Neuropsychological Alterations of Prolactinomas’ Cognitive Flexibility in Task Switching
Abstract
1. Introduction
2. Method
2.1. Participants
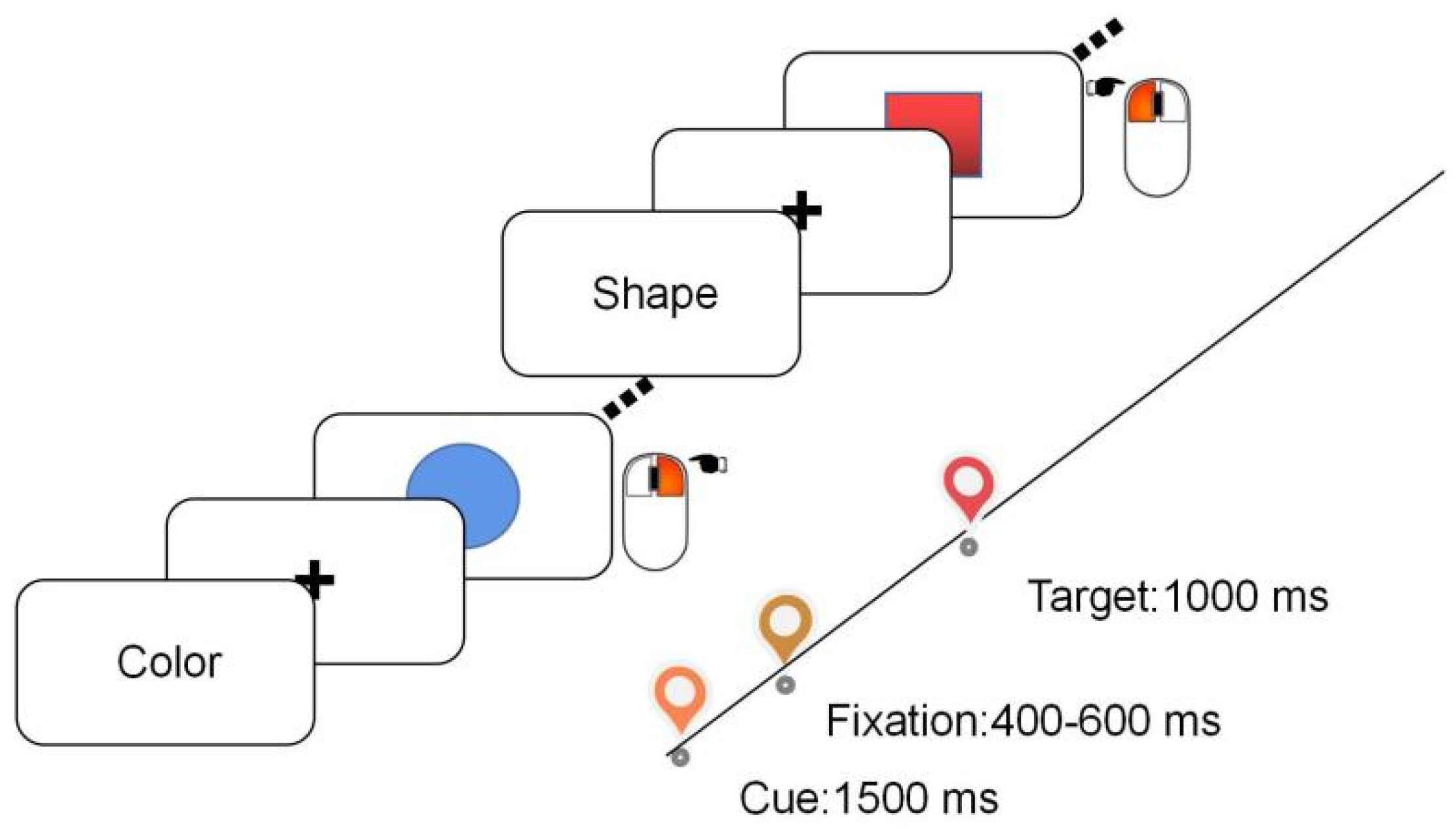
2.2. Stimuli and Procedure
2.3. EEG Recording
2.4. Data Analysis
3. Results
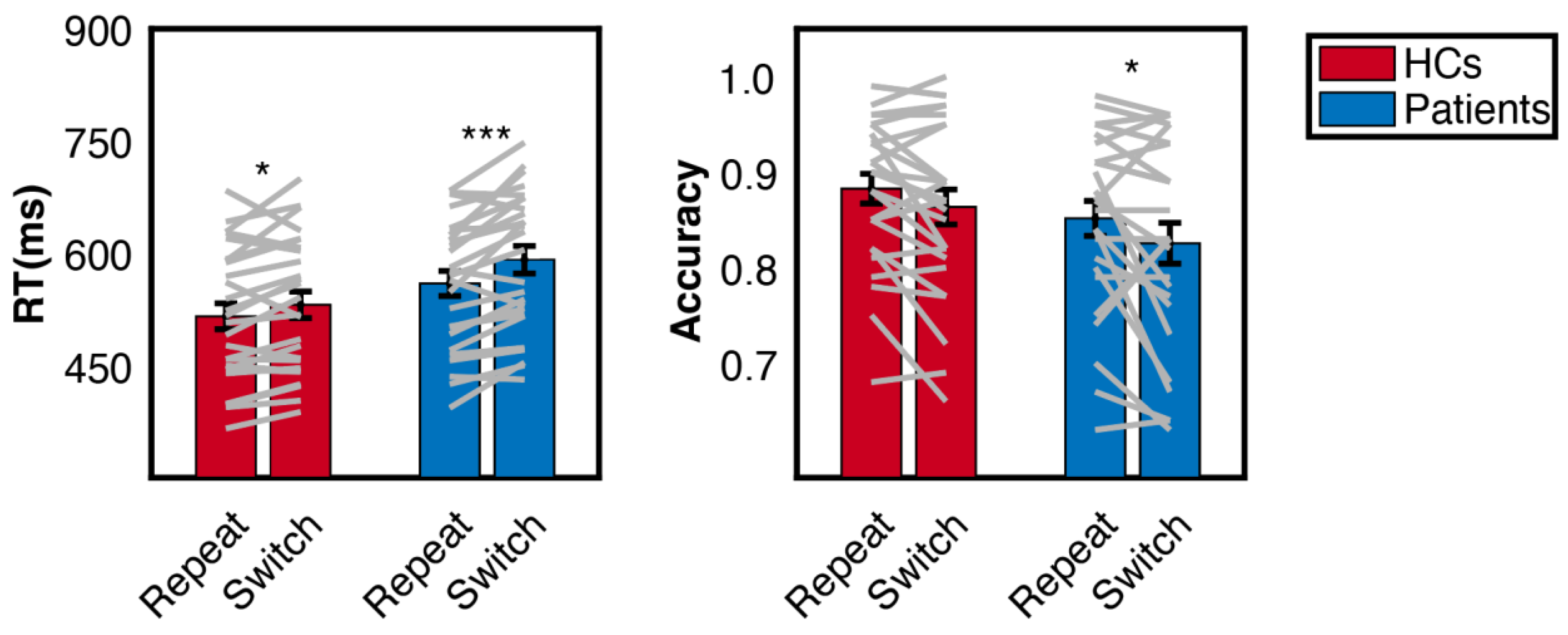
3.1. Behaviour
3.2. Non-Selective Preparatory Brain States in Patients with Prolactinomas
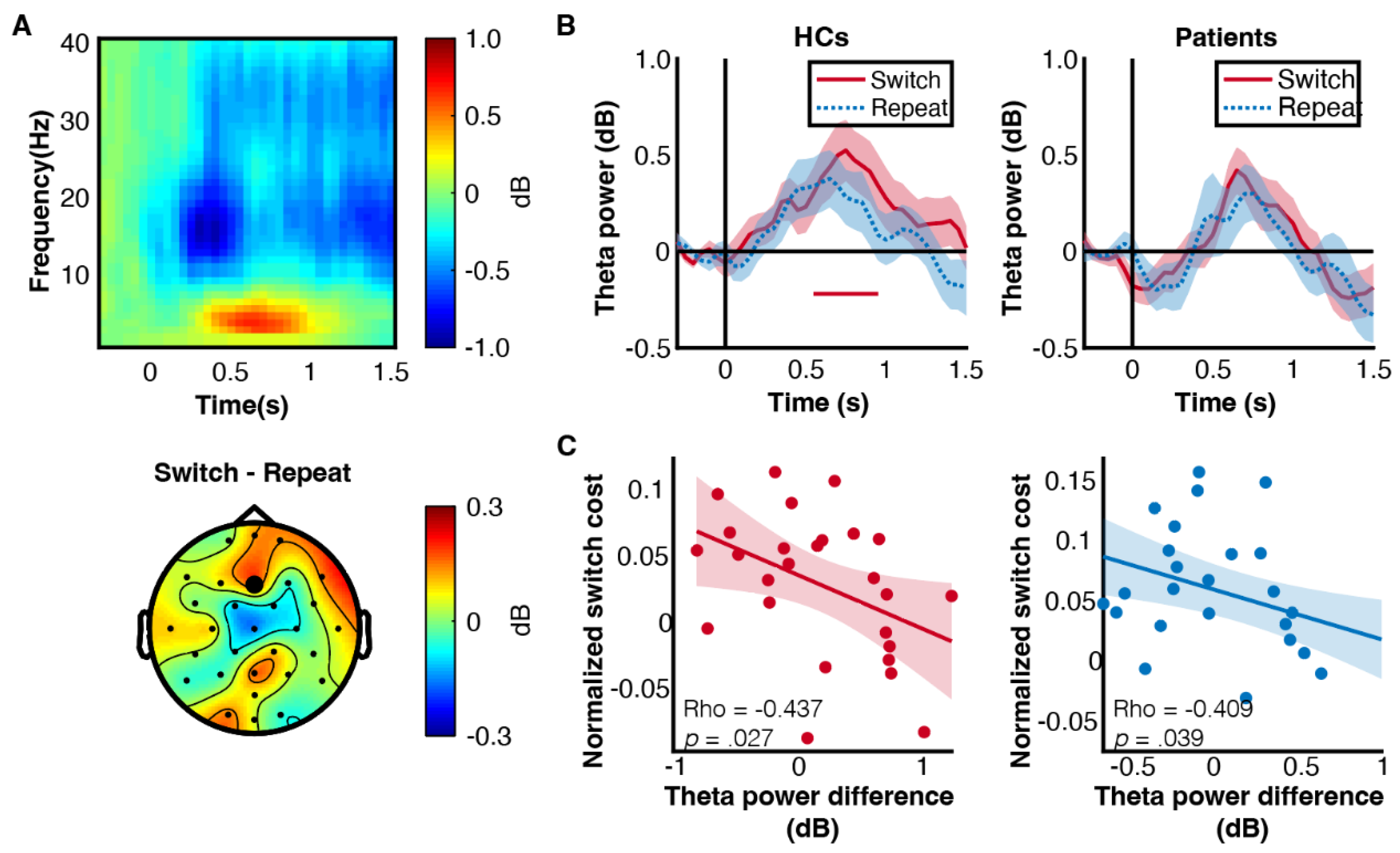
3.3. Patients Showed Decreased Frontal Theta Power
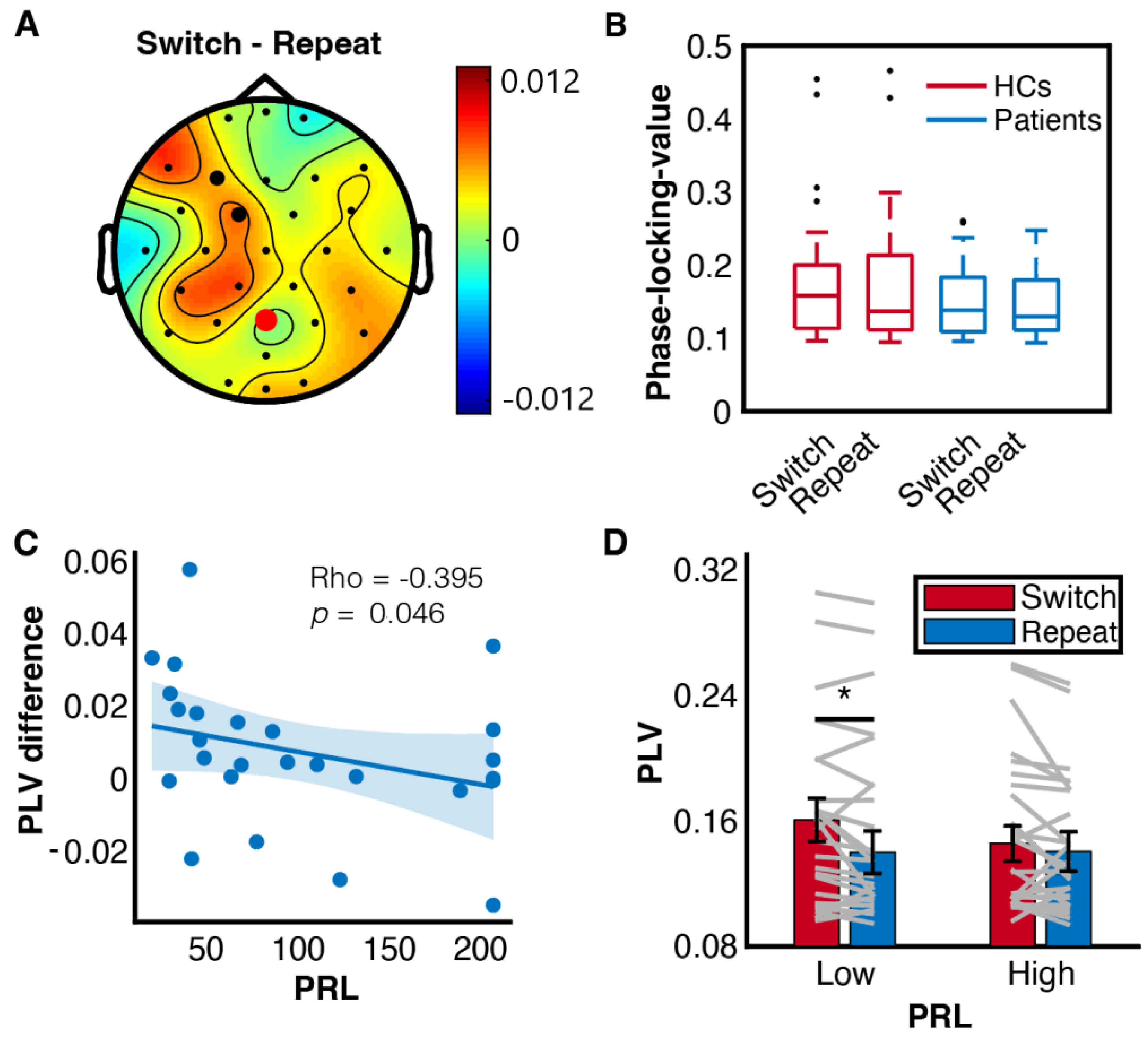
3.4. Prolactinomas Impaired Frontoparietal Synchrony at Theta Band
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Lee, M.; Pineyro, M.M.; Skugor, M.; Reddy, S.K.; Siraj, E.S.; Hamrahian, A.H. Giant invasive pituitary prolactinoma with falsely low serum prolactin: The significance of ‘hook effect’. J. Neuro-Oncol. 2006, 79, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Leaver, L.; Wass, J. Pituitary adenomas. BMJ 2019, 365, l2091. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Lojek, E.; Marchel, A. Cognitive functioning of patients with a PRL-secreting pituitary adenoma: A preliminary report. Neurology 2016, 86, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, Y.; Chen, A.; Xu, G.; Song, J. Improvement in Attention Processing After Surgical Treatment in Functional Pituitary Adenomas: Evidence from ERP Study. Front. Neurol. 2021, 12, 656255. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Y.; Liu, J.; Chen, A.; Lu, J.; Xu, G.; Song, J. Altered Connectivity of the Frontoparietal Network During Attention Processing in Prolactinomas. Front. Neurol. 2021, 12, 638851. [Google Scholar] [CrossRef]
