A Method for the Study of Cerebellar Cognitive Function—Re-Cognition and Validation of Error-Related Potentials
Abstract
:1. Introduction
2. Methods
2.1. Task Description
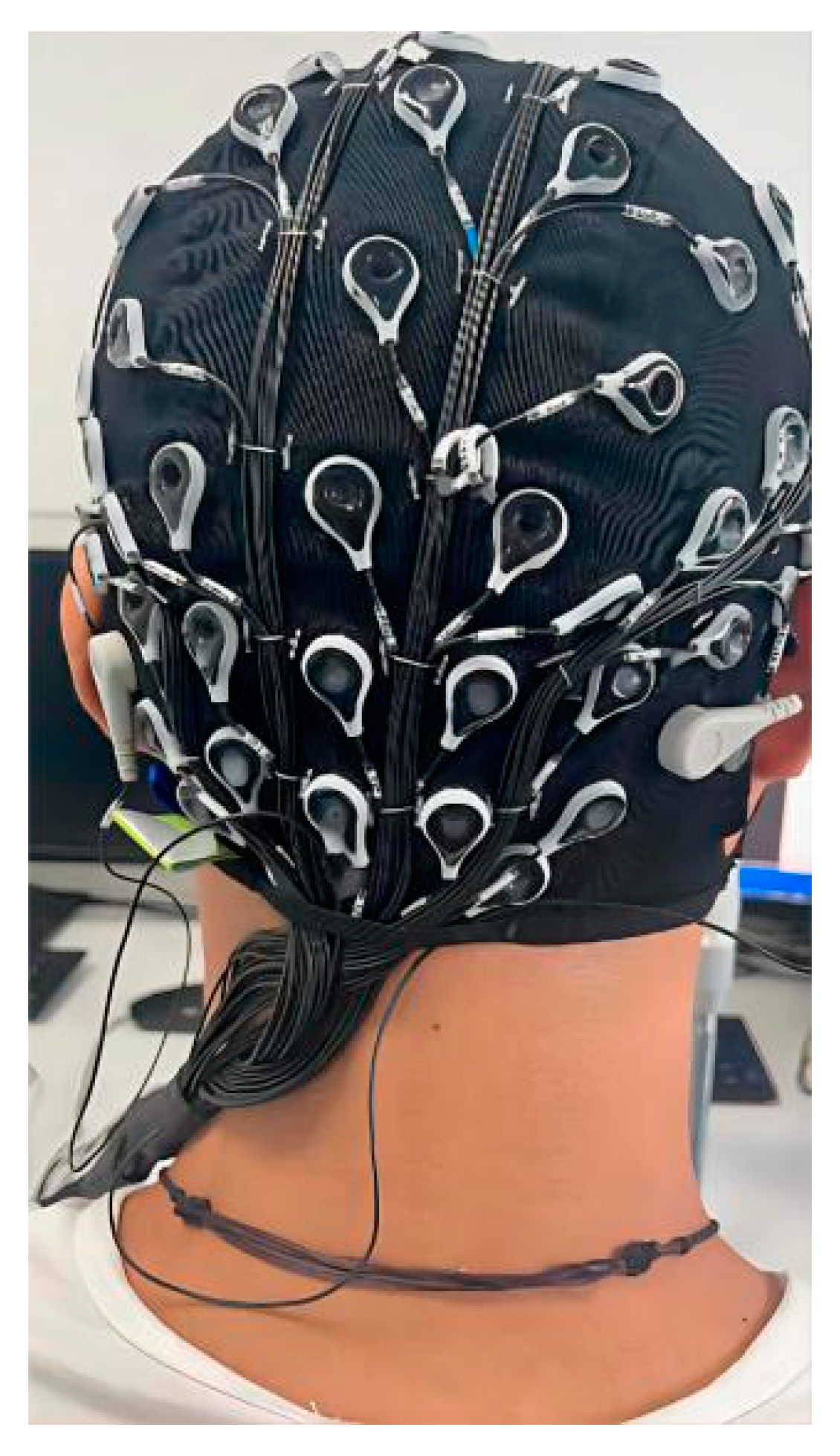
2.2. Subjects and Devices
