Gait Indicators Contribute to Screening Cognitive Impairment: A Single- and Dual-Task Gait Study
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Cognitive Assessment
2.4. Diagnosis
2.5. Gait Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants Stratified by Disease Diagnosis
3.2. Gait Characteristics in Different Severities of Cognitive Impairment
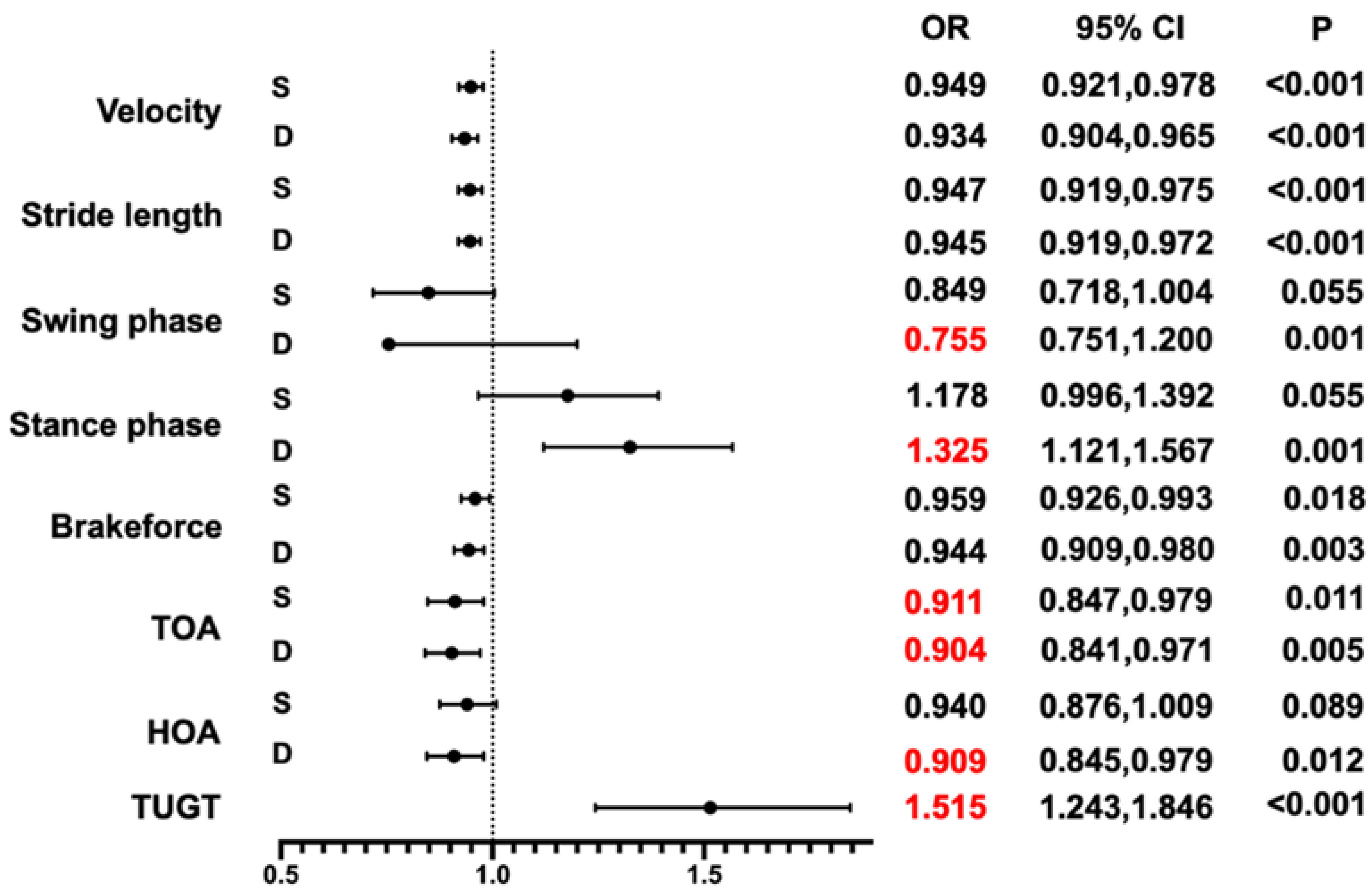
3.3. Association between the Timed “Up & Go” Time (TUGT), TOA, and Cognitive Impairment
| Total | Control | MCI | Mild AD | Moderate AD | p-Value | ||
|---|---|---|---|---|---|---|---|
| Velocity, mean (SD), (cm/s) | S | 75.13 (17.836) | 87.30 (14.721) | 76.33 (15.572) | 69.46 (14.625) | 56.83 (14.620) | <0.001 * |
| D | 64.01 (18.114) | 78.68 (15.099) | 64.28 (14.393) | 55.29 (12.808) | 43.58 (11.321) | <0.001 * | |
| Stride length, mean (SD), (cm/s) | S | 93.12 (18.769) | 105.63 (13.137) | 93.94 (17.652) | 86.70 (16.840) | 76.60 (17.470) | <0.001 * |
| D | 89.51 (21.331) | 105.14 (14.069) | 90.40 (18.884) | 80.17 (19.362) | 66.33 (14.445) | <0.001 * | |
| Stride time (ms) | S | 1.264 (0.143) | 1.215 (0.135) | 1.251 (0.134) | 1.281 (0.131) | 1.364 (0.159) | <0.001 * |
| D | 1.432 (0.209) | 1.355 (0.208) | 1.425 (0.218) | 1.480 (0.188) | 1.545 (0.155) | <0.001 * | |
| Cadence (steps/min) | S | 96.601 (10.154) | 100.157 (10.304) | 97.566 (9.446) | 95.237 (9.061) | 89.299 (9.627) | <0.001 * |
| D | 85.476 (11.354) | 90.007 (11.555) | 86.029 (11.679) | 82.486 (9.885) | 78.785 (7.953) | 0.003 * | |
| Swing phase (%) | S | 32.860 (2.748) | 34.368 (2.325) | 33.381 (2.460) | 32.025 (2.307) | 30.120 (2.524) | <0.001 * |
| D | 31.116 (3.078) | 33.166 (2.314) | 31.358 (2.937) | 29.235 (2.497) | 27.867 (2.411) | <0.001 * | |
| Stance phase (%) | S | 67.140 (2.748) | 65.632 (2.325) | 66.619 (2.460) | 67.975 (2.307) | 69.880 (2.524) | <0.001 * |
| D | 68.884 (3.078) | 66.834 (2.314) | 68.642 (2.937) | 70.146 (2.497) | 72.133 (2.411) | <0.001 * | |
| Back-force | S | 0.687 (0.153) | 0.759 (0.104) | 0.707 (0.160) | 0.648 (0.145) | 0.563 (0.158) | <0.001 * |
| D | 0.661 (0.151) | 0.755 (0.104) | 0.677 (0.145) | 0.599 (0.140) | 0.514 (0.118) | <0.001 * | |
| TOA | S | 37.550 (7.102) | 41.738 (4.934) | 38.365 (7.001) | 35.426 (6.489) | 30.810 (6.253) | <0.001 * |
| D | 35.786 (7.571) | 40.869 (5.268) | 36.748 (7.119) | 32.442 (6.519) | 27.400 (5.085) | <0.001 * | |
| HOA | S | 24.510 (6.713) | 28.018 (5.068) | 25.671 (6.845) | 22.286 (6.150) | 18.883 (5.667) | <0.001 * |
| D | 23.252 (7.107) | 27.876 (5.032) | 24.350 (6.828) | 19.852 (6.217) | 16.038 (4.544) | <0.001 * | |
