Sacrifice of Involved Nerve Root during Surgical Resection of Foraminal and/or Dumbbell Spinal Neurinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Surgical Procedures and Tumor Recurrence
3.3. Neurological Outcomes of the Entire Cohort
3.4. Motor Outcome for Functional Roots
3.5. Histopathology
3.6. Risk Factor Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tish, S.; Habboub, G.; Lang, M.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S.; Recinos, P.F.; Kshettry, V.R. The Epidemiology of Spinal Schwannoma in the United States between 2006 and 2014. J. Neurosurg. Spine 2020, 32, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, K.A.; Propp, J.M.; Villano, J.L.; McCarthy, B.J. Descriptive Epidemiology of Primary Spinal Cord Tumors. J. Neurooncol. 2008, 87, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, J.W.M.; Van Den Hauwe, L.; Özsarlak, Ö.; De Schepper, A.M.A.; Parizel, P.M. Spinal Tumors. Eur. J. Radiol. 2004, 50, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, M.T.; Haltia, M.J.; Sankila, R.J.; Jääskeläinen, J.E.; Heiskanen, O. Long-Term Outcome after Removal of Spinal Schwannoma: A Clinicopathological Study of 187 Cases. J. Neurosurg. 1995, 83, 621–626. [Google Scholar] [CrossRef]
- Kaneko, K.; Kato, Y.; Kojima, T.; Imajyo, Y.; Taguchi, T. Intraoperative Electrophysiologic Studies on the Functions of Nerve Roots Involved in Cervical Dumbbell-Shaped Schwannoma and Their Clinical Utility. J. Spinal Disord. Tech. 2006, 19, 571–576. [Google Scholar] [CrossRef]
- Lot, G.; George, B. Cervical Neuromas with Extradural Components: Surgical Management in a Series of 57 Patients. Neurosurgery 1997, 41, 812–813. [Google Scholar] [CrossRef]