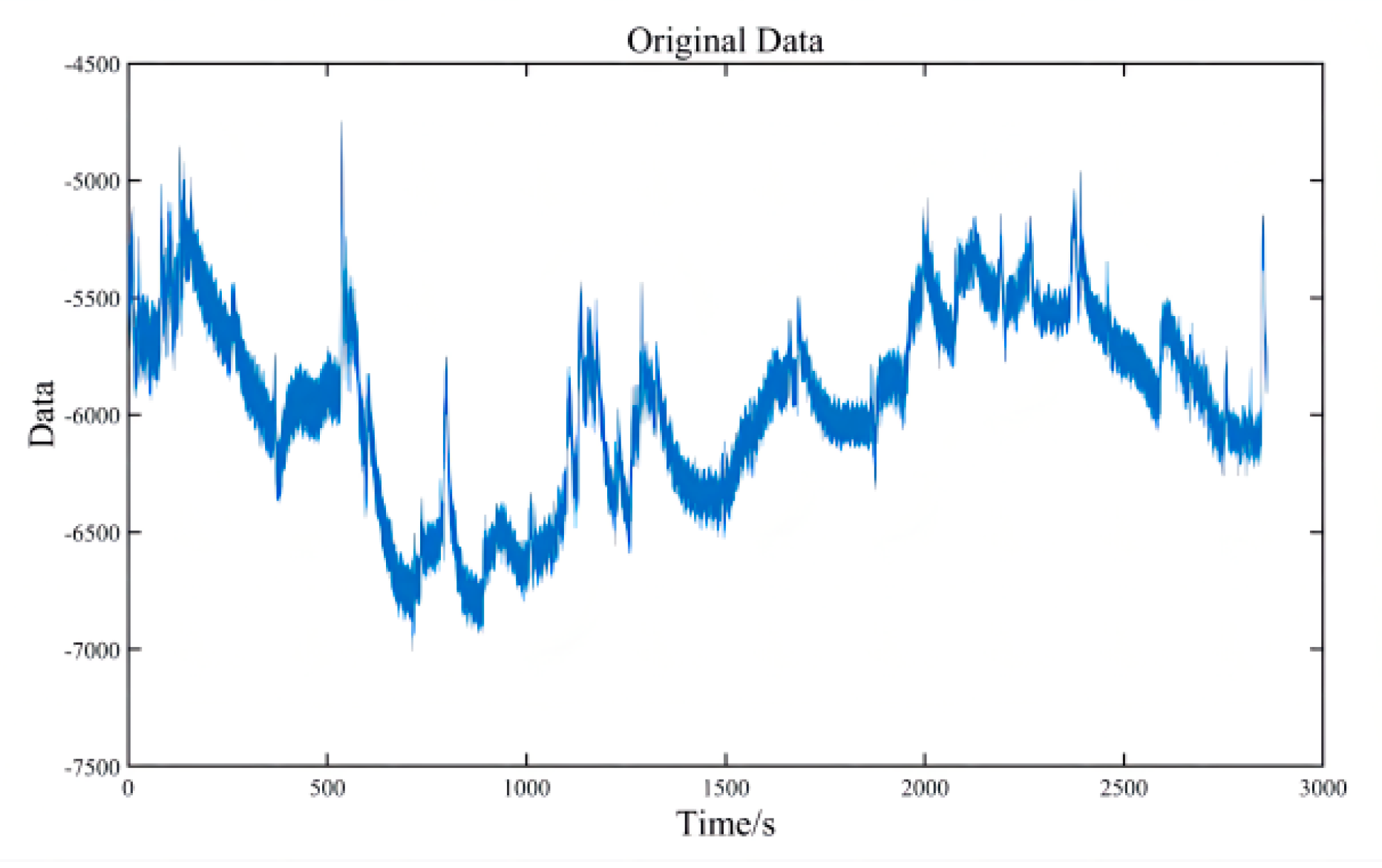
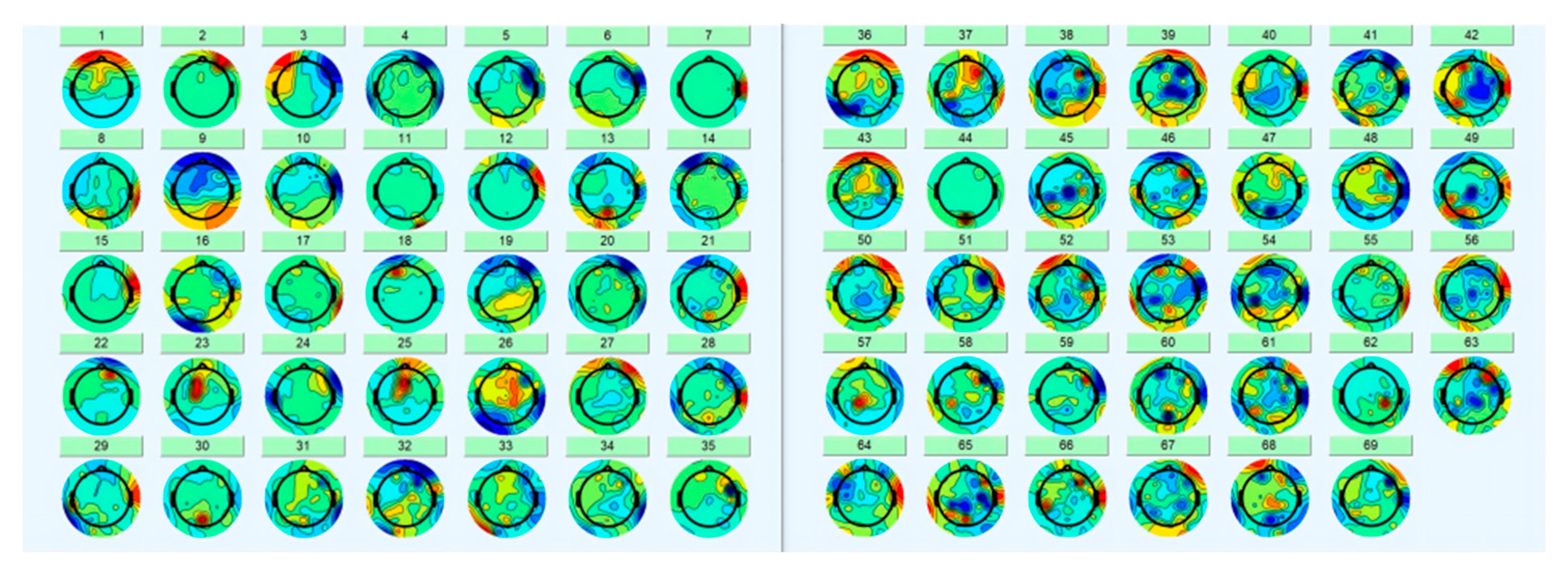
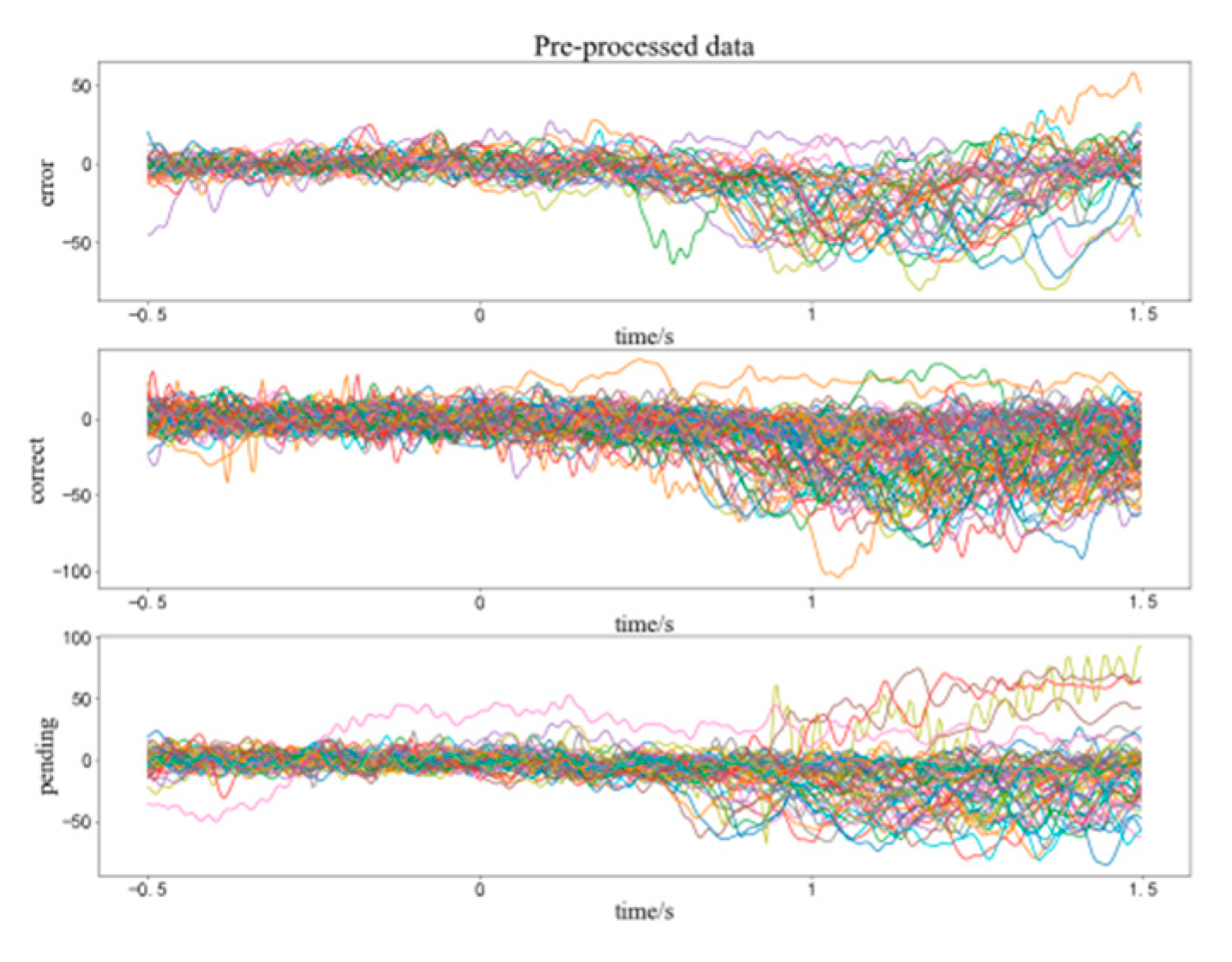
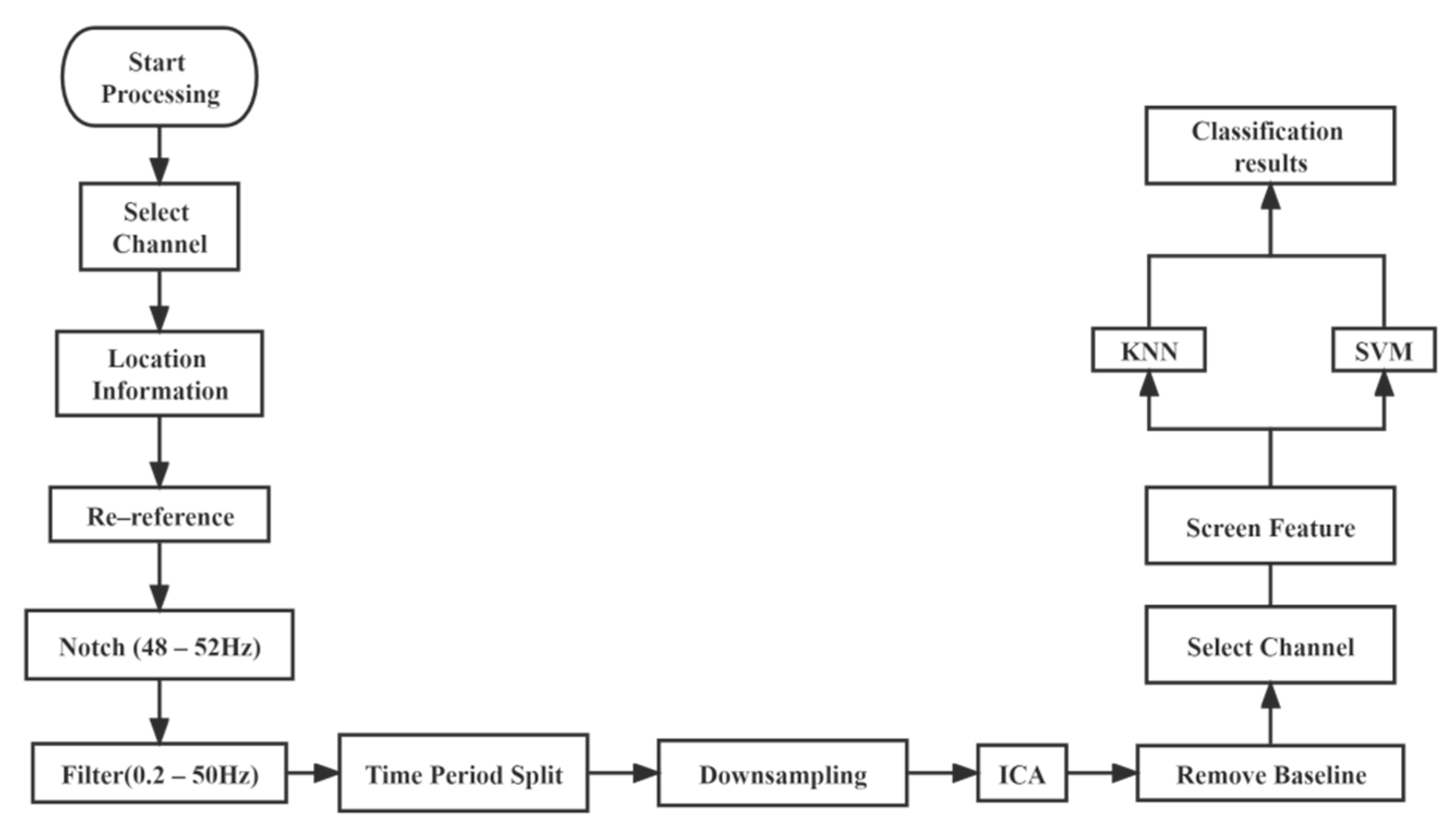
2.3. Pre-Processing
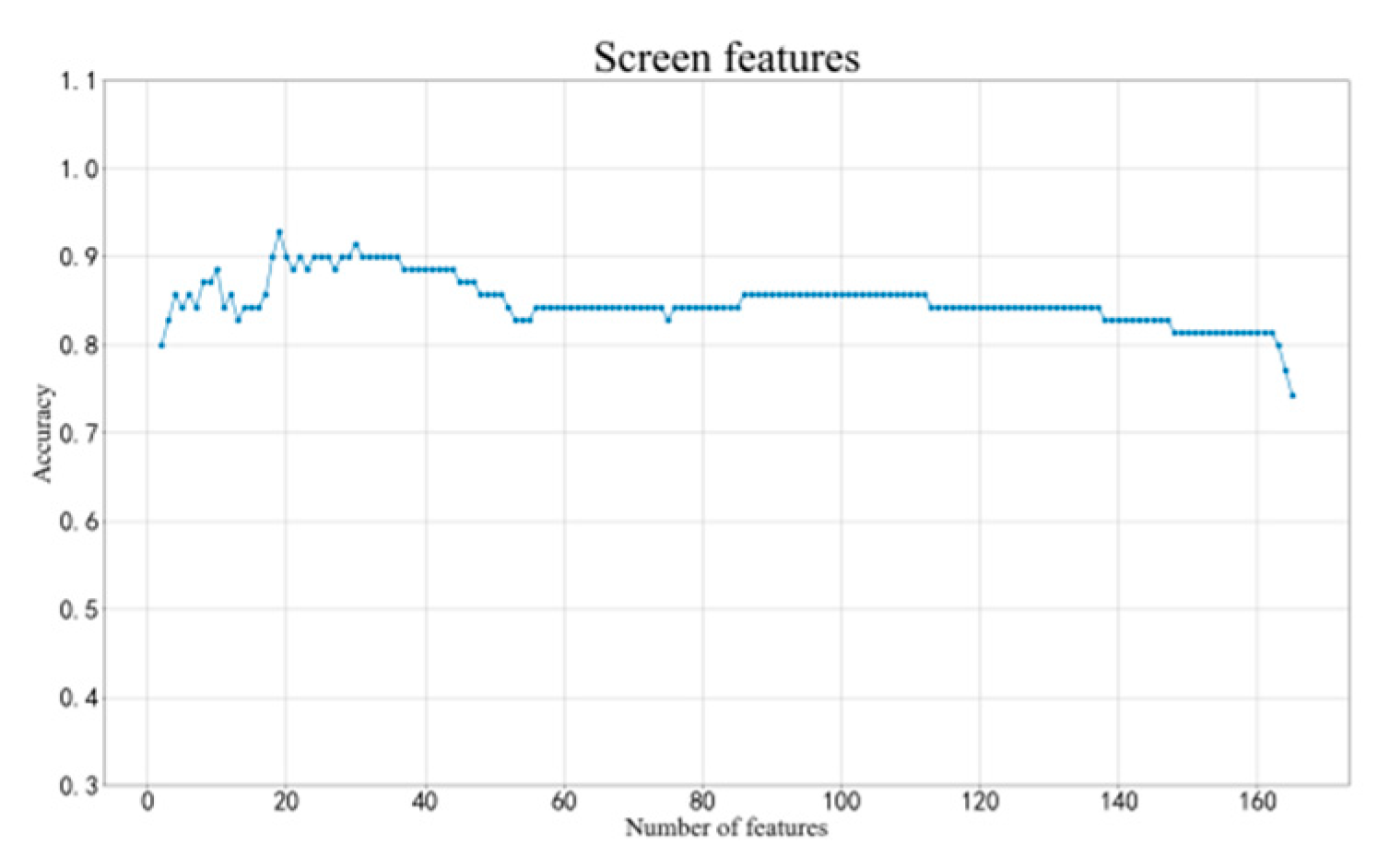
2.4. Method of Classification
- SVM classifier: The SVM technique is a computer algorithm based on statistical theory to learn the labels assigned to objects. These support vectors try to find a hyperplane that separates the different classes of points in the hyperspace [25]. The SVM classifier was implemented in python using scikit-learn packages. We set the hyperparameter C, which controls the error of the class separation, to 0.1 and the kernel function parameter to “poly”, which means polynomial.