- Brummelman, P.; Elderson, M.F.; Dullaart, R.P.; van den Bergh, A.C.; Timmer, C.A.; van den Berg, G.; Koerts, J.; Tucha, O.; Wolffenbuttel, B.H.; van Beek, A.P. Cognitive functioning in patients treated for nonfunctioning pituitary macroadenoma and the effects of pituitary radiotherapy. Clin. Endocrinol. 2011, 74, 481–487. [Google Scholar] [CrossRef]
- Song, J.; Cao, C.; Yang, M.; Yao, S.; Yan, Y.; Peng, G.; Ma, P.; Huang, C.; Ding, H.; Xu, G. The dysfunction of processing task-irrelevant emotional faces in pituitary patients: An evidence from expression-related visual mismatch negativity. Neuroreport 2018, 29, 328–333. [Google Scholar] [CrossRef]
- Cao, C.; Song, J.; Lin, P.; Yan, D.; Yao, S.; Yue, J.; Liu, B.; Lu, Y.; Xu, G. A Longitudinal, Prospective Study to Evaluate the Effects of Treatment on the Inhibitory Control Function After Transsphenoidal Surgery for Pituitary Adenomas. Clin. EEG Neurosci. 2021, 52, 444–454. [Google Scholar] [CrossRef]
- Cao, C.; Song, J.; Yao, S.; Yan, Y.; Li, S.; Peng, G.; Ma, P.; Du, H.; Huang, C.; Ding, H.; et al. The dysfunction of inhibition control in pituitary patients: Evidence from the Go/Nogo event-related potential study. Neuroreport 2017, 28, 272–278. [Google Scholar] [CrossRef]
- Cao, C.; Wen, W.; Liu, B.; Ma, P.; Li, S.; Xu, G.; Song, J. Theta oscillations in prolactinomas: Neurocognitive deficits in executive controls. Neuroimage Clin. 2020, 28, 102455. [Google Scholar] [CrossRef]
