Validating a Portable Device for Blinking Analyses through Laboratory Neurophysiological Techniques
Abstract
1. Introduction
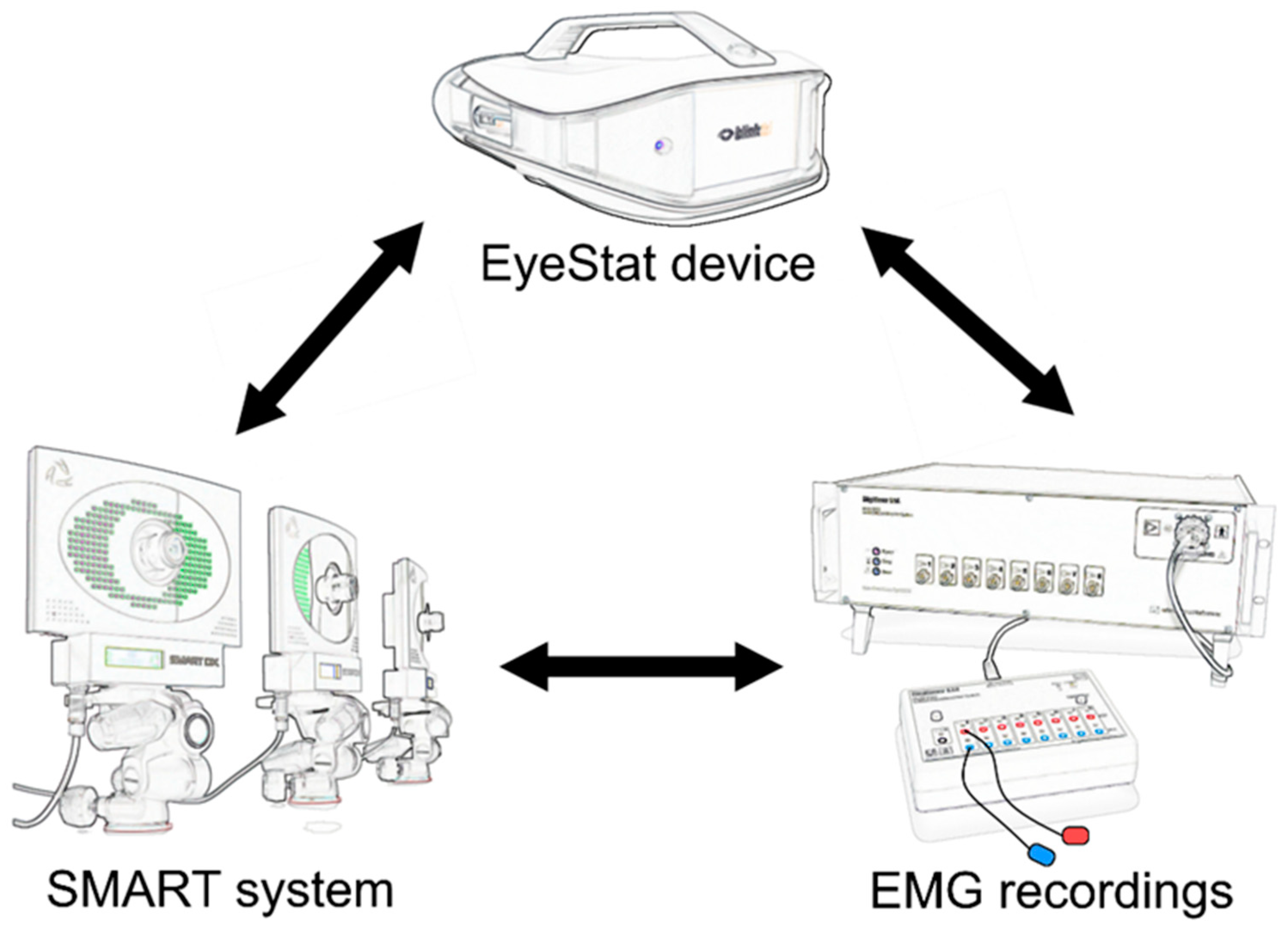
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. EyeStat Recordings and Analysis
2.4. Kinematic Recordings and Analysis
2.5. EMG Recordings and Analysis
2.6. Statistical Analysis
3. Results
3.1. OO-EMG Analysis
3.2. Voluntary Blinking
3.3. Spontaneous Blinking
3.4. Spontaneous Blinking
3.5. Reflex Blinking
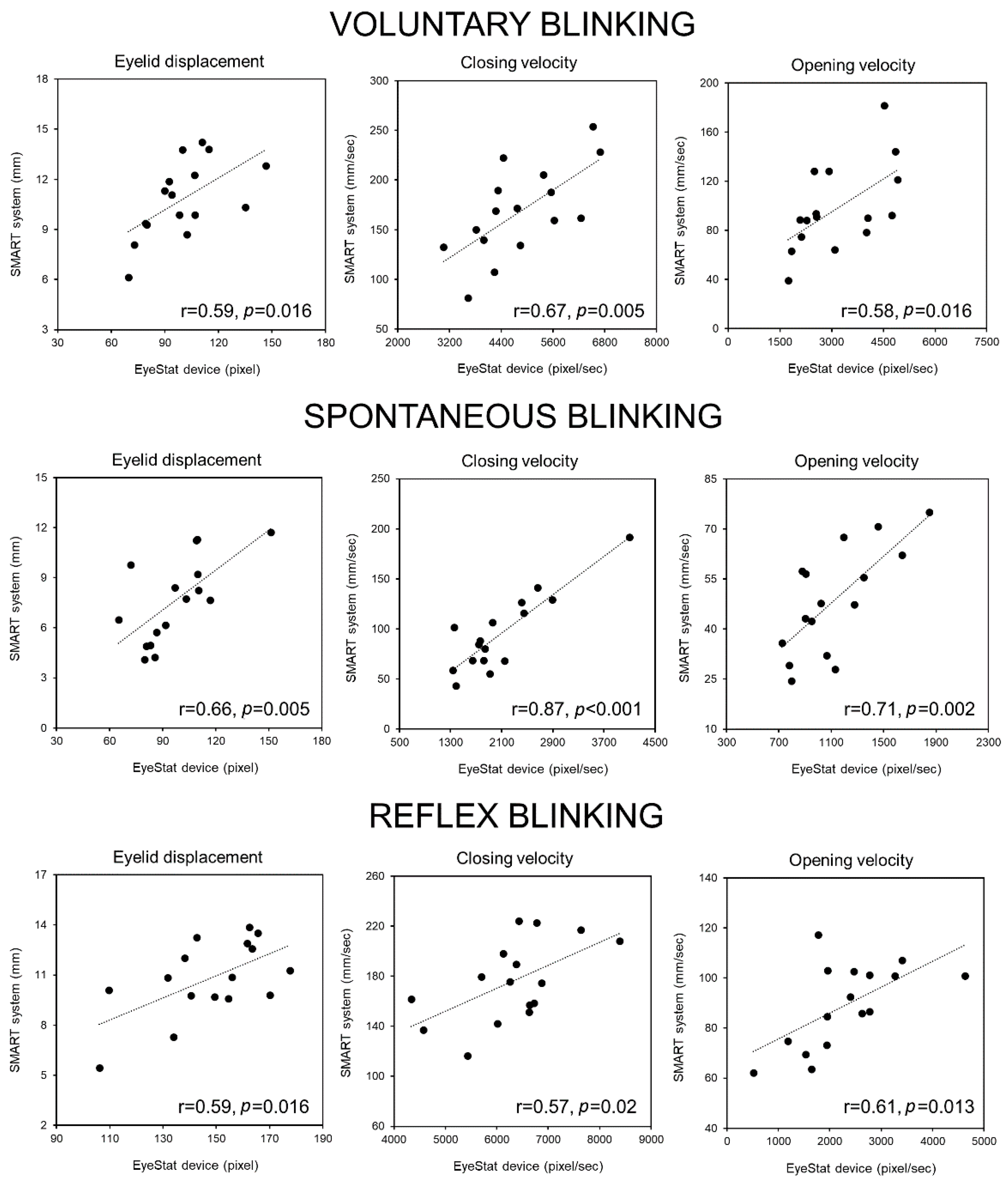
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bologna, M.; Fabbrini, G.; Marsili, L.; Defazio, G.; Thompson, P.D.; Berardelli, A. Facial Bradykinesia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Traba, A.; Prieto, J. Eyelid Movements in Health and Disease. The Supranuclear Impairment of the Palpebral Motility. Neurophysiol. Clin. Clin. Neurophysiol. 2004, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, K.; Büttner-Ennever, J.A. Nervous Control of Eyelid Function. A Review of Clinical, Experimental and Pathological Data. Brain 1992, 115, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Bour, L.J.; Aramideh, M.; de Visser, B.W. Neurophysiological Aspects of Eye and Eyelid Movements during Blinking in Humans. J. Neurophysiol. 2000, 83, 166–176. [Google Scholar] [CrossRef]
- Cruccu, G.; Iannetti, G.D.; Marx, J.J.; Thoemke, F.; Truini, A.; Fitzek, S.; Galeotti, F.; Urban, P.P.; Romaniello, A.; Stoeter, P.; et al. Brainstem Reflex Circuits Revisited. Brain 2005, 128, 386–394. [Google Scholar] [CrossRef]
- Paparella, G.; Di Stefano, G.; Fasolino, A.; Di Pietro, G.; Colella, D.; Truini, A.; Cruccu, G.; Berardelli, A.; Bologna, M. Painful Stimulation Increases Spontaneous Blink Rate in Healthy Subjects. Sci. Rep. 2020, 10, 20014. [Google Scholar] [CrossRef]
- VanderWerf, F.; Brassinga, P.; Reits, D.; Aramideh, M.; de Visser, B.O. Eyelid Movements: Behavioral Studies of Blinking in Humans under Different Stimulus Conditions. J. Neurophysiol. 2003, 89, 2784–2796. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Voller, B.; Dimyan, M.; St Clair Gibson, A.; Hanakawa, T.; Leon-Sarmiento, F.E.; Jung, H.Y.; Hallett, M. Cortical Control of Voluntary Blinking: A Transcranial Magnetic Stimulation Study. Clin. Neurophysiol. 