- KNN classifier: The KNN classification algorithm is one of the data classification technique. The principle is that if the majority of the K nearest neighboring samples in the feature space belong to a certain class, then the sample also belongs to that class, rather than relying on the discriminative class domain [26]. The SVM classifier was implemented in python using scikit-learn packages. We set the number of hyperparameters K to 3.
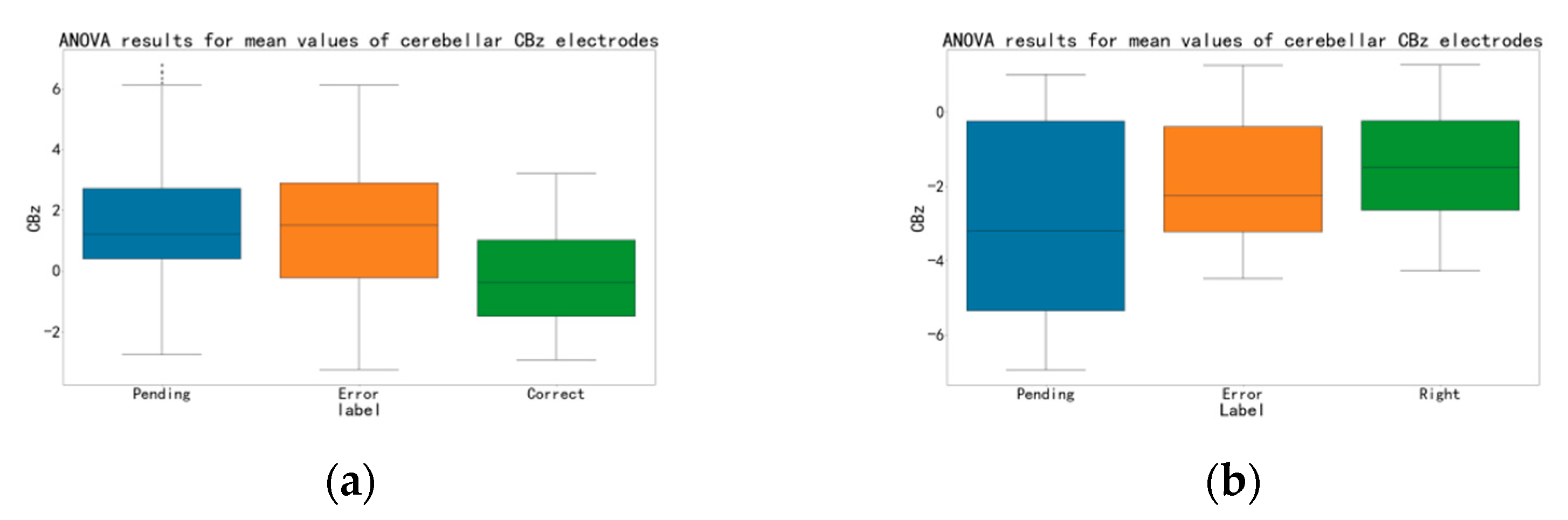
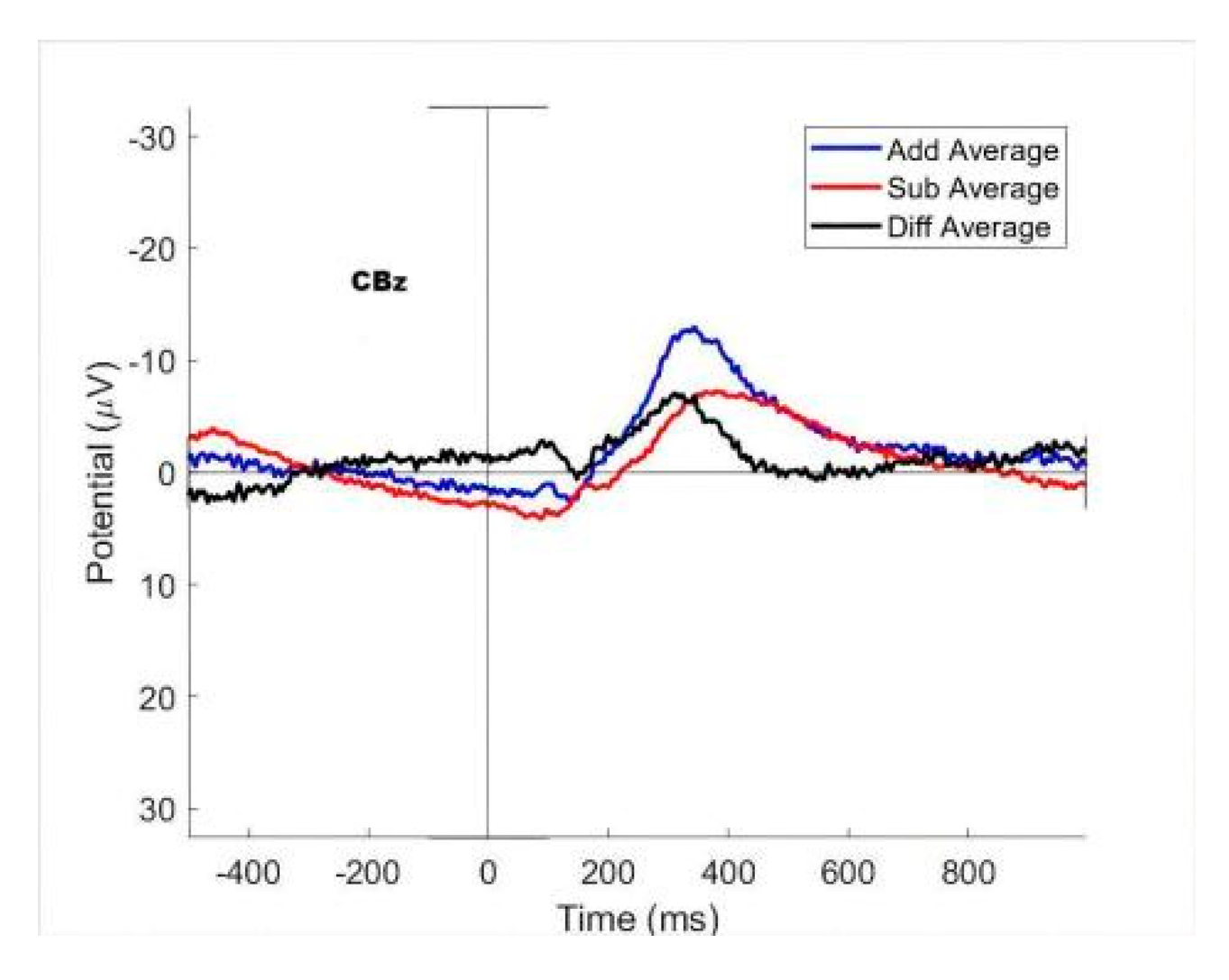
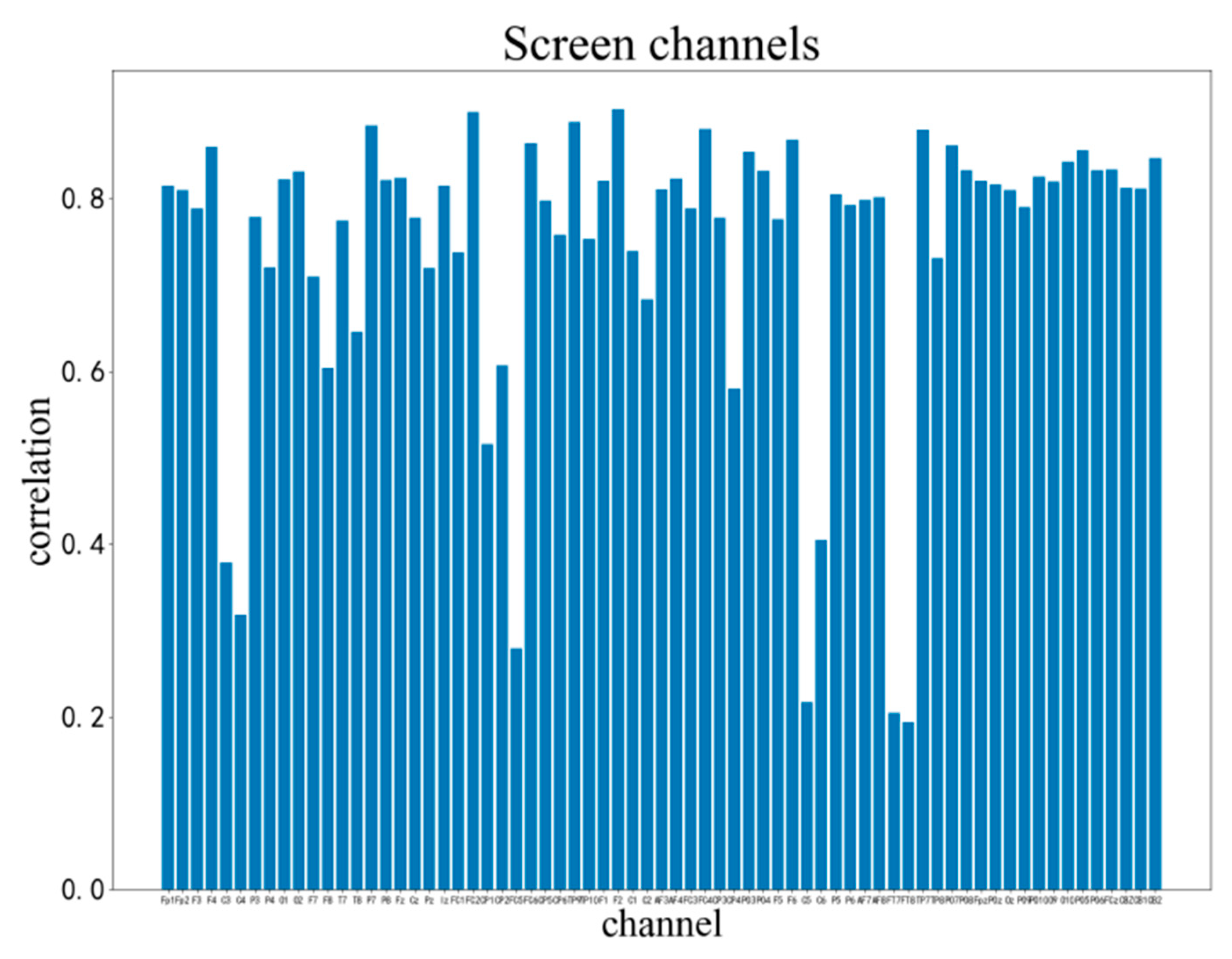
3. Results
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakkenberg, B.; Gundersen, H.J.G. Neocortical neuron number in humans: Effect of sex and age. J. Comp. Neurol. 1997, 384, 312–320. [Google Scholar] [CrossRef]
- Pakkenberg, B.; Pelvig, D.; Marner, L.; Bundgaard, M.J.; Gundersen, H.J.G.; Nyengaard, J.R.; Regeur, L. Aging and the human neocortex. Exp. Gerontol. 2003, 38, 95–99. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 31. [Google Scholar] [CrossRef] [PubMed]
- Wolf, U.; Rapoport, M.J.; Schweizer, T.A. Evaluating the affective component of the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 245–253. [Google Scholar] [CrossRef]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. The human cerebro-cerebellar system: Its computing, cognitive, and language skills. Behav. Brain Res. 1991, 44, 113–128. [Google Scholar] [CrossRef]
- Courchesne, E. Neuroanatomic imaging in autism. Pediatrics 1991, 87, 781–790. [Google Scholar] [CrossRef]
- Gehring, W.J.; Goss, B.; Coles, M.G.; Meyer, D.E.; Donchin, E. A neural system for error detection and compensation. Psychol. Sci. 1993, 4, 385–390. [Google Scholar] [CrossRef]
- Chavarriaga, R.; Sobolewski, A.; Millán, J.D.R. Errare machinale est: The use of error-related potentials in brain-machine interfaces. Front. Neurosci. 2014, 208. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Coles, M.G. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002, 109, 679. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Blankertz, B.; Treder, M.S. Online detection of error-related potentials boosts the performance of mental typewriters. BMC Neurosci. 2012, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Spüler, M.; Bensch, M.; Kleih, S.; Rosenstiel, W.; Bogdan, M.; Kübler, A. Online use of error-related potentials in healthy users and people with severe motor impairment increases performance of a P300-BCI. Clin. Neurophysiol. 2012, 123, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Spüler, M.; Niethammer, C.; Rosenstiel, W.; Bogdan, M. Classification of error-related potentials in eeg during continuous 414 feedback. In Proceedings of the 6th International Brain-Computer Interface Conference, Graz University of Technology, Graz, Austria, 16–19 September 2014; pp. 24–27. [Google Scholar]