- Safaee, M.M.; Lyon, R.; Barbaro, N.M.; Chou, D.; Mummaneni, P.V.; Weinstein, P.R.; Chin, C.T.; Tihan, T.; Ames, C.P. Neurological Outcomes and Surgical Complications in 221 Spinal Nerve Sheath Tumors. J. Neurosurg. Spine 2017, 26, 103–111. [Google Scholar] [CrossRef]
- McCormick, P.C. Surgical Management of Dumbbell Tumors of the Cervical Spine. Neurosurgery 1996, 38, 294–300. [Google Scholar] [CrossRef]
- Moses, Z.B.; Barzilai, O.; O’Toole, J.E. Benign Intradural and Paraspinal Nerve Sheath Tumors: Advanced Surgical Techniques. Neurosurg. Clin. N. Am. 2020, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; O’Doherty, M.J. Neurofibroma and Schwannoma. Curr. Opin. Neurol. 2002, 15, 679–684. [Google Scholar] [CrossRef]
- Farid, M.; Demicco, E.G.; Garcia, R.; Ahn, L.; Merola, P.R.; Cioffi, A.; Maki, R.G. Malignant Peripheral Nerve Sheath Tumors. Oncologist 2014, 19, 193–201. [Google Scholar] [CrossRef]
- Klekamp, J.; Samii, M. Surgery of Spinal Nerve Sheath Tumors with Special Reference to Neurofibromatosis. Neurosurgery 1998, 42, 279–290. [Google Scholar] [CrossRef]
- Celli, P. Treatment of Relevant Nerve Roots Involved in Nerve Sheath Tumors: Removal or Preservation? Neurosurgery 2002, 51, 684–692, discussion 692. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Ebersold, M.J.; Onofrio, B.M.; Quast, L.M. Surgery of Spinal Nerve Schwannoma. Risk of Neurological Deficit after Resection of Involved Root. J. Neurosurg. 1989, 71, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Guan, Y.; Jiang, J.; Lu, F.; Chen, W.; Xia, X.; Wang, L.; Ma, X. Factors Affecting Postoperative Neurological Deficits after Nerve Root Resection for the Treatment of Spinal Intradural Schwannomas. Spine 2016, 41, 384–389. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujisawa, H.; Hayashi, Y.; Tachibana, O.; Kida, S.; Yamashita, J. Surgical Pathology of Spinal Schwannoma: Has the Nerve of Its Origin Been Preserved or Already Degenerated during Tumor Growth? Clin. Neuropathol. 2005, 24, 19–25. [Google Scholar] [PubMed]