2004, 115, 341–347. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kiyosawa, M.; Mochizuki, M.; Ishiwata, K.; Ishii, K. The Pre-Supplementary and Primary Motor Areas Generate Rhythm for Voluntary Eye Opening and Closing Movements. Tohoku J. Exp. Med. 2010, 222, 97–104. [Google Scholar] [CrossRef]
- Agostino, R.; Bologna, M.; Dinapoli, L.; Gregori, B.; Fabbrini, G.; Accornero, N.; Berardelli, A. Voluntary, Spontaneous, and Reflex Blinking in Parkinson’s Disease. Mov. Disord. 2008, 23, 669–675. [Google Scholar] [CrossRef]
- Bologna, M.; Agostino, R.; Gregori, B.; Belvisi, D.; Ottaviani, D.; Colosimo, C.; Fabbrini, G.; Berardelli, A. Voluntary, Spontaneous and Reflex Blinking in Patients with Clinically Probable Progressive Supranuclear Palsy. Brain 2009, 132, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Fasano, A.; Modugno, N.; Fabbrini, G.; Berardelli, A. Effects of Subthalamic Nucleus Deep Brain Stimulation and L-DOPA on Blinking in Parkinson’s Disease. Exp. Neurol. 2012, 235, 265–272. [Google Scholar] [CrossRef]
- Bologna, M.; Marsili, L.; Khan, N.; Parvez, A.K.; Paparella, G.; Modugno, N.; Colosimo, C.; Fabbrini, G.; Berardelli, A. Blinking in Patients with Clinically Probable Multiple System Atrophy. Mov. Disord. 2014, 29, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Berardelli, I.; Paparella, G.; Marsili, L.; Ricciardi, L.; Fabbrini, G.; Berardelli, A. Altered Kinematics of Facial Emotion Expression and Emotion Recognition Deficits Are Unrelated in Parkinson’s Disease. Front. Neurol. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Piattella, M.C.; Upadhyay, N.; Formica, A.; Conte, A.; Colosimo, C.; Pantano, P.; Berardelli, A. Neuroimaging Correlates of Blinking Abnormalities in Patients with Progressive Supranuclear Palsy. Mov. Disord. 2016, 31, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Agostino, R.; Gregori, B.; Belvisi, D.; Manfredi, M.; Berardelli, A. Metaplasticity of the Human Trigeminal Blink Reflex. Eur. J. Neurosci. 2010, 32, 1707–1714. [Google Scholar] [CrossRef]
- Karson, C.N. Spontaneous Eye-Blink Rates and Dopaminergic Systems. Brain 1983, 106, 643–653. [Google Scholar] [CrossRef]
