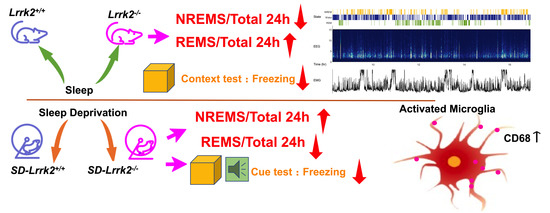
LRRK2 Deficiency Aggravates Sleep Deprivation-Induced Cognitive Loss by Perturbing Synaptic Pruning in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transgenic Mice and Ethics Approval
2.2. Experimental Design
2.3. Surgery Implantation
2.4. EEG/EMG Recordings
2.5. Fear Conditioning
2.6. Open-Field Test
2.7. Tissue Collection and Processing
2.8. RNA Extraction and Real-Time PCR
- IL-1 (sense 5-ATCAGCAACGTCAAGCAACG-3 and anti-sense 5-GGTTGGATGGTCTCTTCCAGA-3),
- IL-6 (sense 5-TAGTCCTTCCTACCCCAATTTCC-3 and anti-sense 5-TTGGTCCTTAGCCACTCCTTC-3),
- IL-4 (sense 5-GCAACGAAGAACACCACAGA-3 and anti-sense 5-TGCAGCTCCATGAGAACACT-3),
- GAPDH (sense 5-CAGTGGCAAAGTGGAGATTGTTG-3 and anti-sense 5-CTCGCTCCTGGAAGATGGTGAT-3).
2.9. Immunohistological Analysis
2.10. Dendritic Spine Analysis
2.11. Statistical Analysis
3. Results
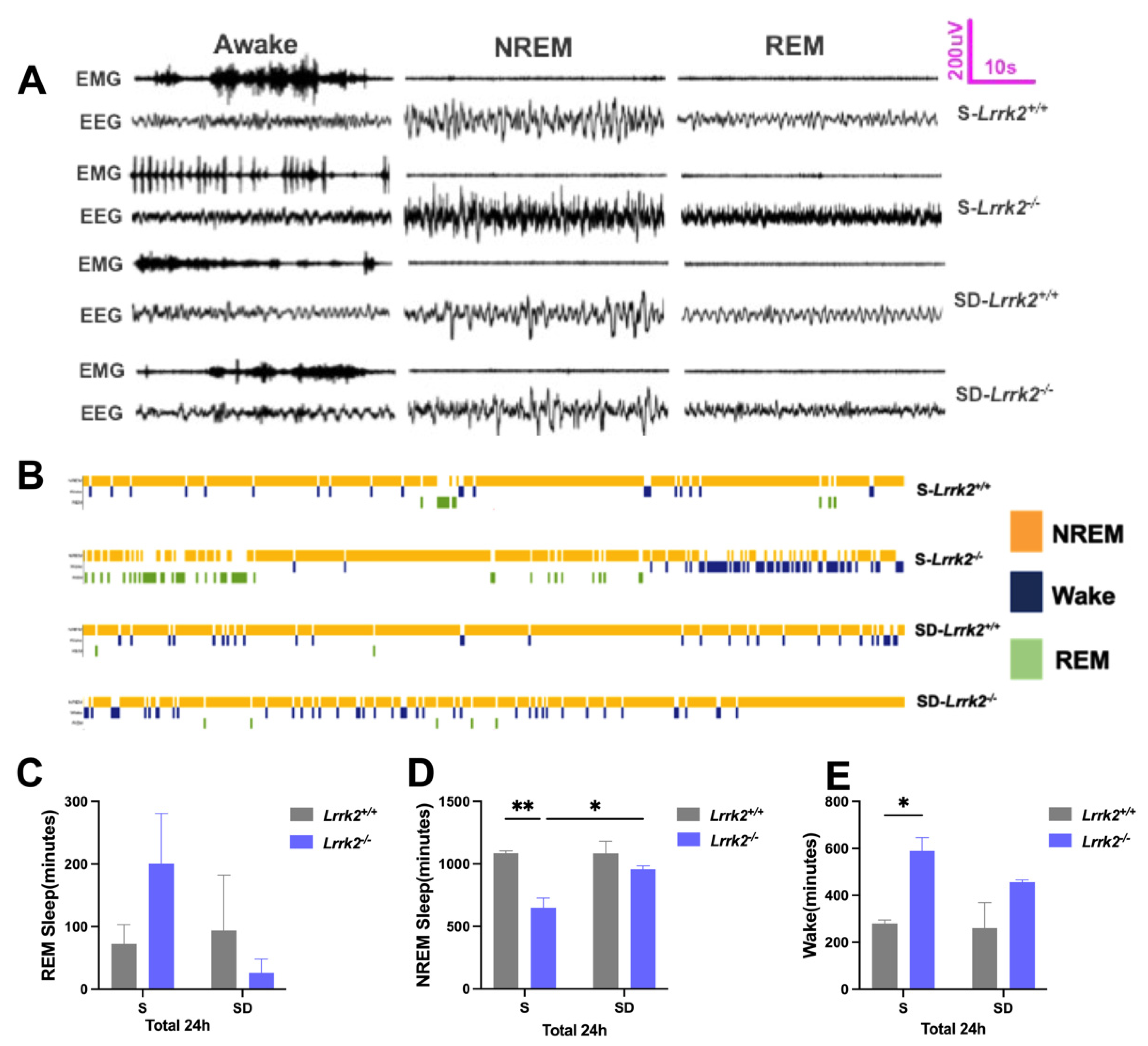
3.1. LRRK2 Deficiency Produces Inverted Effects on the NREM Sleep before and after SD
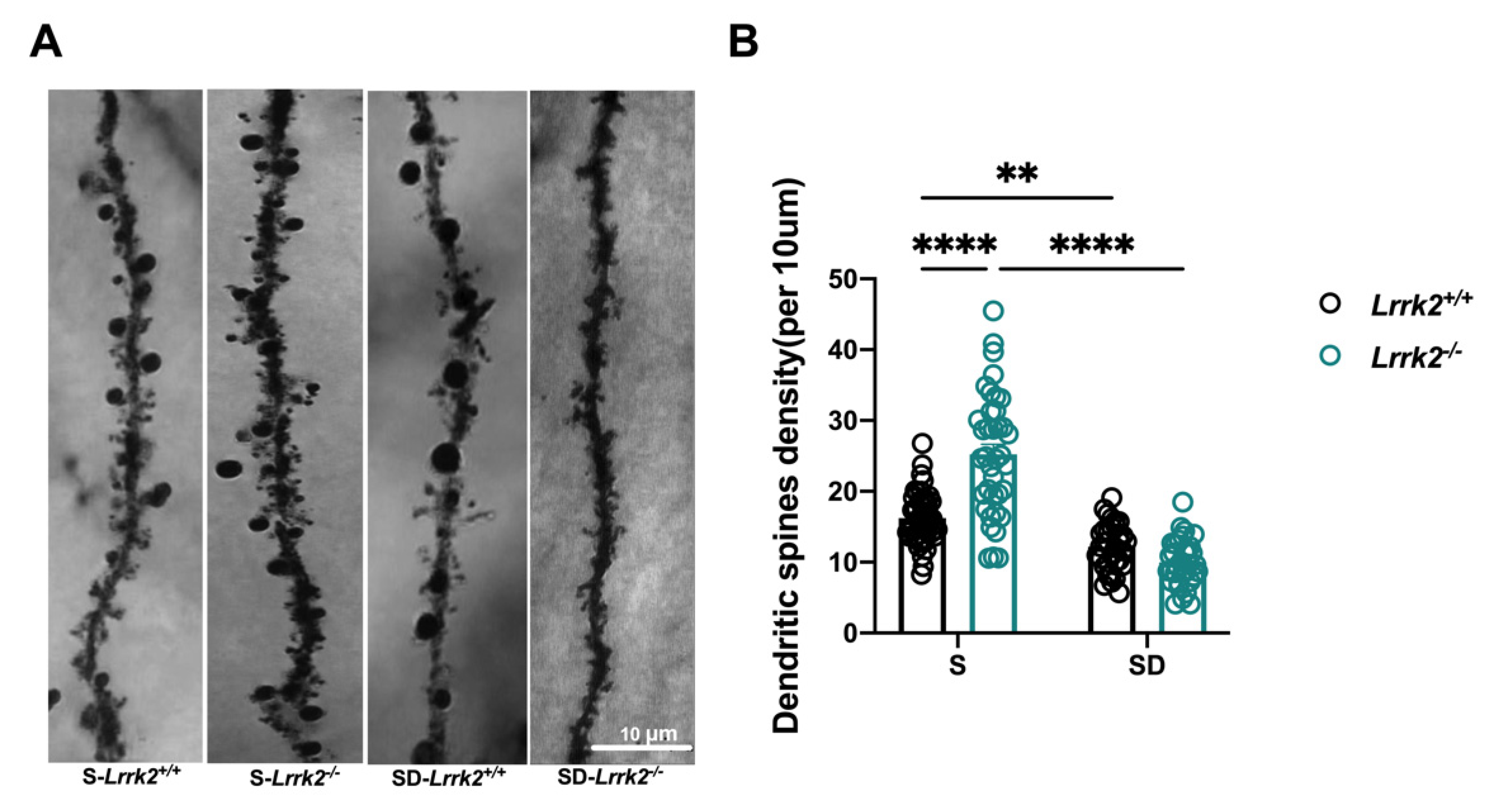
3.2. LRRK2 Deficiency Deceases Dendritic Spine Density after SD
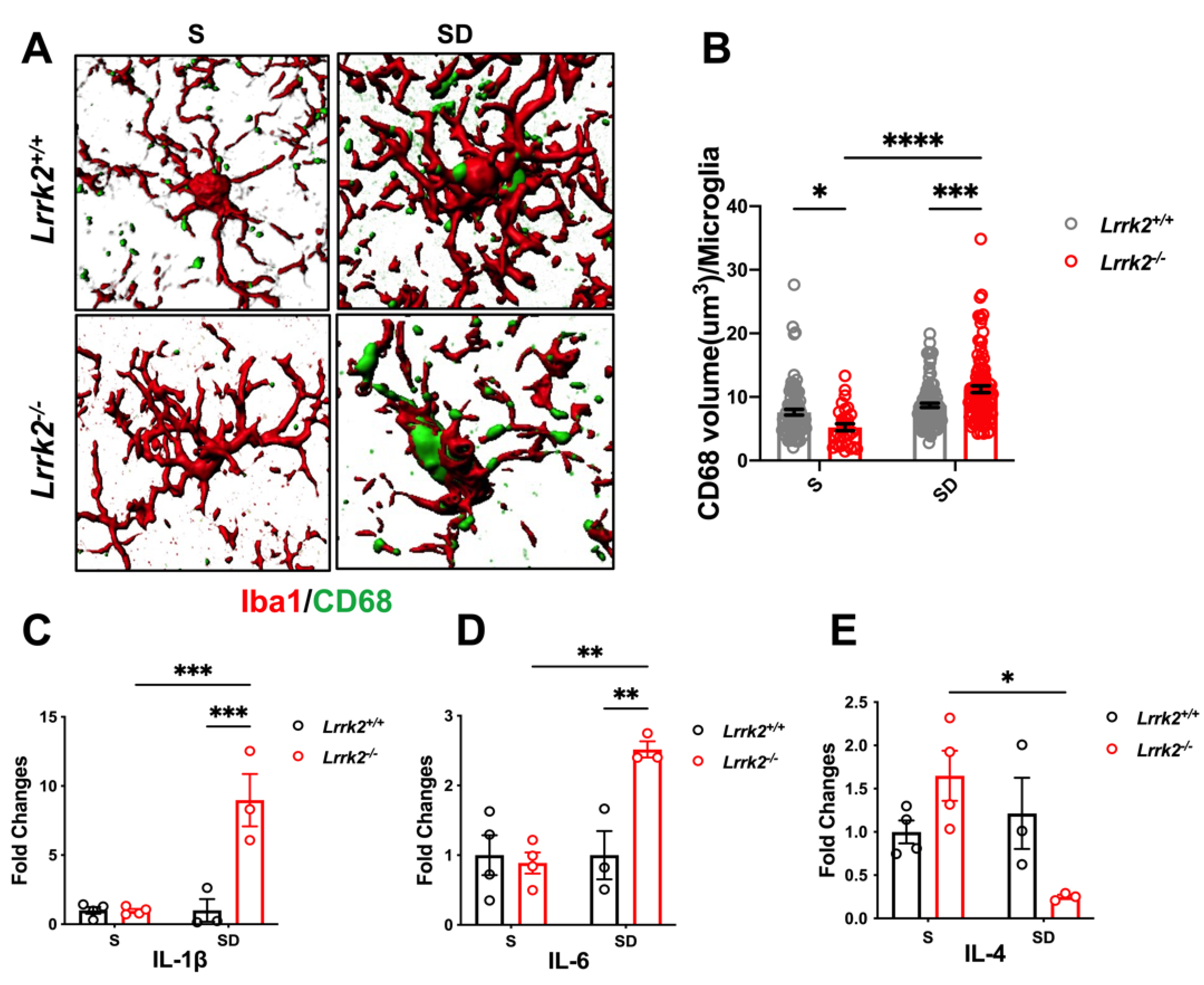
3.3. LRRK2 Deficiency Aggravates Microglial Activation after SD
3.4. LRRK2 Deficiency Increases Synaptic Pruning by Microglia after SD
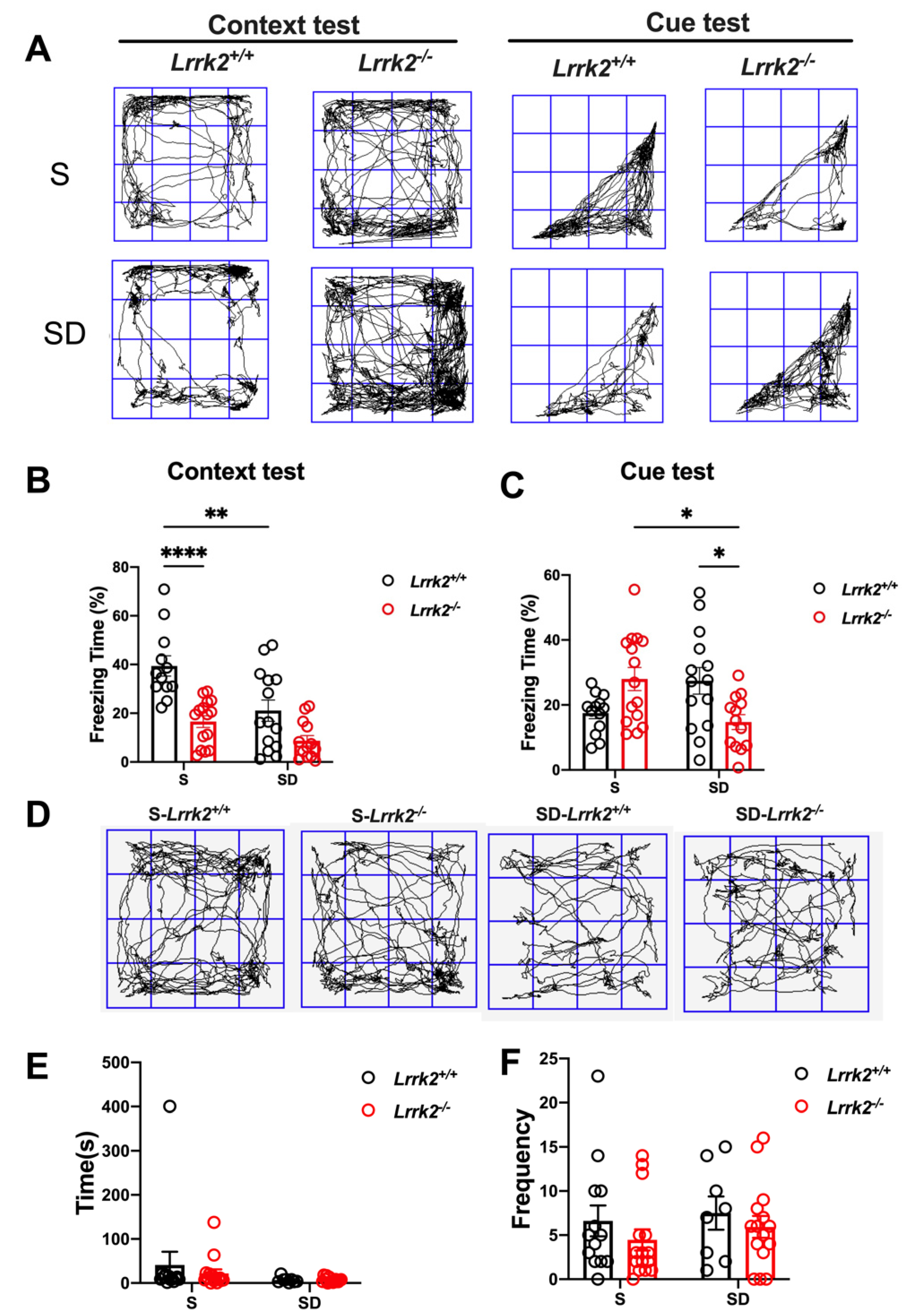
3.5. LRRK2 Deficiency Induces the Abnormalities of Short-Term Memory