- Nanda, A.; Kukreja, S.; Ambekar, S.; Bollam, P.; Sin, A.H. Surgical Strategies in the Management of Spinal Nerve Sheath Tumors. World Neurosurg. 2015, 83, 886–899. [Google Scholar] [CrossRef]
- Safavi-Abbasi, S.; Senoglu, M.; Theodore, N.; Workman, R.K.; Gharabaghi, A.; Feiz-Erfan, I.; Spetzler, R.F.; Sonntag, V.K.H. Microsurgical Management of Spinal Schwannomas: Evaluation of 128 Cases. J. Neurosurg. Spine 2008, 9, 40–47. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Kögl, N.; Meyer, B.; Thomé, C.; Wostrack, M. A Case Series of Surgically Treated Spinal Dumbbell Tumors of Critical Parent Nerve Roots: To Cut or Not to Cut? Oper. Neurosurg. 2021, 20, 260–267. [Google Scholar] [CrossRef]
- Parlak, A.; Oppong, M.D.; Jabbarli, R.; Gembruch, O.; Dammann, P.; Wrede, K.; Rauschenbach, L.; Sure, U.; Özkan, N. Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma? Medicina 2022, 58, 357. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nater, A.; Zamorano, J.J.; Tetreault, L.A.; Varga, P.P.; Gokaslan, Z.L.; Boriani, S.; Fisher, C.G.; Rhines, L.; Bettegowda, C.; et al. Risk Factors for Recurrence of Surgically Treated Conventional Spinal Schwannomas: Analysis of 169 Patients From a Multicenter International Database. Spine 2016, 41, 390–398. [Google Scholar] [CrossRef]
- Sridhar, K.; Ramamurthi, R.; Vasudevan, M.C.; Ramamurthi, B. Giant Invasive Spinal Schwannomas: Definition and Surgical Management. J. Neurosurg. 2001, 94, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, R.; Gullotta, G. Resection of Relevant Nerve Roots in Surgery of Spinal Neurinomas without Persisting Neurological Deficit. Acta Neurochir. 1993, 122, 91–96. [Google Scholar] [CrossRef]