- Song, J.; Cao, C.; Wang, Y.; Yao, S.; Catalino, M.P.; Yan, D.; Xu, G.; Ma, L. Response Activation and Inhibition in Patients with Prolactinomas: An Electrophysiological Study. Front. Hum. Neurosci. 2020, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Monsell, S. Task switching. Trends Cogn. Sci. 2003, 7, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Monsell, S. Costs of a predictible switch between simple cognitive tasks. J. Exp. Psychol. Gen. 1995, 124, 207. [Google Scholar] [CrossRef]
- Rubinstein, J.S.; Meyer, D.; Evans, J. Executive control of cognitive processes in task switching. J. Exp. Psychol. Hum. Percept. Perform. 2001, 27, 763–797. [Google Scholar] [CrossRef]
- Cohen, M.X.; Donner, T.H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013, 110, 2752–2763. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Freunberger, R.; Pecherstorfer, T.; Hanslmayr, S.; Doppelmayr, M. Relevance of EEG alpha and theta oscillations during task switching. Exp. Brain Res. 2006, 170, 295–301. [Google Scholar] [CrossRef]
- Klimesch, W.; Schack, B.; Sauseng, P. The functional significance of theta and upper alpha oscillations. Exp. Psychol. 2005, 52, 99–108. [Google Scholar] [CrossRef]
- McKewen, M.; Cooper, P.S.; Skippen, P.; Wong, A.S.W.; Michie, P.T.; Karayanidis, F. Dissociable theta networks underlie the switch and mixing costs during task switching. Hum. Brain Mapp. 2021, 42, 4643–4657. [Google Scholar] [CrossRef]
- von Stein, A.; Chiang, C.; Konig, P. Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. USA 2000, 97, 14748–14753. [Google Scholar] [CrossRef] [PubMed]
