Chloride Intracellular Channel Protein 2 Promotes Microglial Invasion: A Link to Microgliosis in the Parkinson’s Disease Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Immunoblotting
2.4. Quantitative Real-Time RT-PCR (qPCR)
2.5. Gelatin Zymography
2.6. Transmigration Assay
2.7. Metabolic Flux Analyses
2.8. Immunohistochemical Staining
2.9. High-Performance Liquid Chromatography
2.10. Statistics
3. Results
3.1. 6-OHDA-Responsive Expression of CLIC2 mRNA in Striatal Tissue
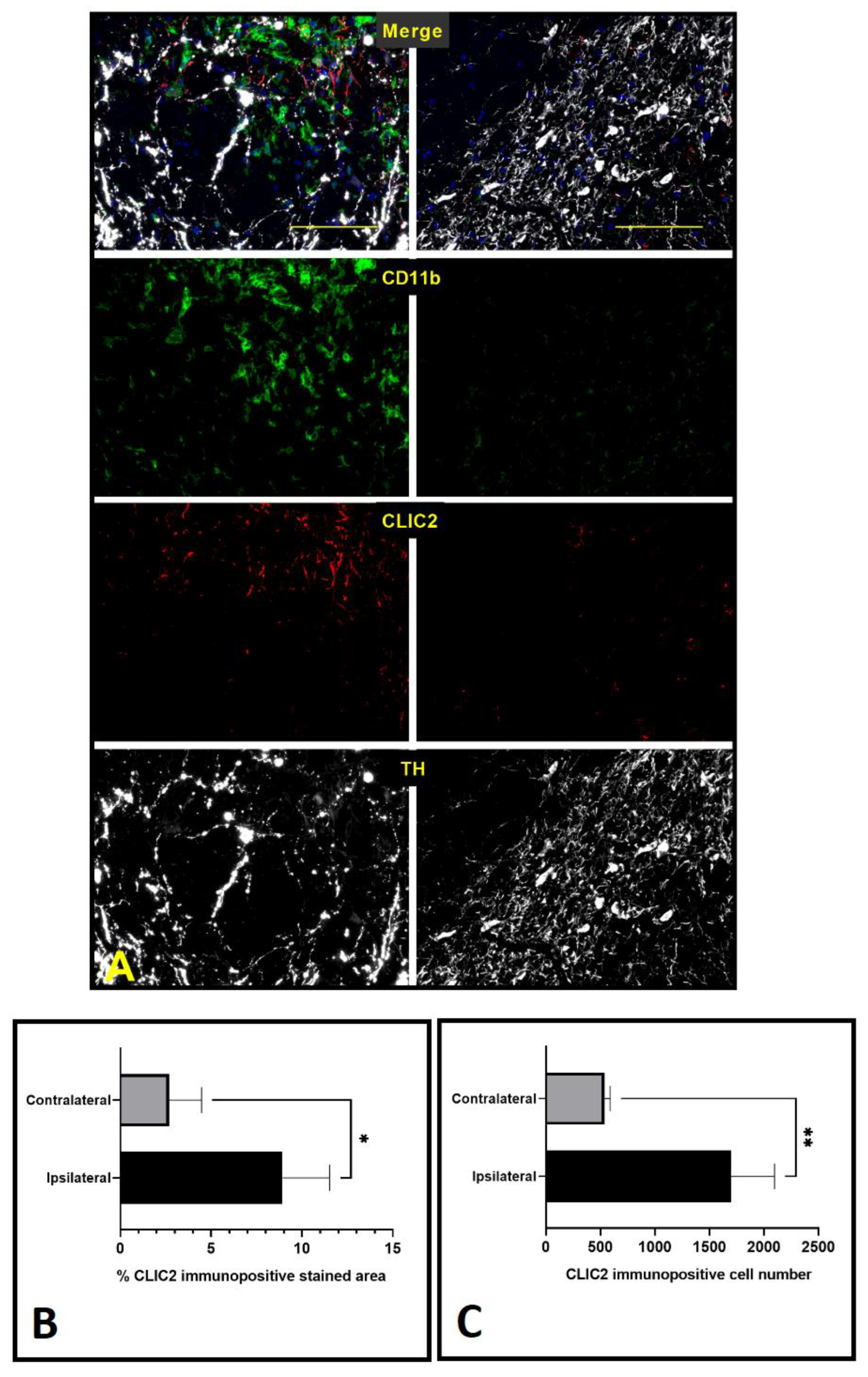
3.2. 6-OHDA Increased Number of CLIC2-Expressing Cells in Substantia Nigra Pars Compacta
3.3. CLIC2 Regulates MMP Expression in Microglial Cells
3.4. CLIC2 Contributes Microglial Release of MMP-9
3.5. CLIC2 Plays Important Role in Microglial Invasion
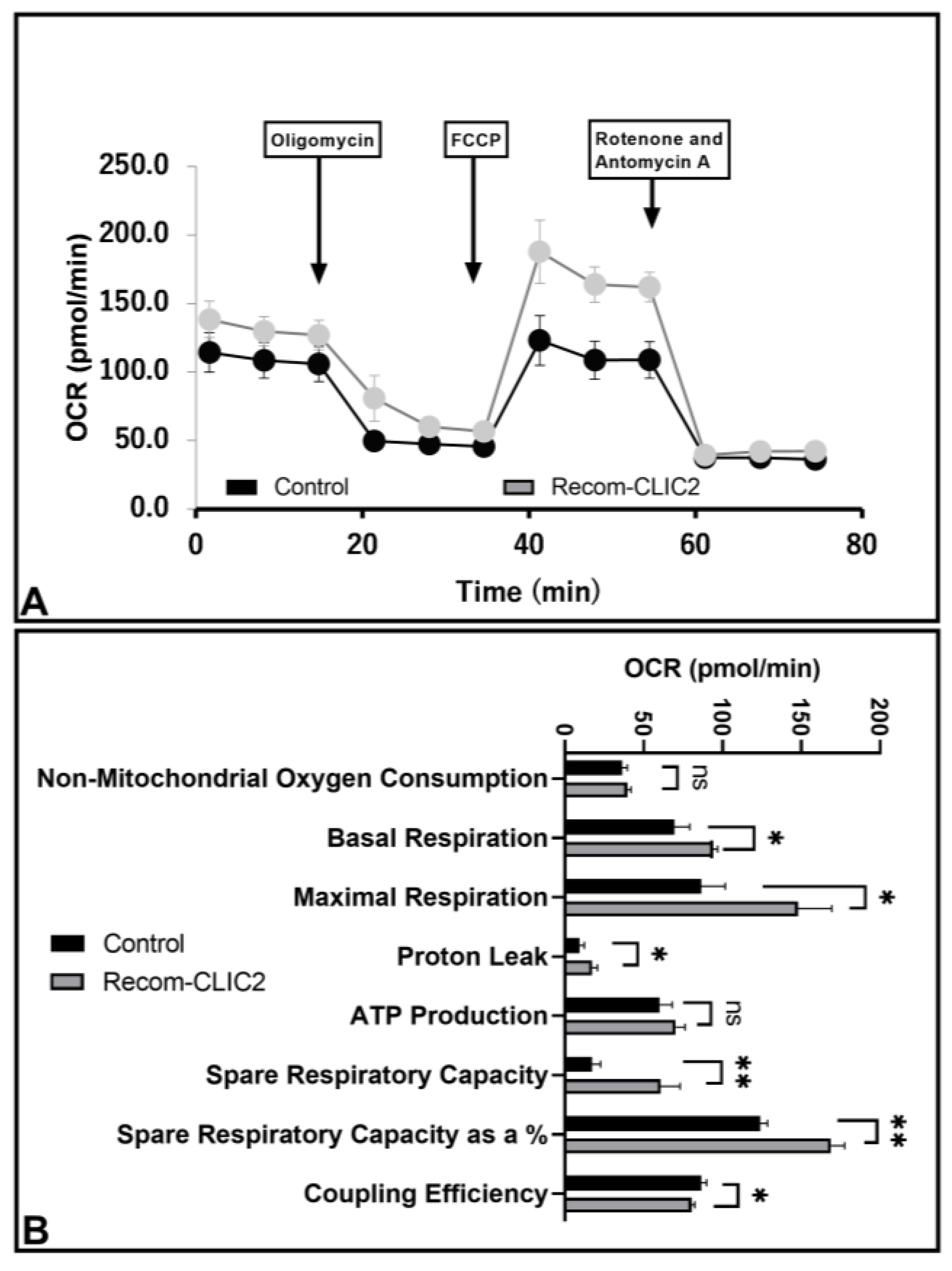
3.6. CLIC2 Plays Vital Role in Microglial Metabolic Programing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higaki, H.; Choudhury, M.E.; Kawamoto, C.; Miyamoto, K.; Islam, A.; Ishii, Y.; Miyanishi, K.; Takeda, H.; Seo, N.; Sugimoto, K.; et al. The hypnotic bromovalerylurea ameliorates 6-hydroxydopamine-induced dopaminergic neuron loss while suppressing expression of interferon regulatory factors by microglia. Neurochem. Int. 2016, 99, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Herrero, M.T.; Di Pentima, M.; Gomez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Anat. Embryol. 2015, 220, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Calingasan, N.; Yang, L.; Albers, D.S.; Shugama, S.; Gregorio, J.; Krell, H.W.; Chirichigno, J.; Joh, T.; Beal, M.F. Matrix Metalloproteinase-9 Is Elevated in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonism in Mice. NeuroMolecular Med. 2004, 5, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W. Metalloproteinases: Mediators of Pathology and Regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Ethell, I.M.; Ethell, D. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, K.; Choudhury, M.E.; Watanabe, M.; Kubo, M.; Nomoto, M.; Yano, H.; Tanaka, J. Behavioral tests predicting striatal dopamine level in a rat hemi-Parkinson’s disease model. Neurochem. Int. 2019, 122, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Argenzio, E.; Moolenaar, W.H. Emerging biological roles of Cl− intracellular channel proteins. J. Cell Sci. 2016, 129, 4165–4174. [Google Scholar] [CrossRef]