- Yamane, K.; Takigawa, T.; Tanaka, M.; Osaki, S.; Sugimoto, Y.; Ozaki, T. Factors Predicting Clinical Impairment after Surgery for Cervical Spinal Schwannoma. Acta Medica Okayama 2013, 67, 343–349. [Google Scholar] [PubMed]
- Butenschoen, V.M.; Nehiba, A.; Meyer, B.; Wostrack, M. Neuropathic Pain after Spinal Intradural Benign Tumor Surgery: An Underestimated Complication? Neurosurg. Rev. 2022, 45, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, D.-C.; Rhim, S.C.; Lee, B.J.; Hong, S.H.; Koo, Y.S.; Park, J.H. Intraoperative Monitoring for Cauda Equina Tumors: Surgical Outcomes and Neurophysiological Data Accrued Over 10 Years. Neurospine 2021, 18, 281–289. [Google Scholar] [CrossRef]
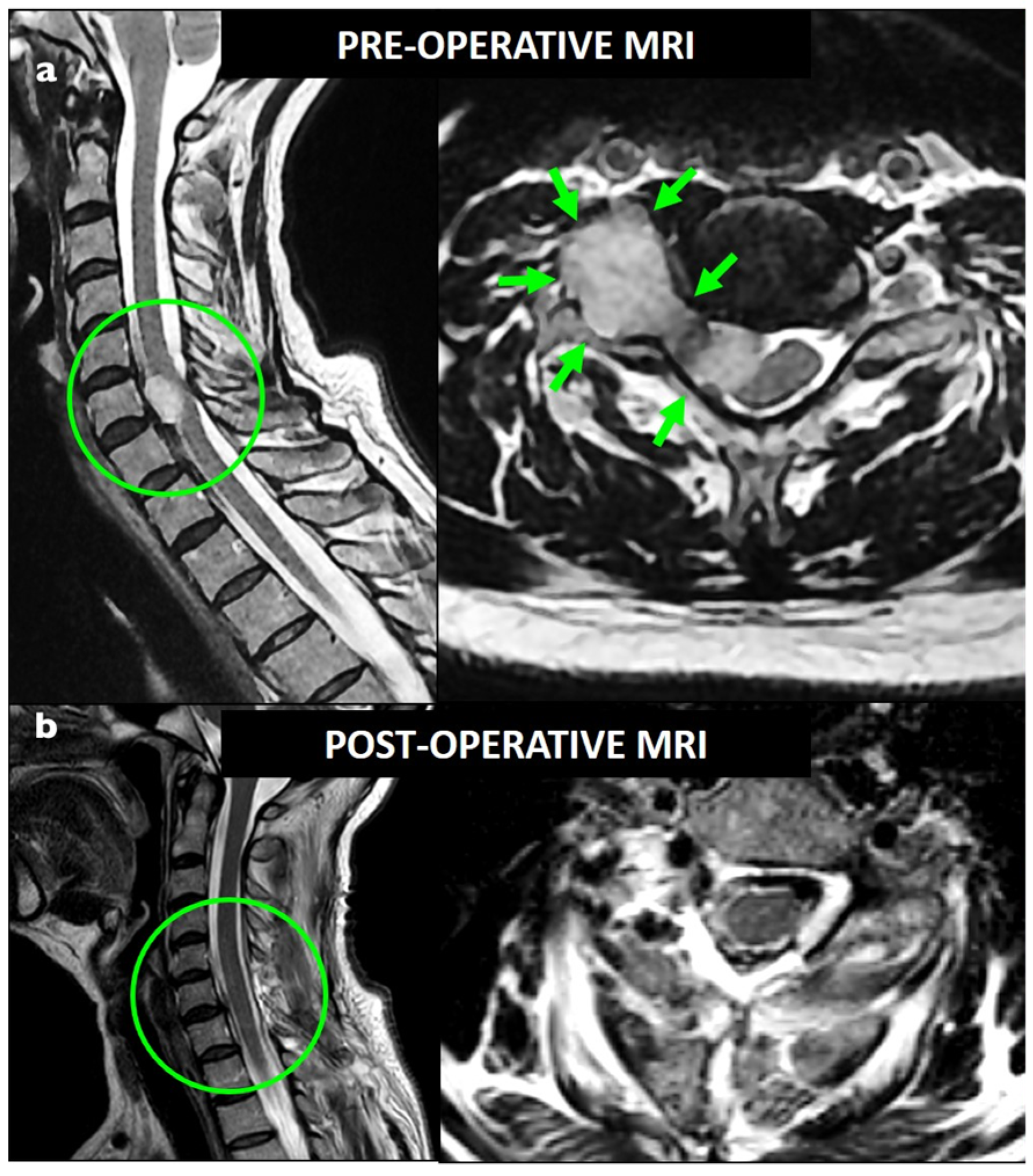
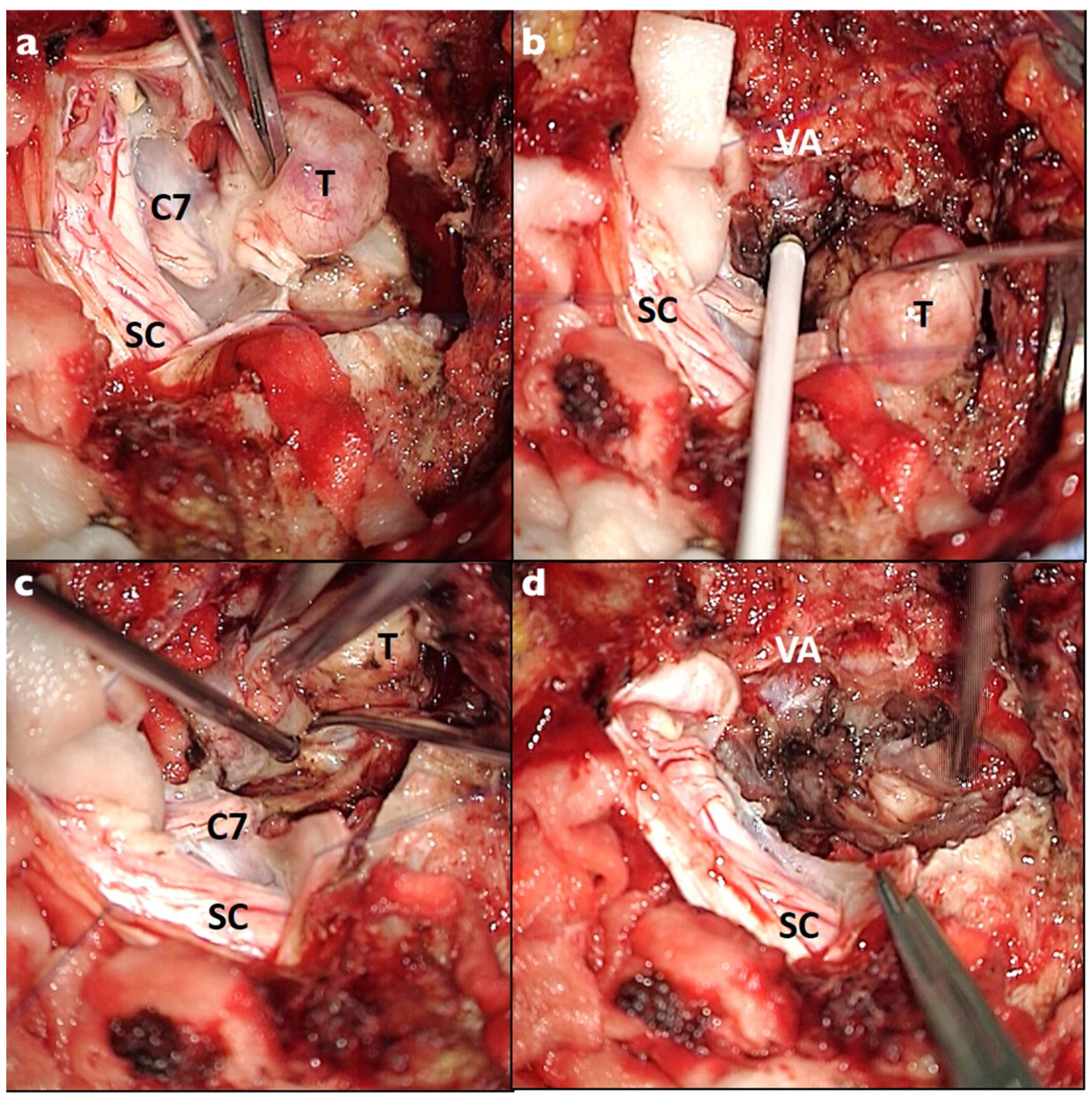

| Characteristics | Total Population, n = 26 |
|---|---|
| Age, mean ± SD, years | 47 ± 16 |
| Sex, male, n (%) | 14 (54) |
| Location, n (%) | |
| Cervical | 10 (38.5) |
| Thoracic | 3 (11.5) |
| Lumbar | 13 (50) |
| Side, n (%) | |
| Left | 12 (46.2) |
| Right | 14 (53.8) |
| Size, mean ± SD, mm | |
| Transverse | 45.4 ± 19.1 |
| Antero-posterior | 34.5 ± 12.8 |
| Foraminal enlargement, n (%) | 20 (77) |
| Extension in multiple foramen, n (%) | 4 (15.4) |
| Symptom duration mean ± SD, months | 21 ± 19.9 |
| Clinical presentation | |
| Radicular pain, n (%) | 11 (42) |
| Motor deficit, n (%) * | 5 (35.7) |
| Sensory loss, n (%) | 1 (3.8) |
| Axial pain, n (%) | 18 (69) |
| Medullary, n (%) | 10 (38.5) |
| Motor, n (%) | 8 (30.8) |
| Sensory, n (%) | 10 (38.5) |
| Sphincteric, n (%) | 1 (3.8) |
| C5-T1, n (%) * | 5 (35.7) |
| L3-S1, n (%) * | 8 (64.3) |