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Srivatsal, S.; Cholerton, B.; Leverenz, J.B.; Wszolek, Z.K.; Uitti, R.J.; Dickson, D.W.; Weintraub, D.; Trojanowski, J.Q.; Van Deerlin, V.M.; Quinn, J.F.; et al. Cognitive profile of LRRK2-related Parkinson’s disease. Mov. Disord. 2015, 30, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Nguyen AP, T.; Moore, D.J. Understanding the GTPase Activity of LRRK2: Regulation, Function, and Neurotoxicity. Adv. Neurobiol. 2017, 14, 71–88. [Google Scholar] [PubMed]
- Ren, C.; Ding, Y.; Wei, S.; Guan, L.; Zhang, C.; Ji, Y.; Wang, F.; Yin, S.; Yin, P. G2019S Variation in LRRK2: An Ideal Model for the Study of Parkinson’s Disease? Front. Hum. Neurosci. 2019, 13, 306. [Google Scholar] [CrossRef]
- Robbins, R.; Quan, S.; Weaver, M.; Bormes, G.; Barger, L.; Czeisler, C. Examining sleep deficiency and disturbance and their risk for incident dementia and all-cause mortality in older adults across 5 years in the United States. Aging 2021, 13, 3254–3268. [Google Scholar] [CrossRef] [PubMed]
- Pont-Sunyer, C.P.-S.; Iranzo, A.; Gaig, C.; Fernández-Arcos, A.; Vilas, D.; Valldeoriola, F.; Compta, Y.; Fernandez-Santiago, R.; Fernandez, M.; Bayes, A.; et al. Sleep Disorders in Parkinsonian and Nonparkinsonian LRRK2 Mutation Carriers. PLoS ONE 2015, 10, e0132368. [Google Scholar] [CrossRef]
- Mufti, K.; Rudakou, U.; Yu, E.; Krohn, L.; Ruskey, J.A.; Asayesh, F.; Laurent, S.B.; Spiegelman, D.; Arnulf, I.; Hu, M.T.M.; et al. Comprehensive Analysis of Familial Parkinsonism Genes in Rapid-Eye-Movement Sleep Behavior Disorder. Mov. Disord. 2021, 36, 235–240. [Google Scholar] [CrossRef]
