A Method for Using Video Presentation to Increase Cortical Region Activity during Motor Imagery Tasks in Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
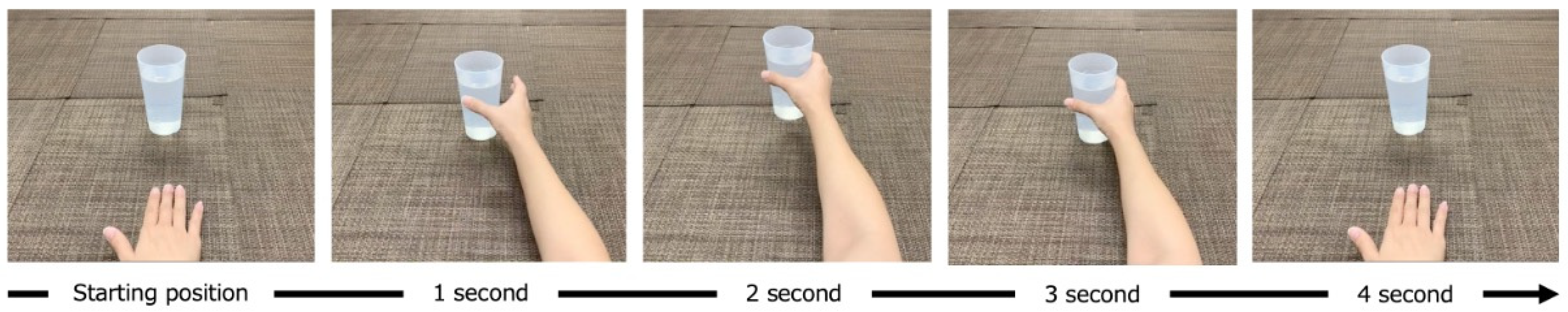
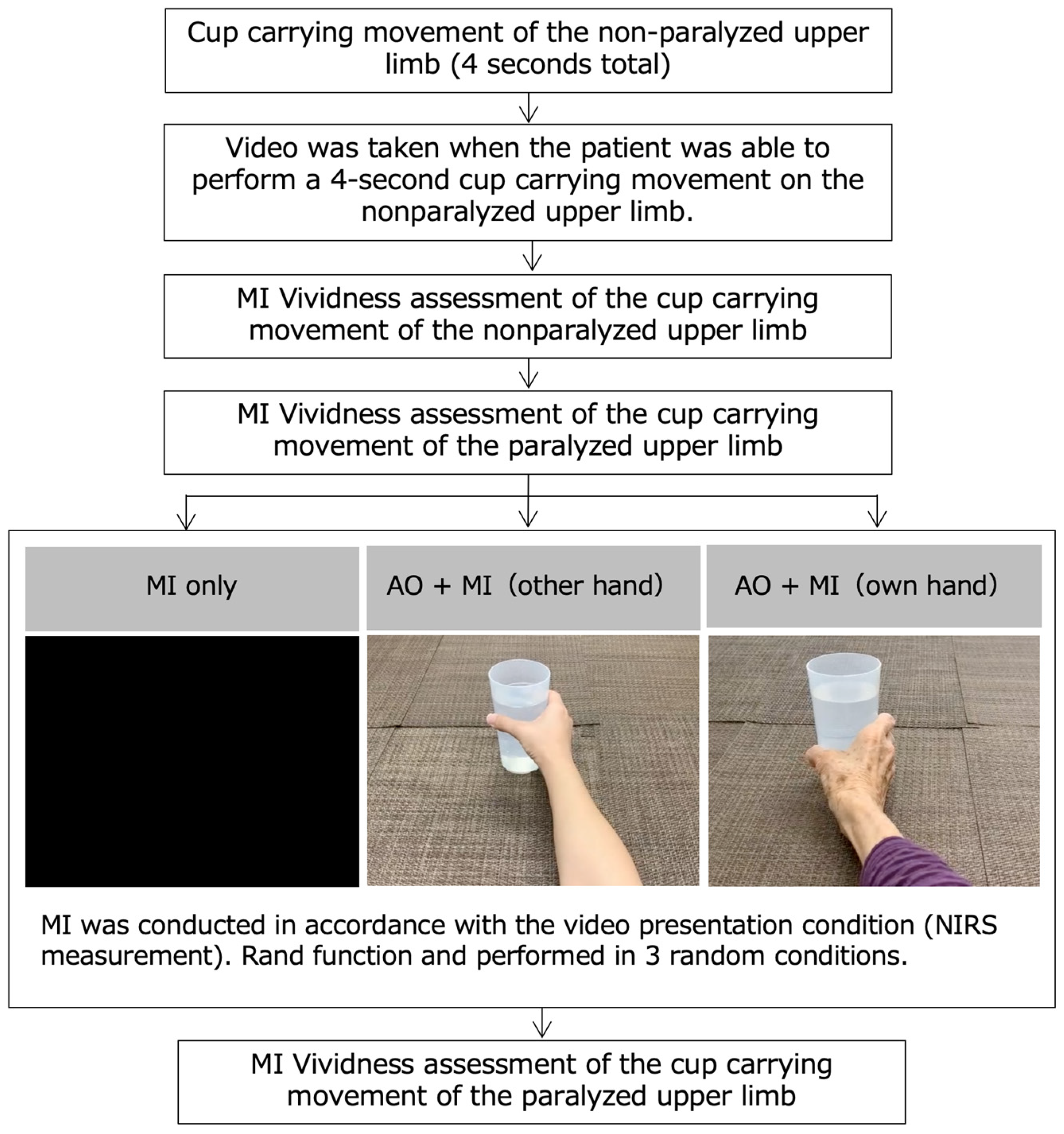
2.2. MI Task
2.3. Near-Infrared Spectroscopy
2.4. Statistical Analysis
3. Results
3.1. Activity of Cortical Regions during MI Task
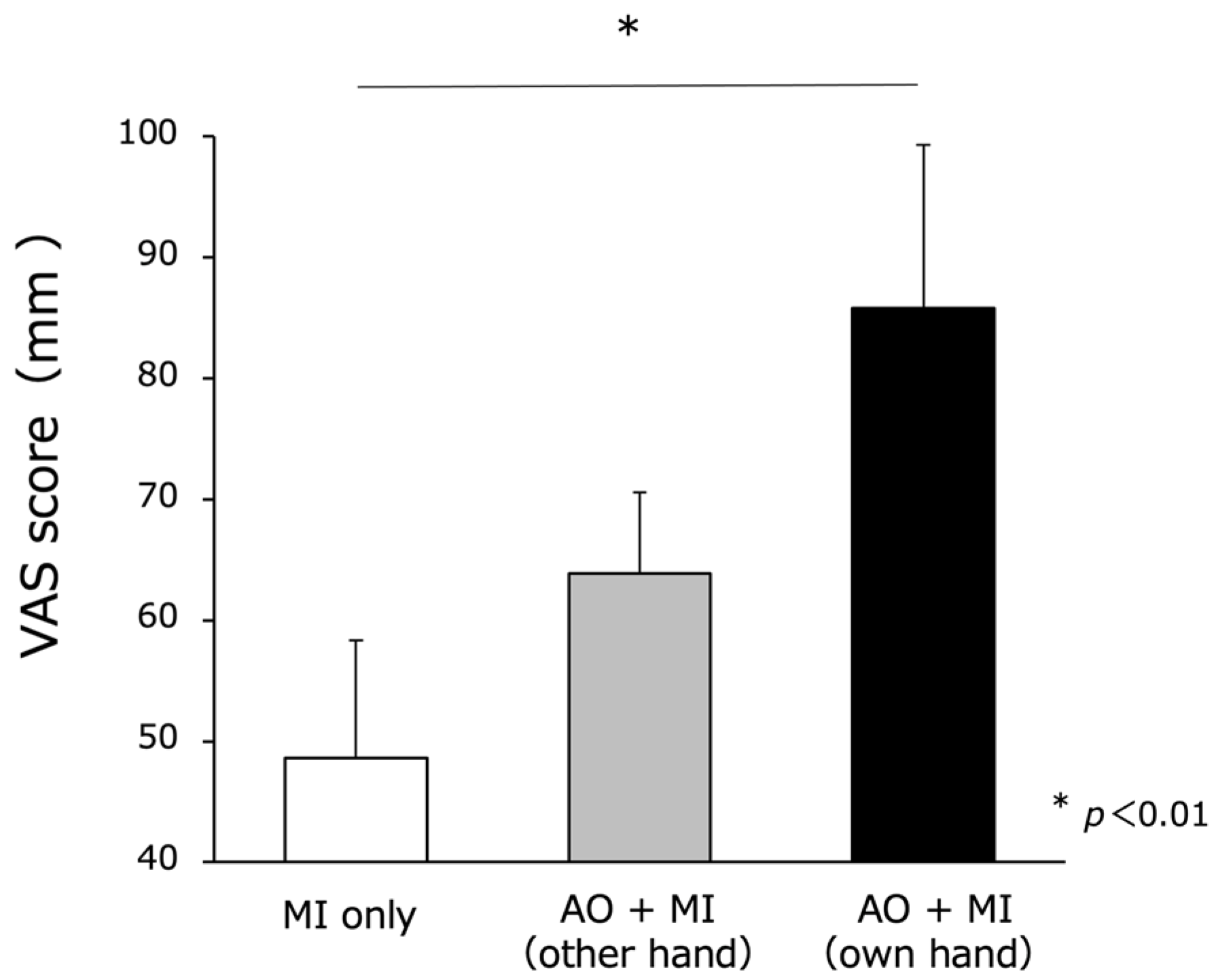
3.2. MI Vividness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, N.; Pomeroy, V.M.; Baron, J.-C. Motor Imagery: A backdoor to the motor system after stroke? Stroke 2006, 37, 1941–1952. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, D.M.; Gillen, G.; Gordon, A.M. Use of Mental Practice to Improve Upper-Limb Recovery After Stroke: A Systematic Review. Am. J. Occup. Ther. 2010, 64, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Buchignani, B.; Beani, E.; Pomeroy, V.; Iacono, O.; Sicola, E.; Perazza, S.; Bieber, E.; Feys, H.; Klingels, K.; Cioni, G.; et al. Action observation training for rehabilitation in brain injuries: A systematic review and meta-analysis. BMC Neurol. 2019, 19, 344. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Dionne, T.P. Interventions to Improve Movement and Functional Outcomes in Adult Stroke Rehabilitation: Review and Evidence Summary. J. Particip. Med. 2018, 10, e3. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. The representing brain: Neural correlates of motor intention and imagery. Behav. Brain Sci. 1994, 17, 187–202. [Google Scholar] [CrossRef]
- Jeannerod, M.; Decety, J. Mental motor imagery: A window into the representational stages of action. Curr. Opin. Neurobiol. 1995, 5, 727–732. [Google Scholar] [CrossRef]
- Decety, J. Do imagined and executed actions share the same neural substrate? Cogn. Brain Res. 1996, 3, 87–93. [Google Scholar] [CrossRef]