| Sridhar classification | |
| I, II (intraspinal) | 0 (0) |
| III (foraminal extension) | 8 (30.8) |
| IVA (dumbbell with extraspinal extension <2.5 cm) | 4 (15.4) |
| IVB (dumbbell with extraspinal extension ≥2.5 cm) | 8 (30.8) |
| V (giant erosive tumor) | 6 (23) |
| Characteristics | Total Population, n = 26 |
|---|---|
| GTR, n (%) | 22 (84.6) |
| STR, n (%) | 4 (15.4) |
| Surgical approach | |
| Posterior, n (%) | 22 (84.6) |
| Combined, n (%) | 4 (15.4) |
| Instrumentation, n (%) | 3 (11.5) |
| Surgical complications other than neurological | |
| CSF leak, n (%) | 2 (7.7) |
| Wound infection, n (%) | 2 (7.7) |
| Others | 0 (0) |
| Follow-up, mean ± SD (min-max), months | 22.4 ± 20.3 (6–85) |
| Characteristics | Total Population, n = 26 |
|---|---|
| Permanent postoperative radicular pain, n (%) | 6 (23) |
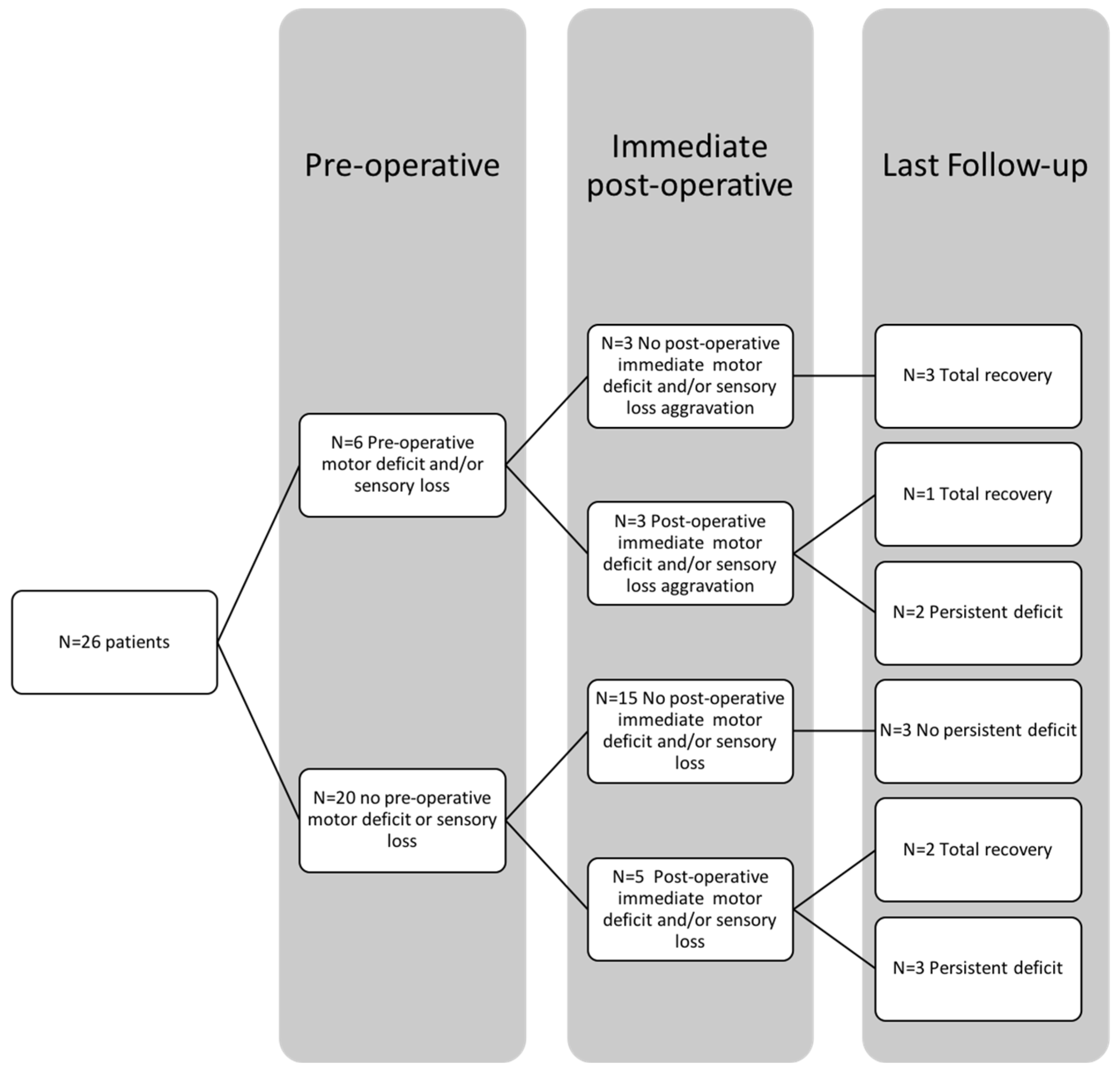
| Immediate postoperative radicular deficit #, n (%) | 8 (30.8) |
| Motor, n (%) | 1 (3.8) |
| Sensory, n (%) | 3 (11.5) |
| Motor and sensory, n (%) | 4 (15.4) |
| Permanent postoperative radicular deficit, n (%) | 5 (19.2) |
| Motor, n (%) | 2 (7.7) |
| Sensory, n (%) | 3 (11.5) |
| Motor and sensory, n (%) | 0 (0) |
| Temporary postoperative myelopathy, n (%) | 2 (7.7) |
| Preoperative myelopathy evolution, n (%) | 10 (40) |
| Recovery *, n (%) | 6 (60) |
| Improvements *, n (%) | 4 (40) |
| Characteristics | Population, n = 14 |
|---|---|
| Preoperative radicular pain, n (%) | 8 (57.1) |
| Preoperative motor deficit, n (%) | 5 (35.7) |
| Immediate postoperative motor deficit #, n (%) | 5 (35.7) |
| Persistent postoperative motor deficit #, n (%) | 2 (14.2) |
| Risk Factors/Variables | Population | Immediate Radicular Deficit | p Value | Persistent Radicular Deficit | p Value |
|---|---|---|---|---|---|