| TUGT | 12.830 (5.289) | 9.588 (2.539) | 12.339 (3.396) | 14.388 (5.227) | 19.927 (7.070) | <0.001 * | |
| OLS-EC | L | 4.03 (2.798) | 4.26 (2.207) | 4.78 (3.589) | 3.56 (2.454) | 2.68 (5.477) | 0.001 * |
| R | 4.06 (2.986) | 4.72 (2.617) | 4.28 (3.796) | 3.56 (2.628) | 2.88 (2.007) | 0.001 * | |
| FPB | T | 149.039 (96.785) | 149.539 (93.665) | 152.436 (107.737) | 158.347 (93.728) | 119.113 (84.543) | 0.346 |
| L | 141.016 (71.117) | 140.374 (71.854) | 149.691 (65.113) | 136.331 (78.519) | 134.430 (65.694) | 0.212 | |
| R | 74.538 (131.111) | 70.606 (128.401) | 77.260 (137.101) | 86.714 (140.232) | 50.606 (102.710) | 0.980 |
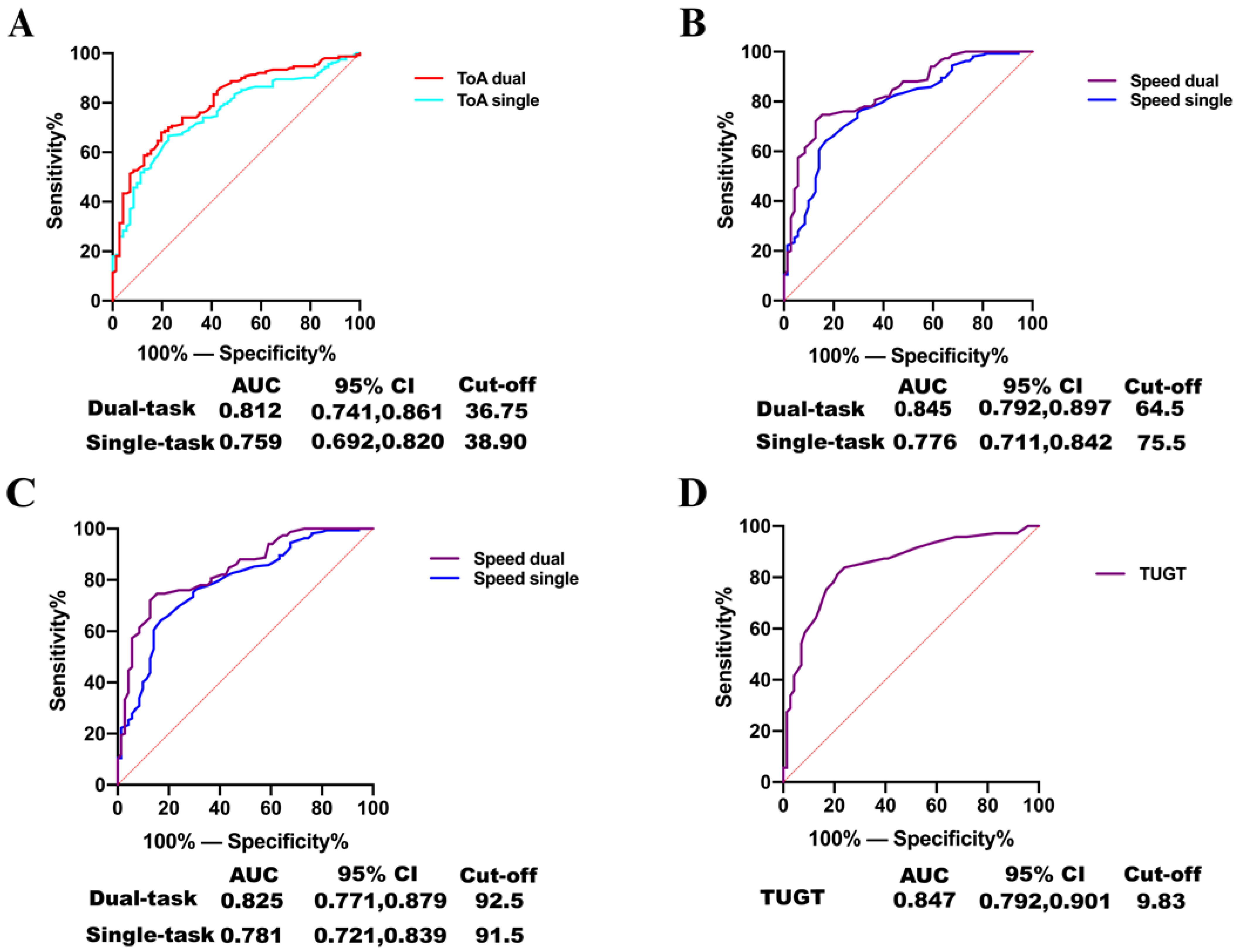
3.4. Accuracy of TOA and TUGT for Predicting Patients with Cognitive Impairment
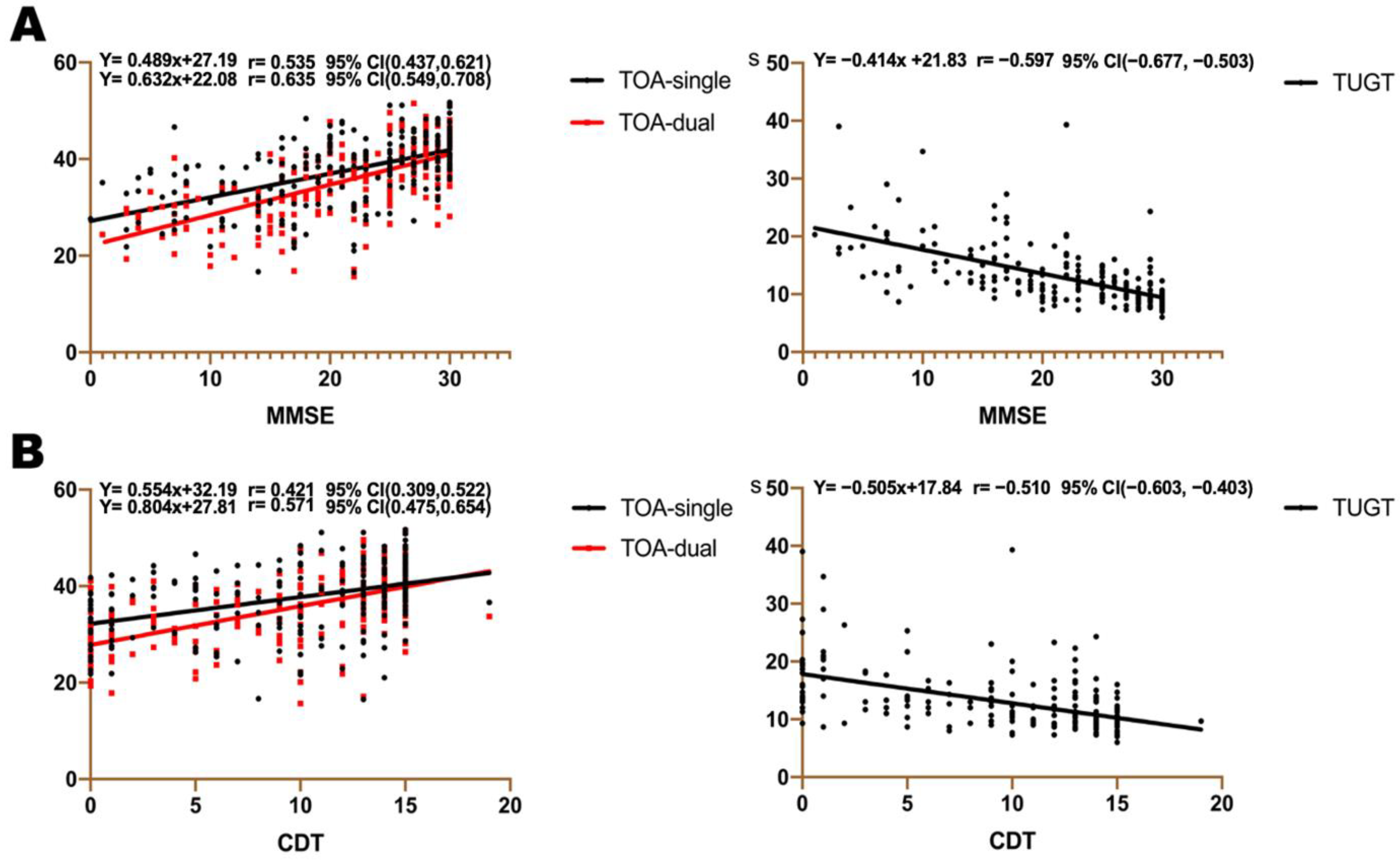
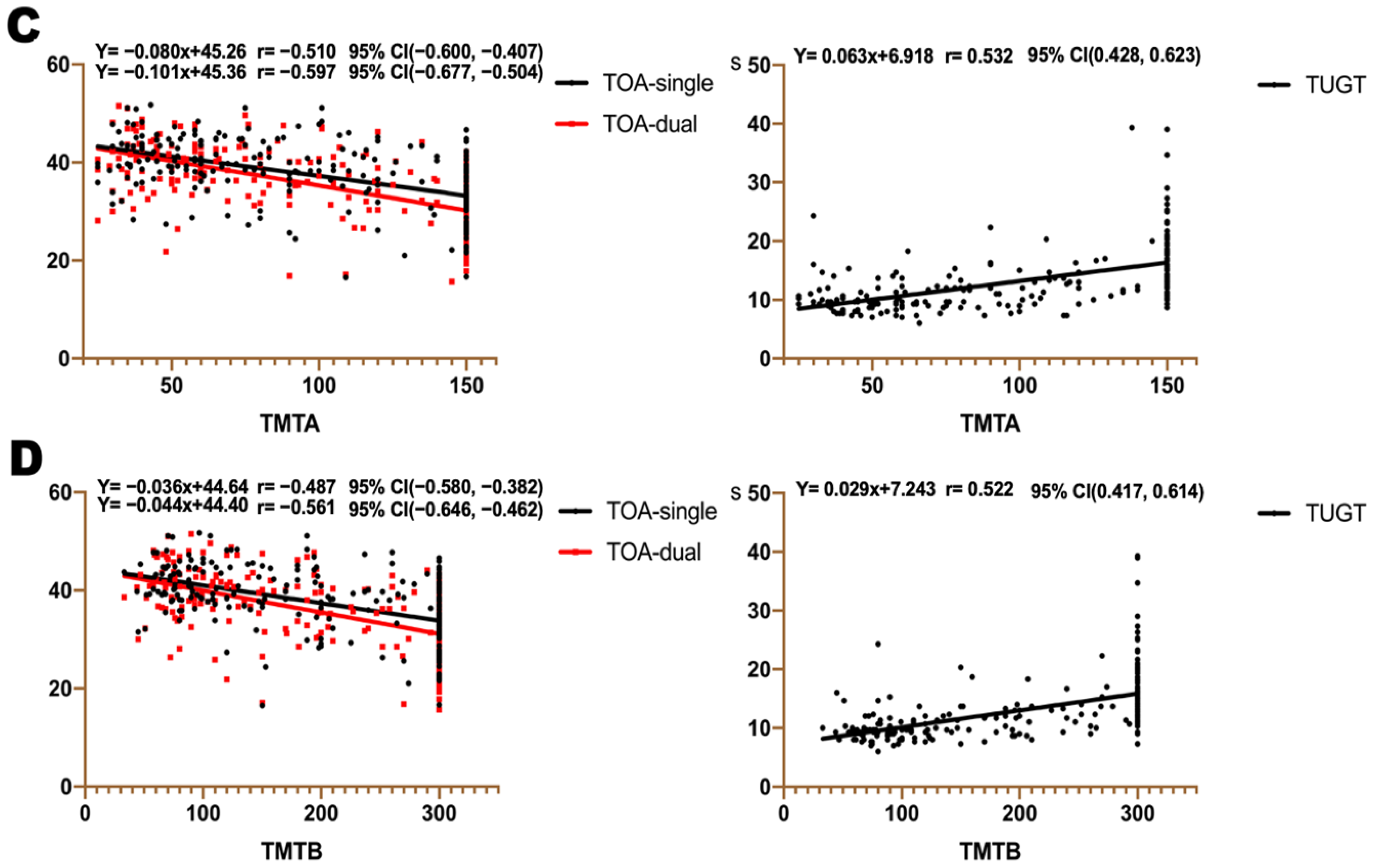
3.5. Association between TOA, TUGT, and Cognitive Assessment Scales
4. Discussion
4.1. Our Findings in This Study
4.2. TOA and TUGT Are Unique and New Gait Indexes
4.3. Cognitive Impairment and Gait Impairment Have a Strong Association
4.4. Balance Function Tests (TUG and the OLS-EC) Are Useful for Cognitive Impairment Patients
4.5. The Gait Paradigms Can Successfully Detect Different Severities of Cognitive Impairment Patients
4.6. Gait Indicators Are More Sensitive in Dual-Task Than Single-Task Assessments
4.7. Gait Facilitates the Clinical Assessment of Patients with Cognitive Impairment
4.8. Strengths
4.9. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Shiri-Feshki, M. Temporal trends in the long term risk of progression of mild cognitive impairment: A pooled analysis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, L.G. Alzheimer Disease. Continuum 2016, 22, 419–434. [Google Scholar] [CrossRef] [Green Version]
- The Need for Early Detection and Treatment in Alzheimer’s Disease. EBioMedicine 2016, 9, 1–2. [CrossRef] [Green Version]
- Reisberg, B.; Gauthier, S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int. Psychogeriatr. 2008, 20, 1–16. [Google Scholar] [CrossRef]
- Cummings, J.; Feldman, H.H.; Scheltens, P. The “rights” of precision drug development for Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Petersen, R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef] [Green Version]
- Woodard, J.L.; Dorsett, E.S.W.; Cooper, J.G.; Hermann, B.P.; Sager, M.A. Development of a Brief Cognitive Screen for Mild Cognitive Impairment and Neurocognitive Disorder. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2005, 12, 299–315. [Google Scholar] [CrossRef]