- Kho, A.Y.; Liu, K.P.Y.; Chung, R.C.K. Meta-analysis on the effect of mental imagery on motor recovery of the hemiplegic upper extremity function. Aust. Occup. Ther. J. 2013, 61, 38–48. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.-P.; Eugène, F.; Michon, P.-E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Ruby, P.; Decety, J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat. Neurosci. 2001, 4, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Bonda, E.; Petrides, M.; Frey, S.; Evans, A. Neural correlates of mental transformations of the body-in-space. Proc. Natl. Acad. Sci. USA 1995, 92, 11180–11184. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.D.; Olivier, E.; Craighero, L.; Fadiga, L.; Duhamel, J.-R.; Sirigu, A. The Influence of Hand Posture on Corticospinal Excitability during Motor Imagery: A Transcranial Magnetic Stimulation Study. Cereb. Cortex 2004, 14, 1200–1206. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Sakamoto, M.; Muraoka, T.; Kanosue, K. Influence of touching an object on corticospinal excitability during motor imagery. Exp. Brain Res. 2009, 196, 529–535. [Google Scholar] [CrossRef]
- Vogt, S.R.; Di Rienzo, F.; Collet, C.; Collins, A.; Guillot, A. Multiple roles of motor imagery during action observation. Front. Hum. Neurosci. 2013, 7, 807. [Google Scholar] [CrossRef]
- Taube, W.; Mouthon, M.; Leukel, C.; Hoogewoud, H.-M.; Annoni, J.-M.; Keller, M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 2015, 64, 102–114. [Google Scholar] [CrossRef]
- Eaves, D.L.; Riach, M.; Holmes, P.S.; Wright, D.J. Motor Imagery during Action Observation: A Brief Review of Evidence, Theory and Future Research Opportunities. Front. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Ruby, P.; Decety, J. What you believe versus what you think they believe: A neuroimaging study of conceptual perspective-taking. Eur. J. Neurosci. 2003, 17, 2475–2480. [Google Scholar] [CrossRef]
- Maeda, F.; Chang, V.Y.; Mazziotta, J.; Iacoboni, M. RETRACTED ARTICLE: Experience-dependent modulation of motor corticospinal excitability during action observation. Exp. Brain Res. 2001, 140, 241–244. [Google Scholar] [CrossRef]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; E Llewellyn, D.M.; Ramachandran, V.S. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhou, Y.; He, H.; Lin, S.; Zhu, R.; Liu, Z.; Liu, J.; Liu, X.; Chen, S.; Zou, J.; et al. Synergistic Effect of Combined Mirror Therapy on Upper Extremity in Patients with Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Iso, N.; Ooso, S.; Yamamoto, N.; Moriuchi, T.; Sagari, A.; Iso, F.; Tanaka, K.; Tabira, T.; Higashi, T. Effect of mental practice using inverse video of the unaffected upper limb in a subject with chronic hemiparesis after stroke. J. Phys. Ther. Sci. 2016, 28, 2984–2987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higashi, T.; Fujiwara, K.; Shibata, M.; Awano, Y.; Shibayama, K.; Iso, N.; Matsuo, M.; Nakashima, A.; Moriuchi, T.; Mitsunaga, W. A method for using video presentation to increase the vividness and activity of cortical regions during motor imagery tasks. Neural Regen. Res. 2021, 16, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–133. [Google Scholar] [CrossRef]
- Avanzino, L.; Lagravinese, G.; Bisio, A.; Perasso, L.; Ruggeri, P.; Bove, M. Action observation: Mirroring across our spontaneous movement tempo. Sci. Rep. 2015, 5, 10325. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L.; Durand, A.; Doyon, J. Clinical Assessment of Motor Imagery After Stroke. Neurorehabilit. Neural Repair 2007, 22, 330–340. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Suezawa, M.; Kanosue, K. Vividness and accuracy: Two independent aspects of motor imagery. Neurosci. Res. 2018, 147, 17–25. [Google Scholar] [CrossRef]
- Jackson, P.L.; Meltzoff, A.N.; Decety, J. Neural circuits involved in imitation and perspective-taking. NeuroImage 2006, 31, 429–439. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2003, 21, 99–111. [Google Scholar] [CrossRef]
- Dely, C.M.D.T. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near in-fra-red transillumination. Med. Biol. Eng. Comput. 1988, 2, 289–294. [Google Scholar]
- Obrig, H.; Villringer, A. Beyond the Visible—Imaging the Human Brain with Light. J. Cereb. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.B.; Parthasarathy, A.B.; Busch, D.R.; Mesquita, R.C.; Greenberg, J.H.; Yodh, A.G. Modified Beer-Lambert law for blood flow. Biomed. Opt. Express 2014, 5, 4053. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Miyai, I.; Mihara, M.; Sakoda, S.; Kubota, K. Frontal regions involved in learning of motor skill—A functional NIRS study. NeuroImage 2007, 34, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sagari, A.; Iso, N.; Moriuchi, T.; Ogahara, K.; Kitajima, E.; Tanaka, K.; Tabira, T.; Higashi, T. Changes in Cerebral Hemodynamics during Complex Motor Learning by Character Entry into Touch-Screen Terminals. PLoS ONE 2015, 10, e0140552. [Google Scholar] [CrossRef]
- Amemiya, K.; Ishizu, T.; Ayabe, T.; Kojima, S. Effects of motor imagery on intermanual transfer: A near-infrared spectroscopy and behavioural study. Brain Res. 2010, 1343, 93–103. [Google Scholar] [CrossRef]
- Iso, N.; Moriuchi, T.; Sagari, A.; Kitajima, E.; Iso, F.; Tanaka, K.; Kikuchi, Y.; Tabira, T.; Higashi, T. Monitoring Local Regional Hemodynamic Signal Changes during Motor Execution and Motor Imagery Using Near-Infrared Spectroscopy. Front. Physiol. 2015, 6, 416. [Google Scholar] [CrossRef][Green Version]
- Hoshi, Y.; Kobayashi, N.; Tamura, M. Interpretation of near-infrared spectroscopy signals: A study with a newly developed perfused rat brain model. J. Appl. Physiol. 2001, 90, 1657–1662. [Google Scholar] [CrossRef]
- Sato, H.; Yahata, N.; Funane, T.; Takizawa, R.; Katura, T.; Atsumori, H.; Nishimura, Y.; Kinoshita, A.; Kiguchi, M.; Koizumi, H.; et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage 2013, 83, 158–173. [Google Scholar] [CrossRef]
- Marumo, K.; Takizawa, R.; Kawakubo, Y.; Onitsuka, T.; Kasai, K. Gender difference in right lateral prefrontal hemodynamic response while viewing fearful faces: A multi-channel near-infrared spectroscopy study. Neurosci. Res. 2009, 63, 89–94. [Google Scholar] [CrossRef]
- Pu, S.; Nakagome, K.; Yamada, T.; Yokoyama, K.; Matsumura, H.; Mitani, H.; Adachi, A.; Nagata, I.; Kaneko, K. The relationship between the prefrontal activation during a verbal fluency task and stress-coping style in major depressive disorder: A near-infrared spectroscopy study. J. Psychiatr. Res. 2012, 46, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, R.; Matsuura, T.; Kanno, I. Frequency Dependence of Local Cerebral Blood Flow Induced by Somatosensory Hind Paw Stimulation in Rat under Normo- and Hypercapnia. Jpn. J. Physiol. 