Attentional Bias for Sleep-Related Words as a Function of Severity of Insomnia Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Insomnia Severity Index (ISI)
2.3. Visual Dot-Probe Task
2.4. Procedure
2.5. Data Analysis
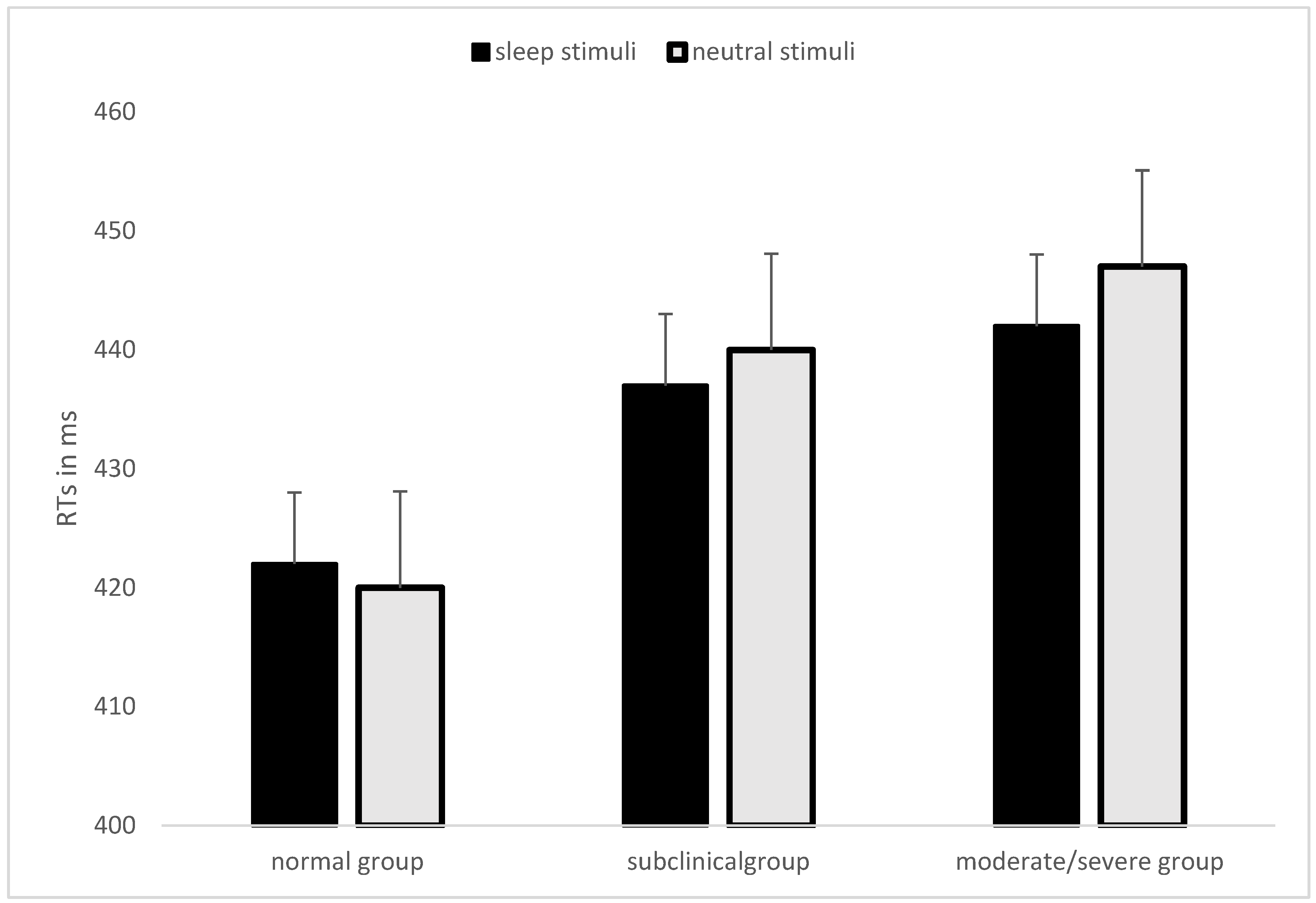
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Couple | Sleep-Related Words | Number of Syllables | Frequency of Occurrence | Word Length | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length |
| 1 | STRESS (distress) | 1 | 99% | 6 | SPRAY (spray) | 1 | 99% | 5 |
| 2 | SOGNO (dream) | 2 | 96% | 5 | PERA (pear) | 2 | 96% | 4 |
| 3 | NOTTE (night) | 2 | 98% | 5 | BAGNO (bathroom) | 2 | 98% | 5 |
| 4 | LETTO (bed) | 2 | 98% | 5 | ACQUA (water) | 2 | 98% | 5 |
| 5 | QUIETE (quiet) | 2 | 85% | 6 | GREZZO (raw) | 2 | 85% | 6 |
| 6 | FIACCO (tired) | 2 | 83% | 6 | SCIOLTO (melt) | 2 | 81% | 7 |
| 7 | PIGRO (lazy) | 2 | 74% | 5 | COMO’ (dresser) | 2 | 75% | 4 |
| 8 | ANSIA (anxiety) | 2 | 93% | 5 | TAZZA (cup) | 2 | 90% | 5 |
| 9 | STANCO (retired) | 2 | 90% | 6 | SCHERZO (trick) | 2 | 92% | 7 |
| 10 | SVEGLIA (alarm) | 2 | 90% | 7 | THERMOS (thermos) | 2 | 94% | 7 |
| 11 | CAFFE’ (coffee) | 2 | 96% | 5 | CURVA (curve) | 2 | 96% | 5 |
| 12 | RIPOSO (rest) | 3 | 93% | 6 | DISPENSA (cupboard) | 3 | 94% | 8 |
| 13 | COMODO (comfy) | 3 | 100% | 6 | NOVITA’ (news) | 3 | 98% | 6 |
| 14 | FARMACO (medication) | 3 | 91% | 7 | ACCANTO (beside) | 3 | 94% | 7 |
| 15 | SOFFRIRE (suffer) | 3 | 87% | 8 | PINGUINO (penguin) | 3 | 91% | 8 |
| 16 | STANCHEZZA (fatigue) | 3 | 85% | 10 | SCORPIONE (scorpion) | 3 | 84% | 9 |
| 17 | RUSSARE (snoring) | 3 | 70% | 7 | FORCINA (hairpin) | 3 | 73% | 7 |
| 18 | SVEGLIO (wakeful) | 3 | 80% | 7 | BORSETTE (handbags) | 3 | 80% | 8 |
| 19 | MALATO (sick) | 3 | 90% | 6 | PASSIVO (passive) | 3 | 92% | 7 |
| 20 | STREMATO (exhausted) | 3 | 66% | 8 | STRETTOIA (bottleneck) | 3 | 64% | 9 |
| 21 | LETARGIA (lethargy) | 3 | 71% | 8 | SENAPE (mustard) | 3 | 74% | 6 |
| 22 | DISTURBO (disorder) | 3 | 90% | 8 | VALIGIA (trolley) | 3 | 90% | 7 |
| 23 | ESAUSTO (exhausted) | 3 | 74% | 7 | RICCHEZZE (riches) | 3 | 76% | 9 |
| 24 | INSONNE (sleepless) | 3 | 75% | 7 | INDIZIO (hint) | 3 | 75% | 7 |
| 25 | INCUBO (nightmare) | 3 | 90% | 6 | PREMERE (pull) | 3 | 91% | 7 |
| 26 | PISOLINI (naps) | 4 | 68% | 8 | INSIPIDO (bland) | 4 | 68% | 8 |
| 27 | RILASSATO (relaxed) | 4 | 88% | 9 | PORTAFOGLI (wallet) | 4 | 90% | 10 |
| 28 | PENNICHELLA (siesta) | 4 | 66% | 11 | ATTUIRE (cushion) | 4 | 64% | 8 |
| 29 | DEBOLEZZA (fatigue) | 4 | 85% | 9 | CUCCHIAINO (teaspoon) | 4 | 82% | 10 |
| 30 | CORICARSI (bedtime) | 4 | 85% | 9 | LAMPADINA (lightbulb) | 4 | 83% | 9 |
| 31 | INQUIETO (tense) | 4 | 90% | 8 | SERBATOIO (tank) | 4 | 92% | 9 |
| 32 | TURBATO (disturbed) | 4 | 80% | 7 | INVENTARE (invents) | 4 | 81% | 9 |
| 33 | SOFFERENZA (ache) | 4 | 90% | 10 | ASTRONAUTA (astronaut) | 4 | 90% | 10 |