- Pinto, E.; Peters, R. Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement. Geriatr. Cogn. Disord. 2009, 27, 201–213. [Google Scholar] [CrossRef]
- Laske, C.; Sohrabi, H.R.; Frost, S.M.; López-de-Ipiña, K.; Garrard, P.; Buscema, M.; Dauwels, J.; Soekadar, S.R.; Mueller, S.; Linnemann, C.; et al. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 561–578. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Ni, L.; Lv, W.; Sun, D.; Sun, Y.; Sun, Y.; Xu, X.; Chang, M.; Han, X.; Tao, S.; Hu, X.; et al. Pathological Gait Signatures of Post-stroke Dementia with Toe-Off and Heel-to-Ground Angles Discriminate From Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 766884. [Google Scholar] [CrossRef]
- Holtzer, R.; Epstein, N.; Mahoney, J.R.; Izzetoglu, M.; Blumen, H.M. Neuroimaging of mobility in aging: A targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1375–1388. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Morris, R.; Wilson, J.; Galna, B.; Thomas, A.J.; Rochester, L. What Can Quantitative Gait Analysis Tell Us about Dementia and Its Subtypes? A Structured Review. J. Alzheimers Dis. 2017, 60, 1295–1312. [Google Scholar] [CrossRef]
- Hirvensalo, M.; Rantanen, T.; Heikkinen, E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J. Am. Geriatr. Soc. 2000, 48, 493–498. [Google Scholar] [CrossRef]
- Grande, G.; Triolo, F.; Nuara, A.; Welmer, A.K.; Fratiglioni, L.; Vetrano, D.L. Measuring gait speed to better identify prodromal dementia. Exp. Gerontol. 2019, 124, 110625. [Google Scholar] [CrossRef]
- Buracchio, T.; Dodge, H.H.; Howieson, D.; Wasserman, D.; Kaye, J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, M.; Deng, Y.; Duan, J.; Tu, Q.; Cao, Y.; Zhu, Q.; Yu, W.; Lü, Y. Does Gait Speed Replace Comprehensive Geriatric Assessment in the Elderly? Int. J. Gerontol. 2016, 10, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Bytyçi, I.; Henein, M.Y. Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2670. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, A.; Tigrini, A.; Fioretti, S.; Verdini, F. Identification of Neurodegenerative Diseases from Gait Rhythm Through Time Domain and Time-Dependent Spectral Descriptors. IEEE J. Biomed. Health Inform. 2022, 26, 5974–5982. [Google Scholar] [CrossRef]
- Lam, F.M.; Huang, M.Z.; Liao, L.R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef]