| Clinical presentation | |||||
| No preoperative motor deficit and/or sensory loss, n (%) | 20 | 5 (25) | 3 (15) | ||
| Preoperative motor deficit and/or sensory loss, n (%) | 6 | 3 (50) | 0.33 | 2 (33) | 0.56 |
| No preoperative radicular pain, n (%) | 15 | 2 (13) | 0 (0) | ||
| Preoperative radicular pain, n (%) | 11 | 6 (55) | 0.03 | 5 (45) | 0.007 |
| No preoperative myelopathy, n (%) | 16 | 6 (37.5) | 4 (25) | ||
| Preoperative myelopathy, n (%) | 10 | 2 (20) | 0.40 | 1 (10) | 0.61 |
| Medical history, mean ± SD, years | 22.7 ± 15.0 * | 17.6 ± 23.3 | 0.50 | ||
| Medical history, mean ± SD, years | 22.1 ± 16.7 ** | 17.54 ± 30.5 | 0.63 | ||
| No foraminal enlargement, n (%) | 6 | 1 (17) | 1 (17) | ||
| Foraminal enlargement, n (%) | 20 | 7 (35) | 0.63 | 4 (20) | 1 |
| No extension in multiple foramen, n (%) | 22 | 6 (27) | 3 (14) | ||
| Extension in multiple foramen, n (%) | 4 | 2 (50) | 0.56 | 2 (50) | 0.15 |
| Location | |||||
| Cervical, n (%) | 10 | 3 (30) | 1 | 1 (10) | 0.61 |
| Thoracic, n (%) | 3 | 0 (0) | 0.52 | 0 (0) | 1 |
| Lumbar, n (%) | 13 | 5 (38.5) | 0.67 | 4 (31) | 0.32 |
| Sex | |||||
| Female, n | 12 | 5 (42) | 4 (33) | ||
| Male, n | 14 | 3 (21) | 0.67 | 1 (7) | 0.14 |
| Age, mean ± SD, years | 45.2 ± 24 * | 50.8 ± 14.8 | 0.60 | ||
| Age, mean ± SD, years | 44.7 ± 16 ** | 56.4 ± 11.6 | 0.13 | ||
| Resection | |||||
| GTR | 22 | 7 (32) | 5 (23) | ||
| STR | 4 | 1 (25) | 1 | 0 (0) | 0.55 |
| Histology | |||||
| Schwannoma WHO I, n (%) | 24 | 7 (29) | 5 (21) | ||
| Neurofibroma WHO I, n (%) | 2 | 1 (50) | 0.53 | 0 (0) | 1 |
| Sridhar grade | |||||
| Sridhar III, n (%) | 8 | 1 (12.5) | 0.36 | 0 (0) | 0.15 |
| Sridhar grade IV, n (%) | 12 | 4 (33) | 1 | 2 (17) | 1 |
| Sridhar grade V, n (%) | 6 | 3 (50) | 0.33 | 3 (50) | 0.06 |