- Ouled Amar Bencheikh, B.; Ruskey, J.A.; Arnulf, I.; Dauvilliers, Y.; Monaca, C.C.; De Cock, V.C.; Gagnon, J.F.; Spiegelman, D.; Hu, M.T.M.; Hogl, B.; et al. LRRK2 protective haplotype and full sequencing study in REM sleep behavior disorder. Parkinsonism Relat. Disord. 2018, 52, 98–101. [Google Scholar] [CrossRef]
- Webb, J.M.; Fu, Y.H. Recent advances in sleep genetics. Curr. Opin. Neurobiol. 2021, 69, 19–24. [Google Scholar] [CrossRef]
- Yamazaki, R.; Toda, H.; Libourel, P.A.; Hayashi, Y.; Vogt, K.E.; Sakurai, T. Evolutionary Origin of Distinct NREM and REM Sleep. Front. Psychol. 2020, 11, 567618. [Google Scholar] [CrossRef]
- Schreiner, S.J.; Imbach, L.L.; Valko, P.O.; Maric, A.; Maqkaj, R.; Werth, E.; Baumann, C.R.; Baumann-Vogel, H. Reduced Regional NREM Sleep Slow-Wave Activity Is Associated With Cognitive Impairment in Parkinson Disease. Front. Neurol. 2021, 12, 618101. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, L.; Zeng, F.; Zhang, T.; Sun, Y.; Shen, Y.; Wang, G.; Ma, J.; Zhang, J. NREM Sleep EEG Characteristics Correlate to the Mild Cognitive Impairment in Patients with Parkinsonism. BioMed Res. Int. 2021, 2021, 5561974. [Google Scholar] [CrossRef] [PubMed]
- Boyce, R.; Glasgow, S.; Williams, S.; Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 2016, 352, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Tuan, L.H.; Lee, L.J. Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol. Dis. 2019, 130, 104517. [Google Scholar] [CrossRef]
- Singh, P.; Donlea, J.M. Bidirectional Regulation of Sleep and Synapse Pruning after Neural Injury. Curr. Biol. 2020, 30, 1063–1076.e1063. [Google Scholar] [CrossRef] [PubMed]