| 34 | IRREQUIETO (restless) | 4 | 81% | 10 | ORGOGLIOSO (proud) | 4 | 80% | 10 |
| 35 | SONNOLENTO (drowsy) | 4 | 55% | 10 | DECOMPORSI (decompose) | 4 | 52% | 10 |
| 36 | ASSONNATO (sleepy) | 4 | 85% | 9 | DECORATO (decorate) | 4 | 83% | 8 |
| 37 | AGITATO (agitated) | 4 | 77% | 7 | URAGANO (hurricane) | 4 | 79% | 7 |
| 38 | MALASSERE (illness) | 4 | 77% | 9 | GROSSOLANO (coarse) | 4 | 76% | 10 |
| 39 | SEDATIVO (sedative) | 4 | 79% | 8 | SAGGISTICA (nonfiction) | 4 | 81% | 10 |
| 40 | ADDORMENTATO (asleep) | 5 | 83% | 12 | CONSIGLIABILE (advisable) | 5 | 86% | 13 |
| Mean (SD) | 3.13 (0.94) | 83.83% (10.39%) | 7.45 (1.81) | Mean (SD) | 3.10 (0.90) | 84.18% (10.77%) | 7.65 (1.98) | |
| Couple | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length |
| 1 | FILM (movie) | 1 | 100% | 4 | TRAM (tram) | 1 | 99% | 4 |
| 2 | ASPRO (sour) | 2 | 81% | 5 | GRUMO (lump) | 2 | 81% | 5 |
| 3 | BACIO (kiss) | 2 | 94% | 5 | FALCO (hawk) | 2 | 95% | 5 |
| 4 | FELCE (fern) | 2 | 71% | 5 | GREGGE (flock) | 2 | 72% | 6 |
| 5 | GRUPPO (group) | 2 | 99% | 6 | GIORNI (days) | 2 | 99% | 6 |
| 6 | MITE (meek) | 2 | 94% | 4 | FOGLIO (sheet) | 2 | 93% | 6 |
| 7 | LIBRO (book) | 2 | 99% | 5 | PUNTO (point) | 2 | 100% | 5 |
| 8 | SFERA (sphere) | 2 | 94% | 5 | LISCIO (smooth) | 2 | 90% | 6 |
| 9 | PALLA (ball) | 2 | 93% | 5 | SPECCHIO (mirror) | 2 | 94% | 8 |
| 10 | SUORA (nun) | 2 | 88% | 5 | STUFA (stove) | 2 | 87% | 5 |
| 11 | TRATTO (trait) | 2 | 95% | 6 | VASCHE (laps) | 2 | 95% | 6 |
| 12 | STUDIARE (study) | 3 | 93% | 8 | ABBRACCIO (hug) | 3 | 93% | 9 |
| 13 | ABETE (fir) | 3 | 86% | 5 | BREVETTO (patent) | 3 | 87% | 8 |
| 14 | STAGNANTE (stagnant) | 3 | 83% | 9 | FORCHETTA (fork) | 3 | 81% | 9 |
| 15 | POSSENTE (mighty) | 3 | 69% | 8 | GIOCOSO (playful) | 3 | 71% | 7 |
| 16 | INNESTO (graft) | 3 | 81% | 7 | VACCINO (vaccine) | 3 | 79% | 7 |
| 17 | FATTURA (invoice) | 3 | 92% | 7 | MARGINE (margin) | 3 | 92% | 7 |
| 18 | NAZIONE (nation) | 3 | 93% | 7 | SEGNALE (signal) | 3 | 93% | 7 |
| 19 | RANCIDO (rancid) | 3 | 62% | 7 | SFREGARE (scrubbing) | 3 | 69% | 8 |
| 20 | OSTILE (hostile) | 3 | 73% | 6 | RASOIO (razor) | 3 | 74% | 6 |
| 21 | SFUGGIRE (wriggles) | 3 | 90% | 8 | SCULTURA (sculpture) | 3 | 90% | 8 |
| 22 | FRAGANZA (fragrance) | 3 | 76% | 9 | SPORCIZIA (filth) | 3 | 73% | 9 |
| 23 | CARAFFA (jug) | 3 | 76% | 7 | TRAPIANTO (graft) | 3 | 79% | 9 |
| 24 | SABATO (Saturday) | 3 | 98% | 6 | UOMINI (males) | 3 | 99% | 6 |
| 25 | VERTICI (tops) | 3 | 91% | 7 | ATTRICE (actress) | 3 | 91% | 7 |
| 26 | CANCELLINO (eraser) | 4 | 65% | 10 | ABBOZZATO (drafted) | 4 | 65% | 9 |
| 27 | AMBIZIONE (ambition) | 4 | 90% | 9 | COCCOLARE (cuddle) | 4 | 88% | 9 |
| 28 | ARROGANTE (arrogant) | 4 | 91% | 9 | CHIRURGIA (surgery) | 4 | 94% | 9 |
| 29 | ATTREZZO (tool) | 4 | 80% | 8 | CARABINA (rifle) | 4 | 83% | 8 |