| Diameter | |||||
| Transverse, mean ± SD, mm | 43.5 ± 18.5 * | 50.3 ± 7.6 | 0.33 | ||
| Transverse, mean ± SD, mm | 41.8 ± 19.2 ** | 58.7 ± 15.3 | 0.08 | ||
| Antero-posterior, mean ± SD, mm | 33.9 ± 14.2 * | 36.3 ± 6.3 | 0.65 | ||
| Antero-posterior, mean ± SD, mm | 33.9 ± 12 ** | 36.3 ± 12 | 0.71 | ||
| Risk Factors/Variables | Population | Immediate Motor Deficit | p Value | Persistent Motor Deficit | p Value |
|---|---|---|---|---|---|
| Clinical presentation | |||||
| No preoperative motor deficit and/or sensory loss, n (%) | 9 | 2 (22) | 1 (11) | ||
| Preoperative motor deficit and/or sensory loss, n (%) | 5 | 3 (60) | 0.26 | 1 (20) | 1 |
| No preoperative radicular pain, n (%) | 6 | 0 (0) | 0 (0) | ||
| Preoperative radicular pain, n (%) | 8 | 5 (63) | 0.03 | 2 (25) | 0.47 |
| No preoperative myelopathy, n (%) | 10 | 5 (50%) | 2 (20) | ||
| Preoperative myelopathy, n (%) | 4 | 0 (0) | 0.22 | 0 (0) | 1 |
| Medical history, mean ± SD, years | 25.5 ± 20.7 * | 21.2 ± 29.8 | 0.75 | ||
| Medical history, mean ± SD, years | 27.8 ± 23.4 ** | 2 ± 0 | 0.15 | ||
| No foraminal enlargement, n (%) | 3 | 1 (33) | 0 (0) | ||
| Foraminal enlargement, n (%) | 11 | 4 (36) | 1 | 2 (18) | 1 |
| No extension in multiple foramen, n (%) | 11 | 4 (36) | 1 (9) | ||
| Extension in multiple foramen, n (%) | 3 | 1 (33) | 1 | 1 (33) | 0.39 |
| Location | |||||
| Cervical, n | 5 | 1 (20) | 0 (0) | ||
| Lumbar, n | 9 | 4 (44) | 0.58 | 2 (22) | 0.50 |
| Sex | |||||
| Female, n | 7 | 4 (57) | 1 (14) | ||
| Male, n | 7 | 1 (14) | 0.26 | 1 (14) | 1 |
| Age, mean ± SD, years | 47.6 ± 19.7 * | 54.4 ± 9.3 | 0.45 | ||
| Age, mean ± SD, years | 49 ± 16.8 ** | 56.5 ± 10.6 | 0.19 | ||
| Resection | |||||
| GTR | 12 | 5 (42) | 2 (17) | ||
| STR | 4 | 0 (0) | 0.10 | 0 (0) | 1 |