- Littler, D.R.; Harrop, S.J.; Goodchild, S.C.; Phang, J.M.; Mynott, A.V.; Jiang, L.; Valenzuela, S.M.; Mazzanti, M.; Brown, L.J.; Breit, S.N.; et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010, 584, 2093–2101. [Google Scholar] [CrossRef]
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017, 8, 202. [Google Scholar] [CrossRef]
- Milton, R.H.; Abeti, R.; Averaimo, S.; DeBiasi, S.; Vitellaro, L.; Jiang, L.; Curmi, P.; Breit, S.N.; Duchen, M.; Mazzanti, M. CLIC1 Function Is Required for -Amyloid-Induced Generation of Reactive Oxygen Species by Microglia. J. Neurosci. 2008, 28, 11488–11499. [Google Scholar] [CrossRef]
- Bogenpohl, J.W.; Weston, R.M.; Foreman, T.N.; Kitchen, K.E.; Miles, M.F. Chloride intracellular channel 4 (CLIC4) expression profile in the mouse medial prefrontal cortex and its regulation by ethanol. Alcohol. Clin. Exp. Res. 2022, 46, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Ozaki, S.; Umakoshi, A.; Yano, H.; Choudhury, M.E.; Abe, N.; Sumida, Y.; Kuwabara, J.; Uchida, R.; Islam, A.; et al. Chloride intracellular channel protein 2 in cancer and non-cancer human tissues: Relationship with tight junctions. Tissue Barriers 2019, 7, 1593775. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Umakoshi, A.; Yano, H.; Ohsumi, S.; Sumida, Y.; Hayase, E.; Usa, E.; Islam, A.; Choudhury, M.E.; Nishi, Y.; et al. Chloride intracellular channel protein 2 is secreted and inhibits MMP14 activity, while preventing tumor cell invasion and metastasis. Neoplasia 2021, 23, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Choudhury, M.E.; Kigami, Y.; Utsunomiya, R.; Matsumoto, S.; Watanabe, H.; Kumon, Y.; Kunieda, T.; Yano, H.; Tanaka, J. Sustained anti-inflammatory effects of TGF-β1 on microglia/macrophages. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 721–734. [Google Scholar] [CrossRef]