2001, 51, 201–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moriuchi, T.; Nakashima, A.; Nakamura, J.; Anan, K.; Nishi, K.; Matsuo, T.; Hasegawa, T.; Mitsunaga, W.; Iso, N.; Higashi, T. The Vividness of Motor Imagery Is Correlated with Corticospinal Excitability During Combined Motor Imagery and Action Observation. Front. Hum. Neurosci. 2020, 14, 581652. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Schlatter, A.; Schuster, C.; A Puhan, M.; Siekierka, E.; Steurer, J. Efficacy of motor imagery in post-stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Pendy, J.T.; Li, W.A.; Du, H.; Zhang, T.; Geng, X.; Ding, Y. Motor Imagery-Based Rehabilitation: Potential Neural Correlates and Clinical Application for Functional Recovery of Motor Deficits after Stroke. Aging Dis. 2017, 8, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Villiger, M.; Estévez, N.; Hepp-Reymond, M.-C.; Kiper, D.; Kollias, S.S.; Eng, K.; Hotz, S. Enhanced Activation of Motor Execution Networks Using Action Observation Combined with Imagination of Lower Limb Movements. PLoS ONE 2013, 8, e72403. [Google Scholar] [CrossRef]
- Batula, A.M.; Mark, J.A.; Kim, Y.E.; Ayaz, H. Comparison of Brain Activation during Motor Imagery and Motor Movement Using fNIRS. Comput. Intell. Neurosci. 2017, 2017, 5491296. [Google Scholar] [CrossRef]
- Abdalmalak, A.; Milej, D.; Diop, M.; Naci, L.; Owen, A.M.; Lawrence, K.S. Assessing the feasibility of time-resolved fNIRS to detect brain activity during motor imagery. In Clinical and Translational Neurophotonics; Neural Imaging and Sensing; and Optogenetics and Optical Manipulation; SPIE: Bellingham, DC, USA, 2016; Volume 9690, p. 969002. [Google Scholar]




| Blood Pressure and Pulse Rate before NIRS Measurement | Upper Limb Functional Assessment | Cognitive Function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease | Paralyzed Side | Gender | Age | Days from Onset to Date of Measurement | Systolic Blood Pressure | Diastolic Blood Pressure | Pulse | FMA | MAL (AOU) | MAL (QOM) | ARAT (Paralyzed Side) | MMSE |