- Iturrate, I.; Montesano, L.; Minguez, J. Task-dependent signal variations in EEG error-related potentials for brain-computer interfaces. J. Neural. Eng. 2013, 10, 026024. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Mo, L.; Wang, J.; Zhang, W.; Zhang, D. Trait anxiety attenuates response inhibition: Evidence from an ERP study using the Go/NoGo task. Front. Behav. Neurosci. 2020, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, R.; Satoh, K.; Itoh, H.; Ono, S.; Furumoto, S.; Gotoh, R.; Koyama, M.; Yoshioka, S.; Takahashi, T.; Takahashi, K.; et al. Functional anatomy of GO/NO-GO discrimination and response selection—A PET study in man. Brain Res. 1996, 728, 79–89. [Google Scholar] [PubMed]
- Sehlmeyer, C.; Konrad, C.; Zwitserlood, P.; Arolt, V.; Falkenstein, M.; Beste, C. ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia 2010, 48, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Ross, T.; Stein, E. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc. Natl. Acad. Sci. USA 1999, 96, 8301–8306. [Google Scholar] [CrossRef]
- Rubia, K.; Overmeyer, S.; Taylor, E.; Brammer, M.; Williams, S.; Simmons, A.; Andrew, C.; Bullmore, E. Prefrontal involvement in temporal bridging and timing movement. Neuropsychologia 1998, 36, 1283–1293. [Google Scholar] [CrossRef]
- Shi, J.; Yin, K.; Ye, X.; Du, W.; Zhao, J. A Whole-Brain Electroencephalogram Cap for Transcranial Magnetic Stimulator Therapy. CN 114366127, 19 April 2022. [Google Scholar]
- Yao, D. A method to standardize a reference of scalp EEG recordings to a point at infinity. Physiol. Meas. 2001, 22, 693. [Google Scholar] [CrossRef]
- Winkler, I.; Debener, S.; Müller, K.R.; Tangermann, M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 4101–4105. [Google Scholar]
- Tatum, W.O. Ellen r. grass lecture: Extraordinary eeg. Neurodiagnostic J. 2014, 54, 3–21. [Google Scholar]
- Wang, M.; Abbass, H.A.; Hu, J.; Merrick, K. Detecting Rare Visual and Auditory Events from EEG Using Pairwise-Comparison Neural Networks. In Advances in Brain Inspired Cognitive Systems. BICS 2016. Lecture Notes in Computer Science; Liu, C.L., Hussain, A., Luo, B., Tan, K., Zeng, Y., Zhang, Z., Eds.; Springer: Cham, Switzerland, 2016; Volume 10023. [Google Scholar] [CrossRef]