- Verlinden, V.J.; van der Geest, J.N.; Hofman, A.; Ikram, M.A. Cognition and gait show a distinct pattern of association in the general population. Alzheimers Dement. 2014, 10, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Park, D.-J.; Kim, M.-K.; Shin, M.-J.; Park, J.-H.; Lee, B.-J.; Lee, E.-L.; Jeong, J.-S.; Ahn, S.-J. Timed up & go quantification algorithm using IMU and sEMG signal. Biomed. Signal Process. Control 2023, 80, 104309. [Google Scholar] [CrossRef]
- Barone, R.; Macaluso, F.; Traina, M.; Leonardi, V.; Farina, F.; Di Felice, V. Soccer players have a better standing balance in nondominant one-legged stance. Open Access J. Sports Med. 2010, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Matsuura, T.; Sakashita, K.; Grushnikov, A.; Okura, F.; Mitsugami, I.; Yagi, Y. Statistical Analysis of Dual-task Gait Characteristics for Cognitive Score Estimation. Sci. Rep. 2019, 9, 19927. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.D.; Go, A.S.; Shilane, D.; Boothroyd, D.B.; Leong, T.K.; Kazi, D.S.; Chang, T.I.; Hlatky, M.A. Comparative effectiveness of clopidogrel in medically managed patients with unstable angina and non-ST-segment elevation myocardial infarction. J. Am. Coll Cardiol. 2014, 63, 2249–2257. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Yu, W.; Liu, X.; Wan, T.; Chen, C.; Xiong, L.; Zhang, W.; Lü, Y. Associations between malnutrition and cognitive impairment in an elderly Chinese population: An analysis based on a 7-year database. Psychogeriatrics 2021, 21, 80–88. [Google Scholar] [CrossRef]
- Hittmair-Delazer, M.; Semenza, C.; Denes, G. Concepts and facts in calculation. Brain 1994, 117, 715–728. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Casas, A.; Hansen, K.T.; Bilski, P.; Gutmanis, I.; Wells, J.L.; Borrie, M.J. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: A reliability study. J. Neuroeng. Rehabil. 2009, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Mathias, S.; Nayak, U.S.; Isaacs, B. Balance in elderly patients: The “get-up and go” test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Jacono, M.; Casadio, M.; Morasso, P.G.; Sanguineti, V. The sway-density curve and the underlying postural stabilization process. Motor Control 2004, 8, 292–311. [Google Scholar] [CrossRef] [Green Version]
- Tigrini, A.; Verdini, F.; Fioretti, S.; Mengarelli, A. Long term correlation and inhomogeneity of the inverted pendulum sway time-series under the intermittent control paradigm. Commun. Nonlinear Sci. Numer. Simul. 2022, 108, 106198. [Google Scholar] [CrossRef]