- Ruge, H.; Jamadar, S.; Zimmermann, U.; Karayanidis, F. The many faces of preparatory control in task switching: Reviewing a decade of fMRI research. Hum. Brain Mapp. 2013, 34, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Slagter, H.A.; Weissman, D.H.; Giesbrecht, B.; Kenemans, J.L.; Mangun, G.R.; Kok, A.; Woldorff, M.G. Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn. Affect. Behav. Neurosci. 2006, 6, 175–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woolgar, A.; Afshar, S.; Williams, M.A.; Rich, A.N. Flexible Coding of Task Rules in Frontoparietal Cortex: An Adaptive System for Flexible Cognitive Control. J. Cogn. Neurosci. 2015, 27, 1895–1911. [Google Scholar] [CrossRef]
- Yao, S.; Song, J.; Gao, J.; Lin, P.; Yang, M.; Zahid, K.R.; Yan, Y.; Cao, C.; Ma, P.; Zhang, H.; et al. Cognitive Function and Serum Hormone Levels Are Associated with Gray Matter Volume Decline in Female Patients with Prolactinomas. Front. Neurol. 2017, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A. Endocrine Society. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Rutland, J.W.; Delman, B.N.; Huang, K.H.; Verma, G.; Benson, N.C.; Villavisanis, D.F.; Lin, H.M.; Bederson, J.B.; Chelnis, J.; Shrivastava, R.K.; et al. Primary visual cortical thickness in correlation with visual field defects in patients with pituitary macroadenomas: A structural 7-Tesla retinotopic analysis. J. Neurosurg. 2019, 133, 1371–1381. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Cohen, M.X. Analyzing neural time series data: Theory and practice. MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Poggiali, D.; Whitaker, K.; Marshall, T.R. Kievit. R.A. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2019, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Ferdinand, N.K.; Kray, J.; Falkenstein, M. Understanding sources of adult age differences in task switching: Evidence from behavioral and ERP studies. Neurosci. Biobehav. Rev. 2018, 92, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Koch, I. Sequential task predictability in task switching. Psychon. Bull. Rev. 2005, 12, 107–112. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Hadland, K.A.; Gaffan, D.; Passingham, R.E. The effect of cingulate cortex lesions on task switching and working memory. J. Cogn. Neurosci. 2003, 15, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.; Hadland, K.A.; Paus, T.; Sipila, P.K. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J. Neurophysiol. 2002, 87, 2577–2592. [Google Scholar] [CrossRef]
- Cooper, P.S.; Darriba, Á.; Karayanidis, F.; Barceló, F. Contextually sensitive power changes across multiple frequency bands underpin cognitive control. Neuroimage 2016, 132, 499–511. [Google Scholar] [CrossRef]
- McKewen, M.; Cooper, P.S.; Wong, A.S.W.; Michie, P.T.; Sauseng, P.; Karayanidis, F. Task-switching costs have distinct phase-locked and nonphase-locked EEG power effects. Psychophysiology 2020, 57, e13533. [Google Scholar] [CrossRef]
- Cooper, P.S.; Karayanidis, F.; McKewen, M.; McLellan-Hall, S.; Wong, A.S.W.; Skippen, P.; Cavanagh, J.F. Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. Neuroimage 2019, 189, 130–140. [Google Scholar] [CrossRef]