- Jung, T.P.; Humphries, C.; Lee, T.W.; Makeig, S.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts: Comparison between ICA and PCA. In Proceedings of the Neural Networks for Signal Processing VIII. Proceedings of the 1998 IEEE Signal Processing Society Workshop (Cat. No. 98TH8378), Cambridge, UK, 2 September 1998; pp. 63–72. [Google Scholar]
- Varone, G.; Boulila, W.; Lo Giudice, M.; Benjdira, B.; Mammone, N.; Ieracitano, C.; Dashtipour, K.; Neri, S.; Gasparini, S.; Morabito, F.C.; et al. A Machine Learning Approach Involving Functional Connectivity Features to Classify Rest-EEG Psychogenic Non-Epileptic Seizures from Healthy Controls. Sensors 2022, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.S. An introduction to kernel and nearest-neighbor nonparametric regression. Am. Stat. 1992, 46, 175–185. [Google Scholar]
- Wu, Q.; Liu, C.; Liu, L. Hybrid Feature Selection Algorithm for Fusion Sequence Backward Selection and Support Vector Machine. Comput. Syst. Appl. 2019, 28, 6. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]



| SVM Pre-Screening | SVM Post-Screening | KNN Pre-Screening | KNN Post-Screening | |
|---|---|---|---|---|
| Two-class Average Accuracy | 45.2% | 82.1% | 34.1% | 84.7% |
| Two-class Standard Deviation | 0.104 | 0.062 | 0.081 | 0.048 |
| Three-class Average Accuracy | 34.1% | 37.8% | 33.2% | 31.7% |
| Three-class Standard Deviation | 0.083 | 0.053 | 0.069 | 0.046 |
| SVM Pre-Screening | SVM Post-Screening | KNN Pre-Screening | KNN Post-Screening | |
|---|---|---|---|---|
| Two-class Average Accuracy | 51.5% | 73.4% | 63.9% | 72.0% |
| Two-class Standard Deviation | 0.019 | 0.025 | 0.033 | 0.028 |
| Three-class Average Accuracy | 47.2% | 63.2% | 57.9% | 60.3% |
| Three-class Standard Deviation | 0.042 | 0.009 | 0.036 | 0.024 |
| Subject | Execution | Feedback | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Screening | Post-Screening | Pre-Screening | Post-Screening | |||||
| Two-Class | Three-Class | Two-Class | Three-Class | Two-Class | Three-Class | Two-Class | Three-Class | |
| 01 | 55.1% | 36.6% | 73.4% | 38.5% | 48.2% | 43.9% | 64.1% | 53.1% |
| 02 | 52.6% | 40.4% | 81.5% | 41.1% | 60.3% | 45.8% | 77.3% | 48.0% |
| 03 | 45.2% | 34.1% | 82.1% | 37.8% | 51.5% | 47.2% | 73.4% | 63.2% |
| 04 | 36.7% | 30.0% | 68.8% | 33.3% | 52.6% | 38.9% | 71.1% | 55.9% |
| 05 | 59.3% | 40.5% | 85.3% | 55.2% | 53.8% | 29.0% | 71.7% | 56.1% |
| 06 | 49.2% | 38.1% | 77.3% | 39.6% | 51.4% | 41.3% | 72.3% | 62.9% |
