 5053–5062. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Prager, E.M.; Pidoplichko, V.; Figueiredo, T.H.; Marini, A.M.; Li, Z.; Eiden, L.E.; Braga, M.F. GABAergic interneuronal loss and reduced inhibitory synaptic transmission in the hippocampal CA1 region after mild traumatic brain injury. Exp. Neurol. 2015, 273, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Huusko, N.; Romer, C.; Ndode-Ekane, X.E.; Lukasiuk, K.; Pitkanen, A. Loss of hippocampal interneurons and epileptogenesis: A comparison of two animal models of acquired epilepsy. Brain Struct. Funct. 2015, 220, 153–191. [Google Scholar] [CrossRef]
- Toth, Z.; Hollrigel, G.S.; Gorcs, T.; Soltesz, I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J. Neurosci. 1997, 17, 8106–8117. [Google Scholar] [CrossRef]
- Drexel, M.; Puhakka, N.; Kirchmair, E.; Hortnagl, H.; Pitkanen, A.; Sperk, G. Expression of GABA receptor subunits in the hippocampus and thalamus after experimental traumatic brain injury. Neuropharmacology 2015, 88, 122–133. [Google Scholar] [CrossRef]
- Mtchedlishvili, Z.; Lepsveridze, E.; Xu, H.; Kharlamov, E.A.; Lu, B.; Kelly, K.M. Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol. Dis. 2010, 38, 464–475. [Google Scholar] [CrossRef]
- O’Dell, D.M.; Gibson, C.J.; Wilson, M.S.; DeFord, S.M.; Hamm, R.J. Positive and negative modulation of the GABA(A) receptor and outcome after traumatic brain injury in rats. Brain Res. 2000, 861, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fedor, M.; Berman, R.F.; Muizelaar, J.P.; Lyeth, B.G. Hippocampal theta dysfunction after lateral fluid percussion injury. J. Neurotrauma 2010, 27, 1605–1615. [Google Scholar] [CrossRef]
- Berryhill, M.E.; Martin, D. Cognitive Effects of Transcranial Direct Current Stimulation in Healthy and Clinical Populations: An Overview. J. ECT 2018, 34, e25–e35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Truong, D.Q.; Esmaeilpour, Z.; Huang, Y.; Badran, B.W.; Bikson, M. Enhanced tES and tDCS computational models by meninges emulation. J. Neural Eng. 2020, 17, 016027. [Google Scholar] [CrossRef] [PubMed]
- Unal, G.; Ficek, B.; Webster, K.; Shahabuddin, S.; Truong, D.; Hampstead, B.; Bikson, M.; Tsapkini, K. Impact of brain atrophy on tDCS and HD-tDCS current flow: A modeling study in three variants of primary progressive aphasia. Neurol. Sci. 2020, 41, 1781–1789. [Google Scholar] [CrossRef]
- Shahid, S.; Wen, P.; Ahfock, T. Assessment of electric field distribution in anisotropic cortical and subcortical regions under the influence of tDCS. Bioelectromagnetics 2014, 35, 41–57. [Google Scholar] [CrossRef]
- Gomez-Tames, J.; Asai, A.; Hirata, A. Significant group-level hotspots found in deep brain regions during transcranial direct current stimulation (tDCS): A computational analysis of electric fields. Clin. Neurophysiol. 2020, 131, 755–765. [Google Scholar] [CrossRef]
- Grover, S.; Fayzullina, R.; Bullard, B.M.; Levina, V.; Reinhart, R.M.G. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl. Med. 2023, 15, eabo2044. [Google Scholar] [CrossRef]
- Senkowski, D.; Sobirey, R.; Haslacher, D.; Soekadar, S.R. Boosting working memory: Uncovering the differential effects of tDCS and tACS. Cereb. Cortex Commun. 2022, 3, tgac018. [Google Scholar] [CrossRef]
- Feltman, K.A.; Hayes, A.M.; Bernhardt, K.A.; Nwala, E.; Kelley, A.M. Viability of tDCS in Military Environments for Performance Enhancement: A Systematic Review. Mil. Med. 2020, 185, e53–e60. [Google Scholar] [CrossRef]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-Analytic Review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Pergher, V.; Au, J.; Alizadeh Shalchy, M.; Santarnecchi, E.; Seitz, A.; Jaeggi, S.M.; Battelli, L. The benefits of simultaneous tDCS and working memory training on transfer outcomes: A systematic review and meta-analysis. Brain Stimul. 2022, 15, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Stephens, J.A.; Alam, M.; Bikson, M.; Berryhill, M.E. Longitudinal neurostimulation in older adults improves working memory. PLoS ONE 2015, 10, e0121904. [Google Scholar] [CrossRef]
- Johnson, E.L.; Arciniega, H.; Jones, K.T.; Kilgore-Gomez, A.; Berryhill, M.E. Individual predictors and electrophysiological signatures of working memory enhancement in aging. Neuroimage 2022, 250, 118939. [Google Scholar] [CrossRef]
- Jones, K.T.; Johnson, E.L.; Berryhill, M.E. Frontoparietal theta-gamma interactions track working memory enhancement with training and tDCS. Neuroimage 2020, 211, 116615. [Google Scholar] [CrossRef]
- Jones, K.T.; Peterson, D.J.; Blacker, K.J.; Berryhill, M.E. Frontoparietal neurostimulation modulates working memory training benefits and oscillatory synchronization. Brain Res. 2017, 1667, 28–40. [Google Scholar] [CrossRef]
- Grover, S.; Nguyen, J.A.; Reinhart, R.M.G. Synchronizing Brain Rhythms to Improve Cognition. Annu. Rev. Med. 2021, 72, 29–43. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 2019, 22, 820–827. [Google Scholar] [CrossRef]