- Cooper, P.S.; Wong, A.S.W.; McKewen, M.; Michie, P.T.; Karayanidis, F. Frontoparietal theta oscillations during proactive control are associated with goal-updating and reduced behavioral variability. Biol. Psychol. 2017, 129, 253–264. [Google Scholar] [CrossRef]
- López, M.E.; Pusil, S.; Pereda, E.; Maestú, F.; Barceló, F. Dynamic low frequency EEG phase synchronization patterns during proactive control of task switching. Neuroimage 2019, 186, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Zambrano-Vazquez, L.; Allen, J. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology 2012, 49, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.; Ysrraelit, M. Role of prolactin in B cell regulation in multiple sclerosis. J. Neuroimmunol. 2014, 269, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, F. The M3 Muscarinic Acetylcholine Receptor Mediates p42mapk Activation and c-fos mRNA Expression in Oligodendrocyte Progenitors. PhD Thesis, McGill University, Montreal, Canada, 1999. [Google Scholar]
- Kars, M.; van der Klaauw, A.A.; Onstein, C.S.; Pereira, A.M.; Romijn, J.A. Quality of life is decreased in female patients treated for microprolactinoma. Eur. J. Endocrinol. 2007, 157, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Reyes, E.A.; Limón-Morales, O.; Rivero-Segura, N.A.; Camacho-Arroyo, I.; Cerbón, M. Prolactin function and putative expression in the brain. Endocrine 2017, 57, 199–213. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Torner, L.; Tinajero, E.; Lajud, N.; Quintanar-Stéphano, A.; Olvera-Cortés, E. Hyperprolactinemia impairs object recognition without altering spatial learning in male rats. Behav. Brain Res. 2013, 252, 32–39. [Google Scholar] [CrossRef]