- Wilson, J.; Allcock, L.; Mc Ardle, R.; Taylor, J.P.; Rochester, L. The neural correlates of discrete gait characteristics in ageing: A structured review. Neurosci. Biobehav. Rev. 2019, 100, 344–369. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Beare, R.; Phan, T.G.; Blizzard, L.; Thrift, A.G.; Chen, J.; Srikanth, V.K. Brain structural change and gait decline: A longitudinal population-based study. J. Am. Geriatr. Soc. 2013, 61, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Dumurgier, J.; Crivello, F.; Mazoyer, B.; Ahmed, I.; Tavernier, B.; Grabli, D.; François, C.; Tzourio-Mazoyer, N.; Tzourio, C.; Elbaz, A. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 2012, 60, 871–878. [Google Scholar] [CrossRef]
- Bast, T.; Pezze, M.; McGarrity, S. Cognitive deficits caused by prefrontal cortical and hippocampal neural disinhibition. Br. J. Pharmacol. 2017, 174, 3211–3225. [Google Scholar] [CrossRef] [Green Version]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef]
- Beh, S.C.; Muthusamy, B.; Calabresi, P.; Hart, J.; Zee, D.; Patel, V.; Frohman, E. Hiding in plain sight: A closer look at posterior cortical atrophy. Pract. Neurol. 2015, 15, 5–13. [Google Scholar] [CrossRef]
- Tosun, D.; Schuff, N.; Mathis, C.A.; Jagust, W.; Weiner, M.W. Spatial patterns of brain amyloid-beta burden and atrophy rate associations in mild cognitive impairment. Brain 2011, 134, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Park, Y. Head Posture and Postural Balance in Community-Dwelling Older Adults Who Use Dentures. Medicina 2020, 56, 529. [Google Scholar] [CrossRef]
- Rubega, M.; Formaggio, E.; Di Marco, R.; Bertuccelli, M.; Tortora, S.; Menegatti, E.; Cattelan, M.; Bonato, P.; Masiero, S.; Del Felice, A. Cortical correlates in upright dynamic and static balance in the elderly. Sci. Rep. 2021, 11, 14132. [Google Scholar] [CrossRef]
- Krishnan, V.; Kanekar, N.; Aruin, A.S. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci. Lett. 2012, 506, 256–260. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. S2), ii7–ii11. [Google Scholar] [CrossRef] [Green Version]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Muir, S.W.; Speechley, M.; Wells, J.; Borrie, M.; Gopaul, K.; Montero-Odasso, M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture 2012, 35, 96–100. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Sarquis-Adamson, Y.; Speechley, M.; Borrie, M.J.; Hachinski, V.C.; Wells, J.; Riccio, P.M.; Schapira, M.; Sejdic, E.; Camicioli, R.M.; et al. Association of Dual-Tas.sk Gait with Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol. 2017, 74, 857–865. [Google Scholar] [CrossRef]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative values for the unipedal stance test with eyes open and closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. 2013, 28, 1520–1533. [Google Scholar] [CrossRef]