- Karson, C.N.; Burns, R.S.; LeWitt, P.A.; Foster, N.L.; Newman, R.P. Blink Rates and Disorders of Movement. Neurology 1984, 34, 677–678. [Google Scholar] [CrossRef]
- Kleinman, J.E.; Karson, C.N.; Weinberger, D.R.; Freed, W.J.; Berman, K.F.; Wyatt, R.J. Eye-Blinking and Cerebral Ventricular Size in Chronic Schizophrenic Patients. Am. J. Psychiatry 1984, 141, 1430–1432. [Google Scholar] [CrossRef]
- Basso, M.A.; Evinger, C. An Explanation for Reflex Blink Hyperexcitability in Parkinson’s Disease. II. Nucleus Raphe Magnus. J. Neurosci. 1996, 16, 7318–7330. [Google Scholar] [CrossRef]
- Berardelli, A.; Cruccu, G.; Manfredi, M.; Rothwell, J.C.; Day, B.L.; Marsden, C.D. The Corneal Reflex and the R2 Component of the Blink Reflex. Neurology 1985, 35, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Accornero, N.; Cruccu, G.; Fabiano, F.; Guerrisi, V.; Manfredi, M. The Orbicularis Oculi Response after Hemispheral Damage. J. Neurol. Neurosurg. Psychiatry 1983, 46, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Frontoni, M.; Cruccu, G. Parkinson’s Disease Related Pain: A Review of Recent Findings. J. Neurol. 2013, 260, 330–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valls-Solé, J.; Hallett, M. Brainstem Functions and Reflexes. J. Clin. Neurophysiol. 2019, 36, 395. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.A.; Powers, A.S.; Evinger, C. An Explanation for Reflex Blink Hyperexcitability in Parkinson’s Disease. I. Superior Colliculus. J. Neurosci. 1996, 16, 7308–7317. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, K.D.; Rider, A.; Potter, W.; Jensen, J.; Fowler, B.T.; Fleming, J.C. Eyelid Spontaneous Blink Analysis and Age-Related Changes Through High-Speed Imaging. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Domenech, B.; Vázquez, C.; Pérez, J.; Mas, D. Blinking Characterization from High Speed Video Records. Application to Biometric Authentication. PLoS ONE 2018, 13, e0196125. [Google Scholar] [CrossRef]
- Godfrey, K.J.; Wilsen, C.; Satterfield, K.; Korn, B.S.; Kikkawa, D.O. Analysis of Spontaneous Eyelid Blink Dynamics Using a 240 Frames per Second Smartphone Camera. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 503–505. [Google Scholar] [CrossRef]