| 30 | BALLERINO (dancer) | 4 | 81% | 9 | CICATRICE (scar) | 4 | 84% | 9 |
| 31 | CAMMINARE (walk) | 4 | 90% | 9 | DISEGNARE (draw) | 4 | 88% | 9 |
| 32 | ATTRAENTE (attractive) | 4 | 82% | 9 | CARAMELLA (candy) | 4 | 81% | 9 |
| 33 | CHEROSENE (kerosene) | 4 | 62% | 9 | DISERTORE (deserter) | 4 | 63% | 9 |
| 34 | INSOLENTE (insolent) | 4 | 82% | 9 | CORRIDORE (runner) | 4 | 84% | 9 |
| 35 | ESULTANTE (elated) | 4 | 57% | 9 | RIVERENTE (reverent) | 4 | 55% | 9 |
| 36 | DIGNITOSO (dignified) | 4 | 82% | 9 | INDUMENTO (garment) | 4 | 85% | 9 |
| 37 | CANDELABRO (candelabrum) | 4 | 79% | 10 | INQUINARE (pollute) | 4 | 79% | 9 |
| 38 | BOLLITORE (kettle) | 4 | 73% | 9 | LAMENTELA (gripe) | 4 | 74% | 9 |
| 39 | RADIATORE (radiator) | 4 | 85% | 9 | APPRENDERE (learn) | 4 | 86% | 10 |
| 40 | DISIDRATATO (blasé) | 5 | 83% | 11 | CENTROTAVOLA (centerpiece) | 5 | 85% | 12 |
| Mean (SD) | 3.10 (0.90) | 84.03% (10.70%) | 7.38 (1.86) | Mean (SD) | 3.10 (0.90) | 84.10% (11.05%) | 7.65 (1.76) | |
Appendix B
| Couple | Sleep-Related Words | Number of Syllables | Frequency of Occurrence | Word Length | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length |
| 1 | BUIO (dark) | 2 | 93% | 4 | PENNA (pencil) | 2 | 94% | 5 |
| 2 | TRISTE (sad) | 2 | 98% | 6 | SPAZI (clearances) | 2 | 98% | 5 |
| 3 | CONFLITTO (conflict) | 3 | 91% | 9 | ARBITRO (referee) | 3 | 93% | 7 |
| 4 | CUSCINO (pillow) | 3 | 89% | 7 | PELLICCIA (fur) | 3 | 88% | 9 |
| 5 | DISPERATO (desperate) | 4 | 84% | 9 | CICLAMINO (cyclamen) | 4 | 81% | 9 |
| 6 | DISASTROSO (disastrous) | 4 | 88% | 10 | SVILUPPATO (developed) | 4 | 93% | 10 |
| 7 | MATERASSO (mattress) | 4 | 87% | 9 | CREATURA (creature) | 4 | 89% | 8 |
| 8 | INADEGUATO (inadequate) | 5 | 88% | 10 | INNAMORATO (valentine) | 5 | 90% | 10 |
| Mean (SD) | 3.38 (1.06) | 89.75% (4.27%) | 8.00 (2.14) | Mean (SD) | 3.38 (1.06) | 90.75% (5.06%) | 7.88 (2.03) | |
| Couple | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length | Neutral Words | Number of Syllables | Frequency of Occurrence | Word Length |
| 1 | CORDA (chord) | 2 | 95% | 5 | PASTI (meals) | 2 | 95% | 5 |
| 2 | STARE (stand) | 2 | 99% | 5 | CREMA (cream) | 2 | 98% | 5 |
| 3 | BOTTIGLIA (bottle) | 3 | 92% | 9 | ACCIAIO (steel) | 3 | 96% | 7 |
| 4 | RINFRESCO (refreshment) | 3 | 87% | 9 | PANINO (sandwich) | 3 | 85% | 6 |
| 5 | SPECULARE (speculates) | 4 | 81% | 9 | OBBEDIRE (obey) | 4 | 84% | 8 |
| 6 | VOLANTINO (brochure) | 4 | 91% | 9 | ADOTTATO (borrowed) | 4 | 91% | 8 |
| 7 | ORCHIDEA (orchid) | 4 | 89% | 8 | PATRIOTA (patriot) | 4 | 90% | 8 |
| 8 | ABITUDINE (habit) | 5 | 89% | 9 | COLLABORAZIONE (featuring) | 6 | 97% | 14 |
| Mean (SD) | 3.38 (1.06) | 90.38% (5.37%) | 7.88 (1.81) | Mean (SD) | 3.50 (1.31) | 92.00% (5.40%) | 7.63 (2.88) | |
References