- Choudhury, M.E.; Sugimoto, K.; Kubo, M.; Nagai, M.; Nomoto, M.; Takahashi, H.; Yano, H.; Tanaka, J. A cytokine mixture of GM-CSF and IL-3 that induces a neuroprotective phenotype of microglia leading to amelioration of (6-OHDA)-induced Parkinsonism of rats. Brain Behav. 2011, 1, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Choudhury, M.E.; Yamanishi, Y.; Kyaw, W.T.; Kubo, M.; Kannou, M.; Nishikawa, N.; Tanaka, J.; Nomoto, M.; Nagai, M. Modafinil alleviates levodopa-induced excessive nighttime sleepiness and restores monoaminergic systems in a nocturnal animal model of Parkinson’s disease. J. Pharmacol. Sci. 2018, 136, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Choudhury, M.E.; Kubo, M.; Ando, R.; Tanaka, J.; Nagai, M. Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models. Brain Sci. 2022, 12, 268. [Google Scholar] [CrossRef]
- Sawasaki, T.; Ogasawara, T.; Morishita, R.; Endo, Y. A cell-free protein synthesis system for high-throughput proteomics. Proc. Natl. Acad. Sci. USA 2002, 99, 14652–14657. [Google Scholar] [CrossRef]
- Crapser, J.D.; Arreola, M.A.; Tsourmas, K.I.; Green, K.N. Microglia as hackers of the matrix: Sculpting synapses and the extracellular space. Cell. Mol. Immunol. 2021, 18, 2472–2488. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflamm. 2013, 10, 75. [Google Scholar] [CrossRef]
- Bernier, L.-P.; York, E.M.; Kamyabi, A.; Choi, H.B.; Weilinger, N.L.; MacVicar, B.A. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 2020, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Pan, R.; Shang, C.; Li, X.; Cheng, J.; Xu, J.; Li, Y. Translocator Protein 18 kDa (TSPO) Deficiency Inhibits Microglial Activation and Impairs Mitochondrial Function. Front. Pharmacol. 2020, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Novarino, G.; Fabrizi, C.; Tonini, R.; Denti, M.A.; Malchiodi-Albedi, F.; Lauro, G.M.; Sacchetti, B.; Paradisi, S.; Ferroni, A.; Curmi, P.M.; et al. Involvement of the Intracellular Ion Channel CLIC1 in Microglia-Mediated β-Amyloid-Induced Neurotoxicity. J. Neurosci. 2004, 24, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, R.K.; Silva, C.; Hader, W.; Bar-Or, A.; Patel, K.D.; Edwards, D.R.; Yong, V.W. Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia 2007, 55, 516–526. [Google Scholar] [CrossRef]
- Lorenzl, S.; Albers, D.S.; Narr, S.; Chirichigno, J.; Beal, M. Expression of MMP-2, MMP-9, and MMP-1 and Their Endogenous Counterregulators TIMP-1 and TIMP-2 in Postmortem Brain Tissue of Parkinson’s Disease. Exp. Neurol. 2002, 178, 13–20. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.-I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e6. [Google Scholar] [CrossRef]