- Rossor, M.N.; Fox, N.C.; Mummery, C.J.; Schott, J.M.; Warren, J.D. The diagnosis of young-onset dementia. Lancet Neurol. 2010, 9, 793–806. [Google Scholar] [CrossRef]

| Variable | Total | Control | MCI | Mild AD | Moderate AD | p-Value |
|---|---|---|---|---|---|---|
| N = 235 | N = 71 | N = 63 | N = 71 | N = 30 | ||
| Age, mean SD | 72.0 (7.836) | 71.01 (6.737) | 70.05 (7.985) | 73.41 (7.989) | 75.10 (8.372) | 0.007 * |
| Female, N (%) | 154 (65.5) | 50 (70.4) | 42 (66.7) | 46 (64.8) | 16 (53.3) | 0.427 |
| Height, mean (SD, (cm) | 1.584 (0.080) | 1.593 (0.0798) | 1.581 (0.0827) | 1.590 (0.0724) | 1.583 (0.0905) | 0.816 |
| Weight, mean (SD, (Kg) | 57.222 (9.872) | 59 (9.368) | 57.854 (10.634) | 55.287 (9.428) | 56.233 (8.721) | 0.169 |
| BMI, mean (SD, (kg/m²) | 22.827 (3.526) | 23.186 (2.740) | 23.079 (3.429) | 21.896 (3.562) | 22.484 (3.215) | 0.112 |
| Education, mean (SD), (y) | 9.3 (4.4) | 11.817 (9.206) | 8.817 (4.180) | 9.282 (4.667) | 6.10 (4.791) | <0.001 * |
| Cognitive tests | ||||||
| MMSE, mean (SD) | 21.23 (7.741) | 28.82 (2.045) | 23.33 (3.910) | 17.31 (4.717) | 8.10 (4.536) | <0.001 * |
| CDT score, mean (SD) | 9.70 (5.387) | 13.83 (2.813) | 10.41 (4.272) | 7.93 (4.894) | 2.60 (3.936) | <0.001 * |
| DSF, mean (SD) | 6.85 (2.790) | 8.56 (1.105) | 7.19 (1.608) | 6.802.326) | 2.03 (3.235) | <0.001 * |
| DSB, mean (SD) | 3.48 (1.943) | 5.06 (1.413) | 3.53 (1.423) | 2.97 (1.307) | 0.76 (1.704) | <0.001 * |
| TMT A, mean (SD), s | 96.72 (44.724) | 51.97 (20.856) | 99.69 (39.203) | 117.29 (35.690) | 147.00 (12.077) | <0.001 * |
| TMT B, mean (SD), s | 199.48 (95.669) | 98.59 (54.673) | 210.74 (82.921) | 247.31 (66.466) | 300.00 (0) | <0.001 * |
| His, mean (SD) | 2.64 (2.080) | 1.87 (1.656) | 2.86 (2.023) | 2.97 (2.169) | 3.28 (2.463) | <0.001 * |
| IADL, mean (SD) | 12.51 (5.430) | 8.62 (1.543) | 10.49 (2.375) | 14.04 (4.680) | 22.37 (3.891) | <0.001 * |
| GDS, mean (SD) | 5.62 (4.664) | 3.69 (3.602) | 7.02 (5.037) | 6.20 (5.157) | 5.88 (3.059) | <0.001 * |
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Gait variable (continuous) | ||||||||||
| Speed | S | 0.952 | 0.928, 0.977 | <0.001 | 0.936 | 0.884, 0.991 | 0.024 | 0.949 | 0.921, 0.978 | <0.001 |
| D | 0.935 | 0.908, 0.963 | <0.001 | 0.928 | 0.899, 0.958 | <0.001 | 0.934 | 0.904, 0.965 | <0.001 | |
| Stride length | S | 0.95 | 0.926, 0.975 | <0.001 | 0.94 | 0.914, 0.967 | <0.001 | 0.947 | 0.919, 0.975 | <0.001 |
| D | 0.947 | 0.924, 0.971 | <0.001 | 0.94 | 0.915, 0.965 | <0.001 | 0.945 | 0.919, 0.972 | <0.001 | |