- Kwon, K.-A.; Shipley, R.J.; Edirisinghe, M.; Ezra, D.G.; Rose, G.; Best, S.M.; Cameron, R.E. High-Speed Camera Characterization of Voluntary Eye Blinking Kinematics. J. R. Soc. Interface 2013, 10, 20130227. [Google Scholar] [CrossRef]
- Sanchis-Jurado, V.; Talens-Estarelles, C.; Esteve-Taboada, J.J.; Pons, Á.M.; García-Lázaro, S. Non-Invasive High-Speed Blinking Kinematics Characterization. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2701–2714. [Google Scholar] [CrossRef]
- Wambier, S.P.F.; Ribeiro, S.F.; Garcia, D.M.; Brigato, R.R.; Messias, A.; Cruz, A.A.V. Two-Dimensional Video Analysis of the Upper Eyelid Motion during Spontaneous Blinking. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.T.; Goodwin, J.S.; Semler, M.E.; Kothera, R.T.; Van Horn, M.; Wolf, B.J.; Garner, D.P. Development of a Non-Invasive Blink Reflexometer. IEEE J. Transl. Eng. Health Med. 2017, 5, 3800204. [Google Scholar] [CrossRef] [PubMed]
- Yengo-Kahn, A.M.; Garner, D.P.; Lessing, N.; Blough, J.; Zuckerman, S.L.; Gifford, K. Normative Blink Reflex Data for the EyeStat Device in Student Athletes. Cogent Eng. 2022, 9, 2024971. [Google Scholar] [CrossRef]
- Kimura, J. Clinical Uses of the Electrically Elicited Blink Reflex. Adv. Neurol. 1983, 39, 773–786. [Google Scholar] [PubMed]
- Hoffland, B.S.; Bologna, M.; Kassavetis, P.; Teo, J.T.H.; Rothwell, J.C.; Yeo, C.H.; van de Warrenburg, B.P.; Edwards, M.J. Cerebellar Theta Burst Stimulation Impairs Eyeblink Classical Conditioning. J. Physiol. 2012, 590, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Bologna, M.; Paparella, G.; Suppa, A.; Colella, D.; Di Lazzaro, V.; Brown, P.; Berardelli, A. Effects of Transcranial Alternating Current Stimulation on Repetitive Finger Movements in Healthy Humans. Neural Plast. 2018, 2018, 4593095. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Alvaro, P.K.; Jackson, M.L.; Berlowitz, D.J.; Swann, P.; Howard, M.E. Prolonged Eyelid Closure Episodes during Sleep Deprivation in Professional Drivers. J. Clin. Sleep Med. 2016, 12, 1099–1103. [Google Scholar] [CrossRef]