- Harvey, A.; Watkins, E.; Mansell, W.; Shafran, R. Cognitive Behavioural Processes Across Psychological Disorders; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Espie, C.A. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu. Rev. Psychol. 2002, 53, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Broomfield, N.M.; Macmahon, K.M.; Macphee, L.M.; Taylor, L.M. The attention-intention-effort pathway in the development of psychophysiological insomnia: A theoretical review. Sleep Med. Rev. 2006, 10, 215–245. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G. A cognitive model of insomnia. Behav. Res. Ther. 2002, 40, 869–893. [Google Scholar] [CrossRef] [PubMed]
- Semler, C.; Harvey, A.G. Monitoring for sleep-related threat: A pilot study of the sleep associated monitoring index (SAMI). Psychosom. Med. 2004, 66, 242–250. [Google Scholar] [CrossRef]
- Lundh, L.G.; Froding, A.; Gyllenhammer, L.; Broman, J.K.; Hetta, J. Cognitive bias and memory performance in patients with persistent insomnia. Scan. J. Behav. Ther. 1997, 26, 27–35. [Google Scholar] [CrossRef]
- Taylor, L.M.; Espie, C.A.; White, C.A. Attentional bias in people with acute versus persistent insomnia secondary to cancer. Behav. Sleep Med. 2003, 1, 200–212. [Google Scholar] [CrossRef]
- Sagaspe, P.; Sanchez-Ortuno, M.; Charles, A.; Taillard, J.; Valtat, C.; Bioulac, B.; Philip, P. Effects of sleep deprivation on color-word, emotional, and specific Stroop interference and on self-reported anxiety. Brain Cog. 2006, 60, 76–87. [Google Scholar] [CrossRef]
- Barclay, N.L.; Ellis, J.G. Sleep-related attentional bias in poor versus good sleepers is independent of affective valence. J. Sleep Res. 2013, 22, 414–421. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Espie, C.A.; Nissen, C.; Riemann, D. Sleep-related attentional bias in patients with primary insomnia compared with sleep experts and healthy controls. J. Sleep Res. 2008, 17, 191–196. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Espie, C.A.; Riemann, D. Is sleep-related attentional bias due to sleepiness or sleeplessness? Cogn. Emot. 2009, 23, 541–550. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Kyle, S.D.; Feige, B.; Prem, M.; Nissen, C.; Espie, C.A.; Riemann, D. The impact of sleep-related attentional bias on polysomnographically measured sleep in primary insomnia. Sleep 2010, 33, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, K.; Baglioni, C.; Regen, W.; Kyle, S.D.; Nissen, C.; Henning, J.; Doerr, J.-P.; Feige, B.; Riemann, D. Brain reactivity and selective attention to sleep-related words in patients with chronic insomnia. Behav. Sleep Med. 2018, 16, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhao, C.; Yang, T.; Du, S.; Yu, M.; Shen, H. Attentional bias towards sleep-related stimuli in insomnia disorder: A behavioural and ERP study. J. Sleep Res. 2018, 27, 1–9. [Google Scholar] [CrossRef]