| Stride time | S | 7.889 | 0.591, 105.305 | 0.118 | ||||||
| D | 4.78 | 0.871, 26.235 | 0.072 | |||||||
| Cadence | S | 0.974 | 0.940, 1.008 | 0.134 | ||||||
| D | 0.971 | 0.942, 1.002 | 0.063 | |||||||
| Swing phase | S | 0.839 | 0.723, 0.974 | 0.021 | 0.805 | 0.686, 0.945 | 0.008 | 0.849 | 0.718, 1.004 | 0.055 |
| D | 0.754 | 0.644, 0.883 | <0.001 | 0.727 | 0.614, 0.859 | <0.001 | 0.755 | 0.751, 1.200 | 0.001 | |
| Stance phase | S | 1.192 | 1.026, 1.384 | 0.021 | 1.242 | 1.058, 1.457 | 0.008 | 1.178 | 0.996, 1.392 | 0.055 |
| D | 1.326 | 1.133, 1.552 | <0.001 | 1.376 | 1.164, 1.628 | <0.001 | 1.325 | 1.121, 1.567 | 0.001 | |
| Brakeforce | S | 0.966 | 0.938, 0.994 | 0.016 | 0.949 | 0.917, 0.981 | 0.002 | 0.959 | 0.926, 0.993 | 0.018 |
| D | 0.951 | 0.923, 0.980 | 0.001 | 0.935 | 0.902, 0.969 | <0.001 | 0.944 | 0.909, 0.980 | 0.003 | |
| TOA | S | 0.907 | 0.852, 0.967 | 0.003 | 0.890 | 0.831, 0.953 | 0.001 | 0.911 | 0.847, 0.979 | 0.011 |
| D | 0.895 | 0.839, 0.954 | 0.001 | 0.885 | 0.827, 0.947 | <0.001 | 0.904 | 0.841, 0.971 | 0.005 | |
| HOA | S | 0.935 | 0.881, 0.993 | 0.028 | 0.916 | 0.857, 0.979 | 0.009 | 0.940 | 0.876, 1.009 | 0.089 |
| D | 0.904 | 0.849, 0.963 | 0.002 | 0.890 | 0.831, 0.953 | 0.001 | 0.909 | 0.845, 0.979 | 0.012 | |
| TUGT | 1.45 | 1.218, 1.727 | <0.001 | 1.550 | 1.278, 1.879 | <0.001 | 1.515 | 1.243, 1.846 | <0.001 | |
| OLS-EC | L | 1.072 | 0.952, 1.208 | 0.252 | ||||||
| R | 0.951 | 0.847, 1.069 | 0.403 | |||||||
| Model 1 (Unadjusted) | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Gait variable (Tertiles) | ||||||
| TOA (single-task) | ||||||
| T1 < 35.244 | 1 | 1 | 1 | |||
| T2 35.244–40.732 | 0.187 | 0.071, 0.489 | 0.173 | 0.065, 0.461 | 0.217 | 0.079, 0.592 |
| T3 > 40.732 | 0.078 | 0.031, 0.201 | 0.071 | 0.026, 0.191 | 0.097 | 0.035, 0.269 |
| p trend | <0.001 * | <0.001 * | <0.001 * | |||
| TOA (dual-task) | ||||||
| T1 < 32.5 | 1 | 1 | 1 | |||
| T2 32.5–39.952 | 0.154 | 0.055, 0.434 | 0.151 | 0.054, 0.428 | 0.174 | 0.061, 0.497 |
| T3 > 39.952 | 0.054 | 0.020, 0.149 | 0.051 | 0.018, 0.146 | 0.068 | 0.023, 0.197 |
| p trend | <0.001 * | <0.001 * | <0.001 * | |||
| TUGT | ||||||
| T1 < 9.67 | 1 | 1 | 1 | |||
| T2 9.67–13.33 | 10.565 | 4.779, 23.358 | 12.381 | 5.269, 29.094 | 11.966 | 4.937, 29.005 |
| T3 > 11.33 | 30.522 | 10.872, 85.686 | 41.262 | 13.393, 127.119 | 36.713 | 11.549, 116.708 |
| p trend | <0.001 * | <0.001 * | <0.001 * | |||
| Variable | TOA | TUGT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-Task | Dual-Task | ||||||||||