- Cori, J.M.; Anderson, C.; Soleimanloo, S.S.; Jackson, M.L.; Howard, M.E. Narrative Review: Do Spontaneous Eye Blink Parameters Provide a Useful Assessment of State Drowsiness? Sleep Med. Rev. 2019, 45, 95–104. [Google Scholar] [CrossRef]
- Rodriguez, J.D.; Lane, K.J.; Ousler, G.W.; Angjeli, E.; Smith, L.M.; Abelson, M.B. Blink: Characteristics, Controls, and Relation to Dry Eyes. Curr. Eye Res. 2018, 43, 52–66. [Google Scholar] [CrossRef]
- Sun, W.S.; Baker, R.S.; Chuke, J.C.; Rouholiman, B.R.; Hasan, S.A.; Gaza, W.; Stava, M.W.; Porter, J.D. Age-Related Changes in Human Blinks. Passive and Active Changes in Eyelid Kinematics. Investig. Ophthalmol. Vis. Sci. 1997, 38, 92–99. [Google Scholar]
- Tsujikawal, M.; Onishil, Y.; Kiuchil, Y.; Ogatsul, T.; Nishino, A.; Hashimoto, S. Drowsiness Estimation from Low-Frame-Rate Facial Videos Using Eyelid Variability Features. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5203–5206. [Google Scholar] [CrossRef]
- Deuschl, G.; Goddemeier, C. Spontaneous and Reflex Activity of Facial Muscles in Dystonia, Parkinson’s Disease, and in Normal Subjects. J. Neurol. Neurosurg. Psychiatry 1998, 64, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Evinger, C.; Shaw, M.D.; Peck, C.K.; Manning, K.A.; Baker, R. Blinking and Associated Eye Movements in Humans, Guinea Pigs, and Rabbits. J. Neurophysiol. 1984, 52, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Kimber, T.E.; Thompson, P.D. Increased Blink Rate in Advanced Parkinson’s Disease: A Form of ’off’-Period Dystonia? Mov. Disord. 2000, 15, 982–985. [Google Scholar] [CrossRef]
- Korosec, M.; Zidar, I.; Reits, D.; Evinger, C.; Vanderwerf, F. Eyelid Movements during Blinking in Patients with Parkinson’s Disease. Mov. Disord. 2006, 21, 1248–1251. [Google Scholar] [CrossRef]
- Defazio, G.; Antonini, A.; Tinazzi, M.; Gigante, A.F.; Pietracupa, S.; Pellicciari, R.; Bloise, M.; Bacchin, R.; Marcante, A.; Fabbrini, G.; et al. Relationship between Pain and Motor and Non-Motor Symptoms in Parkinson’s Disease. Eur. J. Neurol. 2017, 24, 974–980. [Google Scholar] [CrossRef]
- Meyer, P.J.; Morgan, M.M.; Kozell, L.B.; Ingram, S.L. Contribution of Dopamine Receptors to Periaqueductal Gray-Mediated Antinociception. Psychopharmacology 2009, 204, 531–540. [Google Scholar] [CrossRef]