- MacMahon, K.M.A.; Broomfield, N.M.; Espie, C.A. Attention bias for sleep-related stimuli in primary insomnia and delayed sleep phase syndrome using the dot-probe task. Sleep 2006, 29, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Jansson-Fröjmark, M.; Bermås, M.; Kjellén, A. Attentional bias in insomnia: The dot-probe task with pictorial stimuli depicting daytime fatigue/malaise. Cogn. Ther. Res. 2013, 37, 534–546. [Google Scholar] [CrossRef]
- Richardson, C.; Gradisar, M.; Pulford, A. The development of insomnia or the plasticity of good sleep? A preliminary study of acute changes in sleep and insomnia resulting from an analogue trauma. Behav. Sleep Med. 2014, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Akram, U.; Beattie, L.; Ypsilanti, A.; Reidy, J.; Robson, A.; Chapman, A.J.; Barclay, N.L. Sleep-related attentional bias for tired faces in insomnia: Evidence from a dot-probe paradigm. Behav. Res. Ther. 2018, 103, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Poel, L.V.; Raes, F. Pre-sleep arousal can be associated with efficient processing of sleep-related information. J. Behav. Ther. Exp. Psychiatry 2018, 60, 13–21. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, J.; Lin, R.; Yan, Y.; Zhang, R.; Huang, H.; Wang, J.; Huang, R. The impact of negative mood state on sleep-related attentional bias in insomnia. J. Sleep Res. 2019, 28, e12748. [Google Scholar] [CrossRef]
- Woods, H.; Marchetti, L.M.; Biello, S.M.; Espie, C.A. The clock as a focus of selective attention in those with primary insomnia: An experimental study using a modified Posner paradigm. Behav. Res. Ther. 2009, 47, 231–236. [Google Scholar] [CrossRef]
- Jones, B.T.; Macphee, L.M.; Broomfield, N.M.; Jones, B.C.; Espie, C.A. Sleep-related attentional bias in good, moderate and poor (primary insomnia) sleepers. J. Abnorm. Psychol. 2005, 114, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.M.; Biello, S.M.; Broomfield, N.M.; MacMahon, K.M.A.; Espie, C.A. Who is pre-occupied with sleep? A comparison of attention bias in people with psychophysiological insomnia, delayed sleep phase syndrome and good sleepers using the induced change blindness paradigm. J. Sleep Res. 2006, 15, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Koranyi, N.; Meinhard, M.; Bublak, P.; Witte, O.W.; Rupprecht, S. Automatic affective responses towards the bed in patients with primary insomnia: Evidence for a negativity bias. J. Sleep Res. 2018, 27, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Spiegelhalder, K.; Espie, C.A.; MacMahon, K.M.A.; Woods, H.C.; Kyle, S.D. Sleep-related attentional bias in insomnia: A state-of-the-science review. Clin. Psychol. Rev. 2015, 42, 16–27. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Beiger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Akram, U.; Barclay, N.; Milkins, B.; Stevenson, J.; Gardani, M. Sleep-related attentional and interpretive-bias in insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 67, 101713. [Google Scholar] [CrossRef]