| Control | CIM | OR | 95% CI | Interaction | OR | 95% CI | Interaction | OR | 95%CI | Interaction | |
| p-Value | p-Value | p-Value | |||||||||
| Total sample | 71 (30.2) | 164 (69.8) | 0.781, 0.891 | 1.62 | 1.351, 1.944 | ||||||
| Age | |||||||||||
| Young older | 28 (36.8) | 48 (63.2) | 0.884 | 0.811, 0.964 | 0.341 | 0.876 | 0.808, 0.949 | 0.158 | 1.712 | 1.247, 2.351 | 0.662 |
| Old older | 43 (27) | 116 (73) | 0.836 | 0.773, 0.903 | 0.806 | 0.741, 0.876 | 1.62 | 1.334, 1.968 | |||
| Gender | |||||||||||
| Male | 21 (25.9) | 60 (74.1) | 0.898 | 0.827, 0.975 | 0.141 | 0.822 | 0.738, 0.915 | 0.726 | 1.311 | 1.067, 1.612 | 0.022 |
| Female | 50 (32.5) | 104 (67.5) | 0.825 | 0.763, 0.892 | 0.852 | 0.796, 0.912 | 1.891 | 1.495, 2.394 | |||
| Education | |||||||||||
| Lower | 28 (21.7) | 101 (78.3) | 0.837 | 0.768, 0.912 | 0.375 | 0.797 | 0.721, 0.881 | 0.173 | 1.892 | 1.42, 2.521 | 0.147 |
| Higher | 43 (40.6) | 63 (59.4) | 0.882 | 0.816, 0.954 | 0.869 | 0.807, 0.935 | 1.461 | 1.198, 1.782 | |||
| BMI | |||||||||||
| Underweight | 3 (13.6) | 19 (86.4) | 0.866 | 0.705, 1.063 | 0.278 | 0.879 | 0.743, 1.04 | 0.099 | NA | ||
| Normal | 28 (26.9) | 76 (73.1) | 0.824 | 0.747, 0.909 | 0.782 | 0.701, 0.872 | 1.614 | 1.267, 2.056 | 0.118 | ||
| Overweight | 24 (46.2) | 28 (53.8) | 0.852 | 0.764, 0.950 | 0.799 | 0.698, 0.915 | 1.786 | 1.243, 2.567 | |||
| Obese | 18 (28.6) | 45 (71.4) | 0.894 | 0.808, 0.988 | 0.929 | 0.855, 1.01 | 1.358 | 1.041, 1.771 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, W.; Huang, L.; Yan, M.; Zhang, W.; Song, J.; Liu, X.; Yu, W.; Lü, Y. Gait Indicators Contribute to Screening Cognitive Impairment: A Single- and Dual-Task Gait Study. Brain Sci. 2023, 13, 154. https://doi.org/10.3390/brainsci13010154
Wang X, Yu W, Huang L, Yan M, Zhang W, Song J, Liu X, Yu W, Lü Y. Gait Indicators Contribute to Screening Cognitive Impairment: A Single- and Dual-Task Gait Study. Brain Sciences. 2023; 13(1):154. https://doi.org/10.3390/brainsci13010154
Chicago/Turabian StyleWang, Xiaoqin, Wuhan Yu, Lihong Huang, Mengyu Yan, Wenbo Zhang, Jiaqi Song, Xintong Liu, Weihua Yu, and Yang Lü. 2023. "Gait Indicators Contribute to Screening Cognitive Impairment: A Single- and Dual-Task Gait Study" Brain Sciences 13, no. 1: 154. https://doi.org/10.3390/brainsci13010154
APA StyleWang, X., Yu, W., Huang, L., Yan, M., Zhang, W., Song, J., Liu, X., Yu, W., & Lü, Y. (2023). Gait Indicators Contribute to Screening Cognitive Impairment: A Single- and Dual-Task Gait Study. Brain Sciences, 13(1), 154. https://doi.org/10.3390/brainsci13010154