- Garner, D.P.; Goodwin, J.S.; Tsai, N.T.; Kothera, R.T.; Semler, M.E.; Wolf, B.J. Blink Reflex Parameters in Baseline, Active, and Head-Impact Division I Athletes. Cogent Eng. 2018, 5, 1429110. [Google Scholar] [CrossRef]
- Krishnan, S.; Shetty, K.; Puthanveedu, D.K.; Kesavapisharady, K.; Thulaseedharan, J.V.; Sarma, G.; Kishore, A. Apraxia of Lid Opening in Subthalamic Nucleus Deep Brain Stimulation for Parkinson’s Disease-Frequency, Risk Factors and Response to Treatment. Mov. Disord. Clin. Pract. 2021, 8, 587–593. [Google Scholar] [CrossRef]
- Tommasi, G.; Krack, P.; Fraix, V.; Pollak, P. Effects of Varying Subthalamic Nucleus Stimulation on Apraxia of Lid Opening in Parkinson’s Disease. J. Neurol. 2012, 259, 1944–1950. [Google Scholar] [CrossRef]
- do Nascimento, G.C.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira-Junior, N.C. Cannabidiol Increases the Nociceptive Threshold in a Preclinical Model of Parkinson’s Disease. Neuropharmacology 2019, 163, 107808. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-W.; Swanson, L.W. Projections from Bed Nuclei of the Stria Terminalis, Anteromedial Area: Cerebral Hemisphere Integration of Neuroendocrine, Autonomic, and Behavioral Aspects of Energy Balance. J. Comp. Neurol. 2006, 494, 142–178. [Google Scholar] [CrossRef] [PubMed]
- Hasue, R.H.; Shammah-Lagnado, S.J. Origin of the Dopaminergic Innervation of the Central Extended Amygdala and Accumbens Shell: A Combined Retrograde Tracing and Immunohistochemical Study in the Rat. J. Comp. Neurol. 2002, 454, 15–33. [Google Scholar] [CrossRef] [PubMed]

| Voluntary | Spontaneous | Reflex | ||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Stimulation Right | Stimulation Left | |||
| Right | Left | Right | Left | |||||
| EyeStat | 6.42 ± 6.64 | 6.64 ± 6.7 | 2.14 ± 0.96 | 2.18 ± 1.11 | 7.77 ± 4.85 | 7.63 ± 6.39 | 6.38 ± 4.24 | 7.89 ± 5.63 |
| SMART | 7.18 ± 4.52 | 6.47 ± 3.36 | 2.3 ± 1.17 | 2.13 ± 1.03 | 6.58 ± 7.04 | 4.37 ± 3.93 | 4.26 ± 4.04 | 7.34 ± 8.06 |
| Statistical Factors | Voluntary | Spontaneous | Reflex |
|---|---|---|---|
| TECHNIQUE | F1, 15 = 1.39, p = 0.25 | F1, 15 = 0.08, p = 0.77 | F1, 15 = 1.72, p = 0.20 |