- Woods, H.C.; Scheepers, C.; Ross, K.A.; Espie, C.A.; Biello, S.M. What are you looking at? Moving toward an attentional timeline in insomnia: A novel semantic eye tracking study. Sleep 2013, 36, 1491–1499. [Google Scholar] [CrossRef]
- Beattie, L.; Bindermann, M.; Kyle, S.D.; Biello, S.M. Attention to beds in natural scenes by observers with insomnia symptoms. Behav. Res. Ther. 2017, 92, 51–56. [Google Scholar] [CrossRef]
- Akram, U.; Robson, A.; Ypsilanti, A. Sleep-related attentional bias for faces depicting tiredness in insomnia: Evidence from an eye-tracking study. J. Clin. Sleep Med. 2018, 14, 959–965. [Google Scholar] [CrossRef]
- Milkins, B.; Notebaert, L.; MacLeod, C.; Clarke, P.J.F. The potential benefits of targeted attentional bias modification on cognitive arousal and sleep quality in worry-related sleep disturbance. Clin. Psychol. Sci. 2016, 4, 1015–1027. [Google Scholar] [CrossRef]
- Clarke, P.J.F.; Bedford, K.; Notebaert, L.; Bucks, R.S.; Rudaizky, D.; Milkins, B.C.; MacLeod, C. Assessing the therapeutic potential of targeted attentional bias modification for insomnia using smartphone delivery. Psychother. Psychosom. 2016, 85, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Lancee, J.; Yasiney, S.L.; Brendel, R.S.; Boffo, M.; Clarke, P.J.F.; Salemink, E. Attentional bias modification training for insomnia: A double-blind placebo controlled randomized trial. PLoS ONE 2017, 12, e0174531. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Spiegelhalder, K.; Regen, W.; Feige, B.; Nissen, C.; Lombardo, C.; Violani, C.; Hennig, J.; Reimann, D. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 2014, 37, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Bar-Halm, Y.; Lamy, D.; Pergamin, L.; Bakerman-Kranenburg, M.J.; van Jizendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Johnstone, S.J.; Gonsalvez, C.J. Event-related potentials during an emotional Stroop task. Int. J. Psychophysiol. 2007, 63, 221–231. [Google Scholar] [CrossRef]
- Gupta, R.S.; Kujawa, A.; Vago, D.R. The neural chronometry of threat-related attentional bias: Event-related potential (ERP) evidence for early and late stages of selective attentional processing. Int. J. Psychophysiol. 2019, 146, 20–42. [Google Scholar] [CrossRef]
- Mogg, K.; Bradley, B.P. Anxiety and attention to threat: Cognitive mechanisms and treatment with attention bias modification. Behav. Res. Ther. 2016, 87, 76–108. [Google Scholar] [CrossRef]