| 07 | 58.8% | 37.7% | 86.7% | 26.8% | 45.9% | 45.1% | 83.4% | 61.3% |
| 08 | 49.6% | 35.4% | 74.0% | 43.5% | 53.6% | 35.7% | 78.6% | 59.2% |
| 09 | 55.3% | 41.2% | 85.4% | 42.4% | 46.4% | 37.4% | 68.2% | 63.1% |
| 10 | 50.0% | 37.9% | 84.8% | 36.7% | 51.4% | 34.5% | 77.0% | 62.6% |
| 11 | 38.8% | 25.4% | 79.6% | 43.7% | 41.6% | 40.4% | 84.4% | 47.1% |
| 12 | 42.9% | 35.8% | 83.6% | 35.7% | 53.2% | 30.2% | 72.8% | 64.2% |
| 13 | 46.7% | 28.4% | 85.1% | 42.1% | 43.1% | 26.7% | 78.2% | 48.2% |
| 14 | 53.5% | 36.4% | 71.7% | 41.3% | 49.3% | 30.0% | 73.7% | 47.3% |
| 15 | 51.7% | 34.7% | 86.0% | 27.4% | 50.4% | 31.4% | 82.3% | 52.9% |
| 16 | 46.8% | 41.2% | 82.5% | 40.1% | 47.5% | 35.0% | 71.2% | 62.5% |
| 17 | 56.2% | 35.4% | 77.4% | 51.3% | 60.4% | 28.9% | 75.0% | 51.3% |
| 18 | 48.1% | 36.5% | 90.2% | 25.5% | 51.2% | 37.1% | 86.5% | 61.7% |
| 19 | 44.2% | 38.9% | 80.0% | 42.0% | 49.2% | 33.7% | 67.2% | 58.2% |
| 20 | 61.0% | 39.6% | 68.9% | 45.2% | 57.4% | 41.4% | 58.7% | 53.6% |
| 21 | 38.4% | 34.5% | 78.3% | 34.6% | 48.6% | 32.5% | 70.1% | 58.1% |
| 22 | 57.1% | 42.6% | 81.6% | 41.2% | 54.2% | 37.9% | 66.1% | 57.4% |
| 23 | 42.4% | 36.1% | 85.1% | 42.6% | 59.4% | 39.8% | 71.2% | 64.2% |
| 24 | 47.2% | 33.8% | 79.2% | 35.7% | 52.4% | 36.4% | 74.1% | 63.6% |
| 25 | 60.0% | 38.0% | 84.7% | 43.3% | 61.3% | 45.2% | 78.4% | 60.3% |
| 26 | 56.7% | 27.6% | 78.3% | 48.2% | 59.3% | 37.9% | 71.2% | 59.5% |
| average | 50.1% | 36.0% | 80.5% | 39.6% | 52.1% | 36.7% | 74.1% | 62.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, B.; Niu, C.; Shi, J.; Li, R.; Yu, C.; Yin, K. A Method for the Study of Cerebellar Cognitive Function—Re-Cognition and Validation of Error-Related Potentials. Brain Sci. 2022, 12, 1173. https://doi.org/10.3390/brainsci12091173
Mu B, Niu C, Shi J, Li R, Yu C, Yin K. A Method for the Study of Cerebellar Cognitive Function—Re-Cognition and Validation of Error-Related Potentials. Brain Sciences. 2022; 12(9):1173. https://doi.org/10.3390/brainsci12091173
Chicago/Turabian StyleMu, Bo, Chang Niu, Jingping Shi, Rumei Li, Chao Yu, and Kuiying Yin. 2022. "A Method for the Study of Cerebellar Cognitive Function—Re-Cognition and Validation of Error-Related Potentials" Brain Sciences 12, no. 9: 1173. https://doi.org/10.3390/brainsci12091173
APA StyleMu, B., Niu, C., Shi, J., Li, R., Yu, C., & Yin, K. (2022). A Method for the Study of Cerebellar Cognitive Function—Re-Cognition and Validation of Error-Related Potentials. Brain Sciences, 12(9), 1173. https://doi.org/10.3390/brainsci12091173