- Hubbard, J.; Kikumoto, A.; Mayr, U. EEG Decoding Reveals the Strength and Temporal Dynamics of Goal-Relevant Representations. Sci. Rep. 2019, 9, 9051. [Google Scholar] [CrossRef]

| Patients | HCs | Statistic | |
|---|---|---|---|
| Gender | 18 Females | 13 Females | χ2 = 1.997, p = 0.158 a |
| Age | 34.3 (11.94, 18–58) | 34.6 (10.52, 21–56) | t(50) = 0.086, p = 0.931 b |
| Education | 12.7 (2.28, 9–16) | 14.0 (3.48, 6–20) | t(50) = 1.651, p = 0.105 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Wen, W.; Chen, A.; Wang, S.; Xu, G.; Niu, C.; Song, J. Neuropsychological Alterations of Prolactinomas’ Cognitive Flexibility in Task Switching. Brain Sci. 2023, 13, 82. https://doi.org/10.3390/brainsci13010082
Cao C, Wen W, Chen A, Wang S, Xu G, Niu C, Song J. Neuropsychological Alterations of Prolactinomas’ Cognitive Flexibility in Task Switching. Brain Sciences. 2023; 13(1):82. https://doi.org/10.3390/brainsci13010082
Chicago/Turabian StyleCao, Chenglong, Wen Wen, Aobo Chen, Shuochen Wang, Guozheng Xu, Chaoshi Niu, and Jian Song. 2023. "Neuropsychological Alterations of Prolactinomas’ Cognitive Flexibility in Task Switching" Brain Sciences 13, no. 1: 82. https://doi.org/10.3390/brainsci13010082
APA StyleCao, C., Wen, W., Chen, A., Wang, S., Xu, G., Niu, C., & Song, J. (2023). Neuropsychological Alterations of Prolactinomas’ Cognitive Flexibility in Task Switching. Brain Sciences, 13(1), 82. https://doi.org/10.3390/brainsci13010082