| CH | R | F | 59 | 34 | 120 | 70 | 65 | 4 | 0 | 0 | 0 | 29 |
| CI | R | M | 55 | 30 | 140 | 90 | 70 | 54 | 0.38 | 0.61 | 50 | 30 |
| CH | R | F | 61 | 55 | 114 | 84 | 85 | 53 | 2 | 1.8 | 57 | 30 |
| CI | R | M | 78 | 28 | 110 | 72 | 68 | 31 | 0.3 | 0.4 | 17 | 29 |
| CI | R | F | 48 | 35 | 124 | 80 | 80 | 4 | 0 | 0 | 0 | 26 |
| CH | L | F | 81 | 37 | 108 | 60 | 78 | 4 | 0 | 0 | 0 | 29 |
| CI | L | F | 79 | 40 | 138 | 78 | 51 | 34 | 0 | 0 | 10 | 29 |
| CH | L | M | 56 | 25 | 126 | 76 | 80 | 35 | 0 | 0 | 3 | 29 |
| CI | L | M | 53 | 54 | 120 | 84 | 81 | 49 | 0 | 0 | 5 | 30 |
| CI | L | F | 56 | 28 | 132 | 78 | 60 | 16 | 0 | 0 | 6 | 28 |
| AVG | 62.6 | 36.6 | 123.2 | 77.2 | 71.8 | 28.4 | 0.2 | 0.2 | 14.8 | 28.9 | ||
| SD | 12.0 | 10.4 | 11.0 | 8.4 | 10.8 | 20.2 | 0.6 | 0.5 | 21.1 | 1.1 | ||
| SE | 3.8 | 3.31 | 3.4 | 2.6 | 3.4 | 6.4 | 0.1 | 0.1 | 6.6 | 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, K.; Shimoda, R.; Shibata, M.; Awano, Y.; Shibayama, K.; Higashi, T. A Method for Using Video Presentation to Increase Cortical Region Activity during Motor Imagery Tasks in Stroke Patients. Brain Sci. 2023, 13, 29. https://doi.org/10.3390/brainsci13010029
Fujiwara K, Shimoda R, Shibata M, Awano Y, Shibayama K, Higashi T. A Method for Using Video Presentation to Increase Cortical Region Activity during Motor Imagery Tasks in Stroke Patients. Brain Sciences. 2023; 13(1):29. https://doi.org/10.3390/brainsci13010029
Chicago/Turabian StyleFujiwara, Kengo, Rikako Shimoda, Masatomo Shibata, Yoshinaga Awano, Koji Shibayama, and Toshio Higashi. 2023. "A Method for Using Video Presentation to Increase Cortical Region Activity during Motor Imagery Tasks in Stroke Patients" Brain Sciences 13, no. 1: 29. https://doi.org/10.3390/brainsci13010029
APA StyleFujiwara, K., Shimoda, R., Shibata, M., Awano, Y., Shibayama, K., & Higashi, T. (2023). A Method for Using Video Presentation to Increase Cortical Region Activity during Motor Imagery Tasks in Stroke Patients. Brain Sciences, 13(1), 29. https://doi.org/10.3390/brainsci13010029