- Artiushin, G.; Zhang, S.L.; Tricoire, H.; Sehgal, A. Endocytosis at the Drosophila blood-brain barrier as a function for sleep. Elife 2018, 7, e43326. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, N.; Milnerwood, A.J. A Critical LRRK at the Synapse? The Neurobiological Function and Pathophysiological Dysfunction of LRRK2. Front. Mol. Neurosci. 2020, 13, 153. [Google Scholar] [CrossRef]
- Katayama, K.; Mochizuki, A.; Kato, T.; Ikeda, M.; Ikawa, Y.; Nakamura, S.; Nakayama, K.; Wakabayashi, N.; Baba, K.; Inoue, T. Dark/light transition and vigilance states modulate jaw-closing muscle activity level in mice. Neurosci. Res. 2015, 101, 24–31. [Google Scholar] [CrossRef]
- Oishi, Y.; Takata, Y.; Taguchi, Y.; Kohtoh, S.; Urade, Y.; Lazarus, M. Polygraphic Recording Procedure for Measuring Sleep in Mice. J. Vis. Exp. 2016, 107, e53678. [Google Scholar] [CrossRef]
- Nolan, S.O.; Reynolds, C.D.; Smith, G.D.; Holley, A.J.; Escobar, B.; Chandler, M.A.; Volquardsen, M.; Jefferson, T.; Pandian, A.; Smith, T.; et al. Deletion of Fmr1 results in sex-specific changes in behavior. Brain Behav. 2017, 7, e00800. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Wu, W.; Yang, S.; You, H.; Ye, Z.; Liu, N.; Chen, X.; Pan, X. Age-related LRRK2 G2019S Mutation Impacts Microglial Dopaminergic Fiber Refinement and Synaptic Pruning Involved in Abnormal Behaviors. J. Mol. Neurosci. 2021, 72, 527–543. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, e57648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lai CS, W.; Bai, Y.; Li, W.; Zhao, R.; Yang, G.; Frank, M.G.; Gan, W.B. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat. Commun. 2020, 11, 4819. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Delage, C.I.; Tremblay, M.E.; Crespo-Lopez, M.E.; Verkhratsky, A. Plasticity of microglia. Biol. Rev. Camb. Philos. Soc. 2017, 97, 217–250. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.S.; Zito, K. Dendritic Spine Elimination: Molecular Mechanisms and Implications. Neuroscientist 2019, 25, 27–47. [Google Scholar] [CrossRef]
- Naiman, R. Dreamless: The silent epidemic of REM sleep loss. Ann. N. Y. Acad. Sci. 2017, 1406, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Genzel, L.; Spoormaker, V.I.; Konrad, B.N.; Dresler, M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol. Learn. Mem. 2015, 122, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Purple, R.J.; Sakurai, T.; Sakaguchi, M. Auditory conditioned stimulus presentation during NREM sleep impairs fear memory in mice. Sci. Rep. 2017, 7, 46247. [Google Scholar] [CrossRef]
- de Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef]
- Dejanovic, B.; Huntley, M.A.; De Maziere, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e1327. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, B.A.; Jaggard, J.B.; Gratton, M.; Brown, E.B.; Keene, A.C. Sleep Regulates Glial Plasticity and Expression of the Engulfment Receptor Draper Following Neural Injury. Curr. Biol. 2020, 30, 1092–1101.e1093. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef]