| REC SIDE | F1, 15 = 1.13, p = 0.30 | F1, 15 = 0.31, p = 0.58 | F1, 15 = 0.50, p = 0.48 |
| STIM SIDE | - | - | F1, 15 = 0.49, p = 0.82 |
| TECHNIQUE × REC SIDE | F1, 15 = 2.49, p = 0.13 | F1, 15 = 3.44, p = 0.08 | F1, 15 = 0.009, p = 0.92 |
| TECHNIQUE × STIM SIDE | - | - | F1, 15 = 0.59, p = 0.45 |
| REC SIDE × STIM SIDE | - | - | F1, 15 = 8.46, p = 0.01 |
| TECHNIQUE × REC SIDE × STIM SIDE | - | - | F1, 15 = 2.77, p = 0.11 |
| Voluntary | Spontaneous | Reflex | ||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Stimulation Right | Stimulation Left | |||
| Right | Left | Right | Left | |||||
| EyeStat | ||||||||
| Amplitude | 104.59 ± 28.77 [90.49–118.69] | 95.21 ± 25.84 [82.46–107.78] | 99.38 ± 24.12 [87.56–111.2] | 94.61 ± 20.69 [84.47–104.75] | 146.12 ± 23.25 | 143.39 ± 23.26 | 144.87 ± 19.32 | 147.44 ± 19.87 |
| Open Vel | 3351.1 ± 1365.02 | 2987.09 ± 1094.06 | 1169.7 ± 346.12 | 1069.64 ± 316.65 | 2513.31 ± 1237.23 | 2149.15 ± 651.69 | 2356.94 ± 885.02 | 2164.35 ± 824.53 |
| Clos Vel | 5061.37 ± 1254.7 | 4614.09 ± 1300 | 2105.47 ± 719.53 | 2068.22 ± 709.8 | 6436.65 ± 1153.66 | 5819.11 ± 1191.43 | 6107.45 ± 1143.32 | 6111.58 ± 969.33 |
| SMART | ||||||||
| Amplitude | 11.1 ± 2.75 [9.75–12.45] | 10.71 ± 2.85 [9.31– 12.11] | 7.64 ± 2.78 [6.28–9] | 5.13 ± 2.54 [3.88–6.37] | 10.47 ± 2.69 | 9.76 ± 2.2 | 10.86 ± 2.3 | 10.84 ± 2.38 |
| Open Vel | 100.5 ± 35.2 | 89.72 ± 36.29 | 48.29 ± 15.81 | 48.59 ± 18.78 | 91.63 ± 18.84 | 77.31 ± 16.96 | 82.13 ± 17.73 | 86.56 ± 20.13 |
| Clos Vel | 170.38 ± 46.57 | 166.44 ± 46.23 | 96.1 ± 39.27 | 94.96 ± 41.05 | 187.46 ± 41.65 | 155.81 ± 40.27 | 155.41 ± 33.74 | 176.7 ± 33.91 |
| Statistical Factors | Voluntary EyeStat
(Velocity) | Voluntary SMART
(Velocity) | Spontaneous EyeStat
(Velocity) | Spontaneous SMART (Velocity) |
|---|---|---|---|---|
| PHASE | F1, 15 = 66.31, p < 0.001 | F1, 15 = 70.51, p < 0.001 | F1, 15 = 61.73, p < 0.001 | F1, 15 = 41.92, p < 0.001 |
| REC SIDE | F1, 15 = 2.49, p = 0.13 | F1, 15 = 3.42, p = 0.07 | F1, 15 = 2.47, p = 0.14 | F1, 15 = 0.16, p = 0.90 |
| PHASE × REC SIDE | F1, 15 = 0.08, p = 0.77 | F1, 15 = 0.82, p = 0.38 | F1, 15 = 2.79, p = 0.11 | F1, 15 = 0.35, p = 0.85 |