- Akram, U.; Milkins, B.; Ypsilanti, A.; Reidy, J.; Lazuras, L.; Stevenson, J.; Notabaert, L.; Barclay, N.L. The therapeutic potential of attentional bias modification training for insomnia: Study protocol for a randomized controlled trial. Trials 2018, 19, 567. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 192–213. [Google Scholar] [CrossRef]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring subjective sleep quality: A review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ree, M.J.; Pollitt, A.; Harvey, A.G. An investigation of interpretive bias in insomnia: An analog study comparing normal and poor sleepers. Sleep 2006, 29, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Thomson, A.; Gregory, A.M.; Sterr, A. Biased processing of sleep-related stimuli in children of parents with insomnia. Behav. Sleep Med. 2013, 11, 108–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoet, G. PsyToolkit–A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- Granello, D.H.; Wheaton, J.E. Online data collection: Strategies for research. J. Couns. Dev. 2004, 82, 387–393. [Google Scholar] [CrossRef]
- Castronovo, V.; Galbiati, A.; Marelli, S.; Brombin, C.; Cugnata, F.; Giarolli, L.; Anelli, M.M.; Rinaldi, F.; Ferini-Strambi, L. Validatiom study of the Italian version of the insomnia Severity Index (ISI). Neurol. Sci. 2016, 37, 1517–1524. [Google Scholar] [CrossRef]
- Fortier-Brochu, E.; Morin, C.M. Cognitive impairment in individuals with insomnia: Clinical significance and correlates. Sleep 2014, 37, 1787–1798. [Google Scholar] [CrossRef]
- Wardle-Pinkston, S.; Slavish, D.C.; Taylor, D.J. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 48, 101205. [Google Scholar] [CrossRef]
- De Ruiter, C.; Brosschol, J.F. The emotional Stroop interference effect in anxiety: Attentional bias or cognitive avoidance? Behav. Res. Ther. 1994, 32, 315–319. [Google Scholar] [CrossRef]
- Koster, E.; Crombez, G.; Verschuere, B.; Houwer, J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behav. Res. Ther. 2004, 42, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, Y.J.; Kim, N.; Kim, S.; Choi, J.-W.; Park, J.; Gwak, A.R.; Kang, C.-K.; Kang, S.-G.; Jeong, D.-U. Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci. Rep. 2017, 7, 12528. [Google Scholar] [CrossRef] [PubMed]
- Bar-Haim, Y. Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. J. Child Psychol. Psychiatry 2010, 51, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Sholtes, D. A minsdfulness-based approach to the treatment of insomnia. J. Clin. Psychol. 2010, 66, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.N.; Zhou, E.S.; Gonzalez, B.D.; Rodriguez, N. The quest for mindful sleep: A critical synthesis of the impact of mindfulness-based interventions for insomnia. Curr. Sleep Med. Rep. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Simione, L.; Raffone, A.; Mirolli, M. Acceptance and not its interaction with attention monitoring, increases psychological well-being: Testing the Monitoring and Acceptance Theory of mindfulness. Mindfulness 2021, 12, 1398–1411. [Google Scholar] [CrossRef]