- Xie, Y.; Ba, L.; Wang, M.; Deng, S.Y.; Chen, S.M.; Huang, L.F.; Zhang, M.; Wang, W.; Ding, F.F. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: Endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neurosci. Ther. 2020, 26, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, X.; Li, Y.; Zhao, J.; Liu, Z.; Hu, Z.; Wang, Y.; Yao, Y.; Miller, A.W.; Su, B.; et al. LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J. Exp. Med. 2017, 214, 3051–3066. [Google Scholar] [CrossRef]
- Erb, M.L.; Moore, D.J. LRRK2 and the Endolysosomal System in Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, 1271–1291. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Chen, L.; Chen, L.; Tsirka, S.E.; Ge, S.; Xiong, Q. Microglia modulate stable wakefulness via the thalamic reticular nucleus in mice. Nat. Commun. 2021, 12, 4646. [Google Scholar] [CrossRef] [PubMed]
- Goo, M.S.; Sancho, L.; Slepak, N.; Boassa, D.; Deerinck, T.J.; Ellisman, M.H.; Bloodgood, B.L.; Patrick, G.N. Activity-dependent trafficking of lysosomes in dendrites and dendritic spines. J. Cell Biol. 2017, 216, 2499–2513. [Google Scholar] [CrossRef]
- Lee, H.; Flynn, R.; Sharma, I.; Haberman, E.; Carling, P.J.; Nicholls, F.J.; Stegmann, M.; Vowles, J.; Haenseler, W.; Wade-Martins, R.; et al. LRRK2 Is Recruited to Phagosomes and Co-recruits RAB8 and RAB10 in Human Pluripotent Stem Cell-Derived Macrophages. Stem. Cell Rep. 2020, 14, 940–955. [Google Scholar] [CrossRef]