| Statistical Factors | Reflex EyeStat
(Velocity) | Reflex EyeStat
(Amplitude) | Reflex SMART
(Velocity) | Reflex SMART
(Amplitude) |
|---|---|---|---|---|
| PHASE | F1, 15 = 386.74, p < 0.001 | - | F1, 15 = 183.26, p < 0.001 | - |
| REC SIDE | F1, 15 = 3.88, p = 0.07 | F1, 15 = 0.03, p = 0.87 | F1, 15 = 1.50, p = 0.24 | F1, 15 = 1.29, p = 0.27 |
| STIM SIDE | F1, 15 = 0.39, p = 0.53 | F1, 15 = 0.47, p = 0.50 | F1, 15 = 0.51, p = 0.49 | F1, 15 = 3.33, p = 0.09 |
| PHASE × REC SIDE | F1, 15 = 0.11, p = 0.74 | - | F1, 15 = 0.01, p = 0.95 | - |
| PHASE × STIM SIDE | F1, 15 = 0.43, p = 0.52 | - | F1, 15 = 2.69, p = 0.12 | - |
| REC SIDE × STIM SIDE | F1, 15 = 21.21, p = 0.004 | F1, 15 = 2.43, p = 0.14 | F1, 15 = 47.90, p < 0.001 | F1, 15 = 1.37, p = 0.26 |
| PHASE × REC SIDE × STIM SIDE | F1, 15 = 2.31, p = 0.14 | - | F1, 15 = 32.36, p < 0.001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparella, G.; De Biase, A.; Cannavacciuolo, A.; Colella, D.; Passaretti, M.; Angelini, L.; Guerra, A.; Berardelli, A.; Bologna, M. Validating a Portable Device for Blinking Analyses through Laboratory Neurophysiological Techniques. Brain Sci. 2022, 12, 1228. https://doi.org/10.3390/brainsci12091228
Paparella G, De Biase A, Cannavacciuolo A, Colella D, Passaretti M, Angelini L, Guerra A, Berardelli A, Bologna M. Validating a Portable Device for Blinking Analyses through Laboratory Neurophysiological Techniques. Brain Sciences. 2022; 12(9):1228. https://doi.org/10.3390/brainsci12091228
Chicago/Turabian StylePaparella, Giulia, Alessandro De Biase, Antonio Cannavacciuolo, Donato Colella, Massimiliano Passaretti, Luca Angelini, Andrea Guerra, Alfredo Berardelli, and Matteo Bologna. 2022. "Validating a Portable Device for Blinking Analyses through Laboratory Neurophysiological Techniques" Brain Sciences 12, no. 9: 1228. https://doi.org/10.3390/brainsci12091228
APA StylePaparella, G., De Biase, A., Cannavacciuolo, A., Colella, D., Passaretti, M., Angelini, L., Guerra, A., Berardelli, A., & Bologna, M. (2022). Validating a Portable Device for Blinking Analyses through Laboratory Neurophysiological Techniques. Brain Sciences, 12(9), 1228. https://doi.org/10.3390/brainsci12091228