| Histology | |||||
| Schwannoma WHO I, n (%) | 12 | 4 (33) | 2 (17) | ||
| Neurofibroma WHO I, n (%) | 2 | 1 (50) | 1 | 0 (0) | 1 |
| Tumor extension | |||||
| Sridhar grade III | 3 | 0 (0) | 0.25 | 0 (0) | 1 |
| Sridhar grade IV | 7 | 3 (43) | 1 | 0 (0) | 0.46 |
| Sridhar grade V | 4 | 2 (50) | 0.53 | 2 (50) | 0.06 |
| Diameter | |||||
| Transverse, mean ± SD, mm | 38.6 ± 20.2 * | 58.7 ± 9.3 | 0.06 | ||
| Transverse, mean ± SD, mm | 41.9 ± 21 ** | 55.5 ± 10.6 | 0.39 | ||
| Antero-posterior, mean ± SD, mm | 30.6 ± 11.2 * | 36.3 ± 7.7 | 0.32 | ||
| Antero-posterior, mean ± SD, mm | 30.5 ± 10.3 ** | 39.5 ± 7.8 | 0.26 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandenbulcke, A.; D’Onofrio, G.F.; Capo, G.; Baassiri, W.; Barrey, C.Y. Sacrifice of Involved Nerve Root during Surgical Resection of Foraminal and/or Dumbbell Spinal Neurinomas. Brain Sci. 2023, 13, 109. https://doi.org/10.3390/brainsci13010109
Vandenbulcke A, D’Onofrio GF, Capo G, Baassiri W, Barrey CY. Sacrifice of Involved Nerve Root during Surgical Resection of Foraminal and/or Dumbbell Spinal Neurinomas. Brain Sciences. 2023; 13(1):109. https://doi.org/10.3390/brainsci13010109
Chicago/Turabian StyleVandenbulcke, Alberto, Ginevra Federica D’Onofrio, Gabriele Capo, Wassim Baassiri, and Cédric Y. Barrey. 2023. "Sacrifice of Involved Nerve Root during Surgical Resection of Foraminal and/or Dumbbell Spinal Neurinomas" Brain Sciences 13, no. 1: 109. https://doi.org/10.3390/brainsci13010109
APA StyleVandenbulcke, A., D’Onofrio, G. F., Capo, G., Baassiri, W., & Barrey, C. Y. (2023). Sacrifice of Involved Nerve Root during Surgical Resection of Foraminal and/or Dumbbell Spinal Neurinomas. Brain Sciences, 13(1), 109. https://doi.org/10.3390/brainsci13010109