- Song, S.; Yu, L.; Hasan, N.; Paruchuri, S.S.; Mullett, S.J.; Sullivan, M.L.G.; Fiesler, V.M.; Young, C.B.; Stolz, D.B.; Wendell, S.G.; et al. Elevated microglial oxidative phosphorylation and phagocytosis stimulate post-stroke brain remodeling and cognitive function recovery in mice. Commun. Biol. 2022, 5, 35. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. Methods Enzymol. 2014, 547, 309–354. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Turovsky, E.A.; Babenko, V.A.; Plotnikov, E.Y. The Mechanisms Underlying the Protective Action of Selenium Nanoparticles against Ischemia/Reoxygenation Are Mediated by the Activation of the Ca2+ Signaling System of Astrocytes and Reactive Astrogliosis. Int. J. Mol. Sci. 2021, 22, 12825. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, M.E.; Ozaki, S.; Miyaue, N.; Matsuura, T.; Mikami, K.; Islam, A.; Kubo, M.; Ando, R.; Yano, H.; Kunieda, T.; et al. Chloride Intracellular Channel Protein 2 Promotes Microglial Invasion: A Link to Microgliosis in the Parkinson’s Disease Brain. Brain Sci. 2023, 13, 55. https://doi.org/10.3390/brainsci13010055
Choudhury ME, Ozaki S, Miyaue N, Matsuura T, Mikami K, Islam A, Kubo M, Ando R, Yano H, Kunieda T, et al. Chloride Intracellular Channel Protein 2 Promotes Microglial Invasion: A Link to Microgliosis in the Parkinson’s Disease Brain. Brain Sciences. 2023; 13(1):55. https://doi.org/10.3390/brainsci13010055
Chicago/Turabian StyleChoudhury, Mohammed E., Saya Ozaki, Noriyuki Miyaue, Taisei Matsuura, Kanta Mikami, Afsana Islam, Madoka Kubo, Rina Ando, Hajime Yano, Takeharu Kunieda, and et al. 2023. "Chloride Intracellular Channel Protein 2 Promotes Microglial Invasion: A Link to Microgliosis in the Parkinson’s Disease Brain" Brain Sciences 13, no. 1: 55. https://doi.org/10.3390/brainsci13010055
APA StyleChoudhury, M. E., Ozaki, S., Miyaue, N., Matsuura, T., Mikami, K., Islam, A., Kubo, M., Ando, R., Yano, H., Kunieda, T., Nagai, M., & Tanaka, J. (2023). Chloride Intracellular Channel Protein 2 Promotes Microglial Invasion: A Link to Microgliosis in the Parkinson’s Disease Brain. Brain Sciences, 13(1), 55. https://doi.org/10.3390/brainsci13010055