- Mirolli, M.; Simione, L.; Martoni, M.; Fabbri, M. Accept anxiety to improve sleep: The impact of the COVID-19 lockdown on the relationships between mindfulness, distress and sleep quality. Int. J. Environ. Res. Public Health 2021, 18, 13149. [Google Scholar] [CrossRef]
- Fabbri, M.; Simione, L.; Martoni, M.; Mirolli, M. The relationship between acceptance and sleep-wake quality before, during, and after the first Italian COVID-19 lockdown. Clock Sleep 2022, 4, 172–184. [Google Scholar] [CrossRef]
- Merritt, P.; Hirshman, E.; Wharton, W.; Stangl, B.; Devlin, J.; Lenz, A. Evidence for gender differences in visual selective attention. Pers. Individ. Differ. 2007, 43, 597–609. [Google Scholar] [CrossRef]
| Sleep Group | Age ± SD Years | Male | Female | Middle-School Diploma | High-School Diploma | Bachelor’s Degree | Master’s Degree | University Master | PhD |
|---|---|---|---|---|---|---|---|---|---|
| Normal groups | 26.59 ± 7.05 | 53.80% | 52.10% | 0.90% | 27.20% | 49.10% | 16.70% | 4.40% | 1.80% |
| Subthreshold group | 25.72 ± 6.35 | 25.00% | 35.20% | 2.80% | 31.00% | 42.30% | 18.30% | 4.20% | 1.40% |
| Moderate/Severe group | 26.59 ± 7.22 | 21.20% | 12.70% | 0.00% | 31.20% | 43.80% | 21.90% | 0.00% | 3.10% |
| Total Sample | 26.30 ± 6.84 | 24.00% | 76.00% | 1.40% | 29.00% | 46.10% | 18.00% | 3.70% | 1.80% |
| Neutral Word–Probe Below | Neutral Word–Probe Above | Sleep Word–Probe Below | Sleep Word -Probe Above | Task RTs | Mean Neutral-Neutral Words Couple RTs | Task Accuracy | |
|---|---|---|---|---|---|---|---|
| Normal group (N = 114) | 425 (59.04) | 420 (58.92) | 423 (61.07) | 418 (60.05) | 421 (55.68) | 420 (55.17) | 96.54% (1.60%) |
| Subclinical group (N = 71) | 441 (75.61) | 433 (65.76) | 440 (75.79) | 440 (73.65) | 439 (68.77) | 439 (70.18) | 96.37% (1.69%) |
| Moderate/Severe group (N = 32) | 447 (69.20) | 438 (61.99) | 450 (73.62) | 444 (65.90) | 447 (65.95) | 448 (67.81) | 96.35% (2.22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, M.; Simione, L.; Catalano, L.; Mirolli, M.; Martoni, M. Attentional Bias for Sleep-Related Words as a Function of Severity of Insomnia Symptoms. Brain Sci. 2023, 13, 50. https://doi.org/10.3390/brainsci13010050
Fabbri M, Simione L, Catalano L, Mirolli M, Martoni M. Attentional Bias for Sleep-Related Words as a Function of Severity of Insomnia Symptoms. Brain Sciences. 2023; 13(1):50. https://doi.org/10.3390/brainsci13010050
Chicago/Turabian StyleFabbri, Marco, Luca Simione, Laura Catalano, Marco Mirolli, and Monica Martoni. 2023. "Attentional Bias for Sleep-Related Words as a Function of Severity of Insomnia Symptoms" Brain Sciences 13, no. 1: 50. https://doi.org/10.3390/brainsci13010050
APA StyleFabbri, M., Simione, L., Catalano, L., Mirolli, M., & Martoni, M. (2023). Attentional Bias for Sleep-Related Words as a Function of Severity of Insomnia Symptoms. Brain Sciences, 13(1), 50. https://doi.org/10.3390/brainsci13010050