- Srihagulang, C.; Vongsfak, J.; Vaniyapong, T.; Chattipakorn, N.; Chattipakorn, S.C. Potential roles of vagus nerve stimulation on traumatic brain injury: Evidence from in vivo and clinical studies. Exp. Neurol. 2022, 347, 113887. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.; Nemmi, F.; Karlsson, K.; Klingberg, T. Transcranial Electric Stimulation Can Impair Gains during Working Memory Training and Affects the Resting State Connectivity. Front. Hum. Neurosci. 2017, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljevic, V.; Fecteau, S.; Ferreri, F.; Floel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Giacino, J.; Fins, J.J.; Machado, A.; Schiff, N.D. Central thalamic deep brain stimulation to promote recovery from chronic posttraumatic minimally conscious state: Challenges and opportunities. Neuromodulation 2012, 15, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Chudy, D.; Deletis, V.; Almahariq, F.; Marcinkovic, P.; Skrlin, J.; Paradzik, V. Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: Experience in 14 patients. J. Neurosurg. 2018, 128, 1189–1198. [Google Scholar] [CrossRef]
- Verisezan Rosu, O.; Jemna, N.; Hapca, E.; Benedek, I.; Vadan, I.; Muresanu, I.; Chira, D.; Radu, C.; Chereches, R.; Strilciuc, S.; et al. Cerebrolysin and repetitive transcranial magnetic stimulation (rTMS) in patients with traumatic brain injury: A three-arm randomized trial. Front. Neurosci. 2023, 17, 1186751. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, M.K. Effect of Low Frequency Repetitive Transcranial Magnetic Stimulation on Depression and Cognition of Patients with Traumatic Brain Injury: A Randomized Controlled Trial. Med. Sci. Monit. 2018, 24, 8789–8794. [Google Scholar] [CrossRef]
- Franke, L.M.; Gitchel, G.T.; Perera, R.A.; Hadimani, R.L.; Holloway, K.L.; Walker, W.C. Randomized trial of rTMS in traumatic brain injury: Improved subjective neurobehavioral symptoms and increases in EEG delta activity. Brain Inj. 2022, 36, 683–692. [Google Scholar] [CrossRef]
- Rodrigues, P.A.; Zaninotto, A.L.; Ventresca, H.M.; Neville, I.S.; Hayashi, C.Y.; Brunoni, A.R.; de Paula Guirado, V.M.; Teixeira, M.J.; Paiva, W.S. The Effects of Repetitive Transcranial Magnetic Stimulation on Anxiety in Patients with Moderate to Severe Traumatic Brain Injury: A Post-hoc Analysis of a Randomized Clinical Trial. Front. Neurol. 2020, 11, 564940. [Google Scholar] [CrossRef]
- Neville, I.S.; Zaninotto, A.L.; Hayashi, C.Y.; Rodrigues, P.A.; Galhardoni, R.; Ciampi de Andrade, D.; Brunoni, A.R.; Amorim, R.L.O.; Teixeira, M.J.; Paiva, W.S. Repetitive TMS does not improve cognition in patients with TBI: A randomized double-blind trial. Neurology 2019, 93, e190–e199. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E.; Lu, L.H.; Reid, M.W.; Leal, F.O.; Cooper, D.B. Correlates of Depression in U.S. Military Service Members with a History of Mild Traumatic Brain Injury. Mil. Med. 2019, 184, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Stilling, J.; Paxman, E.; Mercier, L.; Gan, L.S.; Wang, M.; Amoozegar, F.; Dukelow, S.P.; Monchi, O.; Debert, C. Treatment of Persistent Post-Traumatic Headache and Post-Concussion Symptoms Using Repetitive Transcranial Magnetic Stimulation: A Pilot, Double-Blind, Randomized Controlled Trial. J. Neurotrauma 2020, 37, 312–323. [Google Scholar] [CrossRef]
- Leung, A.; Metzger-Smith, V.; He, Y.; Cordero, J.; Ehlert, B.; Song, D.; Lin, L.; Shahrokh, G.; Tsai, A.; Vaninetti, M.; et al. Left Dorsolateral Prefrontal Cortex rTMS in Alleviating MTBI Related Headaches and Depressive Symptoms. Neuromodulation 2018, 21, 390–401. [Google Scholar] [CrossRef]
- Vaninetti, M.; Lim, M.; Khalaf, A.; Metzger-Smith, V.; Flowers, M.; Kunnel, A.; Yang, E.; Song, D.; Lin, L.; Tsai, A.; et al. fMRI findings in MTBI patients with headaches following rTMS. Sci. Rep. 2021, 11, 9573. [Google Scholar] [CrossRef] [PubMed]
- Charvet, L.; Harrison, A.T.; Mangold, K.; Moore, R.D.; Guo, S.; Zhang, J.; Datta, A.; Androulakis, X.M. Remotely supervised at-home tDCS for veterans with persistent post-traumatic headache: A double-blind, sham-controlled randomized pilot clinical trial. Front. Neurol. 2023, 14, 1184056. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chou, P.-H.; Chuang, H.-Y.; Yao, C.-Y.; Chen, W.-J.; Tsai, H.-C. Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 6030. [Google Scholar] [CrossRef]
- Moussavi, Z.; Suleiman, A.; Rutherford, G.; Ranjbar Pouya, O.; Dastgheib, Z.; Zhang, W.; Salter, J.; Wang, X.; Mansouri, B.; Lithgow, B. A Pilot Randomised Double-Blind Study of the Tolerability and Efficacy of Repetitive Transcranial Magnetic Stimulation on Persistent Post-Concussion Syndrome. Sci. Rep. 2019, 9, 5498. [Google Scholar] [CrossRef]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philos. Ethics Humanit. Med. 2009, 4, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).