- Nirujogi, R.S.; Tonelli, F.; Taylor, M.; Lis, P.; Zimprich, A.; Sammler, E.; Alessi, D.R. Development of a multiplexed targeted mass spectrometry assay for LRRK2-phosphorylated Rabs and Ser910/Ser935 biomarker sites. Biochem. J. 2021, 478, 299–326. [Google Scholar] [CrossRef]
- Eguchi, T.; Kuwahara, T.; Sakurai, M.; Komori, T.; Fujimoto, T.; Ito, G.; Yoshimura, S.I.; Harada, A.; Fukuda, M.; Koike, M.; et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, E9115–E9124. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Wu, X.; Zhang, Y.; Li, W.; Feng, L.; You, H.; Yang, S.; Yang, D.; Chen, X.; Pan, X. LRRK2 Deficiency Aggravates Sleep Deprivation-Induced Cognitive Loss by Perturbing Synaptic Pruning in Mice. Brain Sci. 2022, 12, 1200. https://doi.org/10.3390/brainsci12091200
Cheng X, Wu X, Zhang Y, Li W, Feng L, You H, Yang S, Yang D, Chen X, Pan X. LRRK2 Deficiency Aggravates Sleep Deprivation-Induced Cognitive Loss by Perturbing Synaptic Pruning in Mice. Brain Sciences. 2022; 12(9):1200. https://doi.org/10.3390/brainsci12091200
Chicago/Turabian StyleCheng, Xiaojuan, Xilin Wu, Yuying Zhang, Weian Li, Linjuan Feng, Hanlin You, Siyu Yang, Dongping Yang, Xiaochun Chen, and Xiaodong Pan. 2022. "LRRK2 Deficiency Aggravates Sleep Deprivation-Induced Cognitive Loss by Perturbing Synaptic Pruning in Mice" Brain Sciences 12, no. 9: 1200. https://doi.org/10.3390/brainsci12091200
APA StyleCheng, X., Wu, X., Zhang, Y., Li, W., Feng, L., You, H., Yang, S., Yang, D., Chen, X., & Pan, X. (2022). LRRK2 Deficiency Aggravates Sleep Deprivation-Induced Cognitive Loss by Perturbing Synaptic Pruning in Mice. Brain Sciences, 12(9), 1200. https://doi.org/10.3390/brainsci12091200