Hyperprolactinemia Associated with Attentional Processing and Interference Control Impairments in Patients with Prolactinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
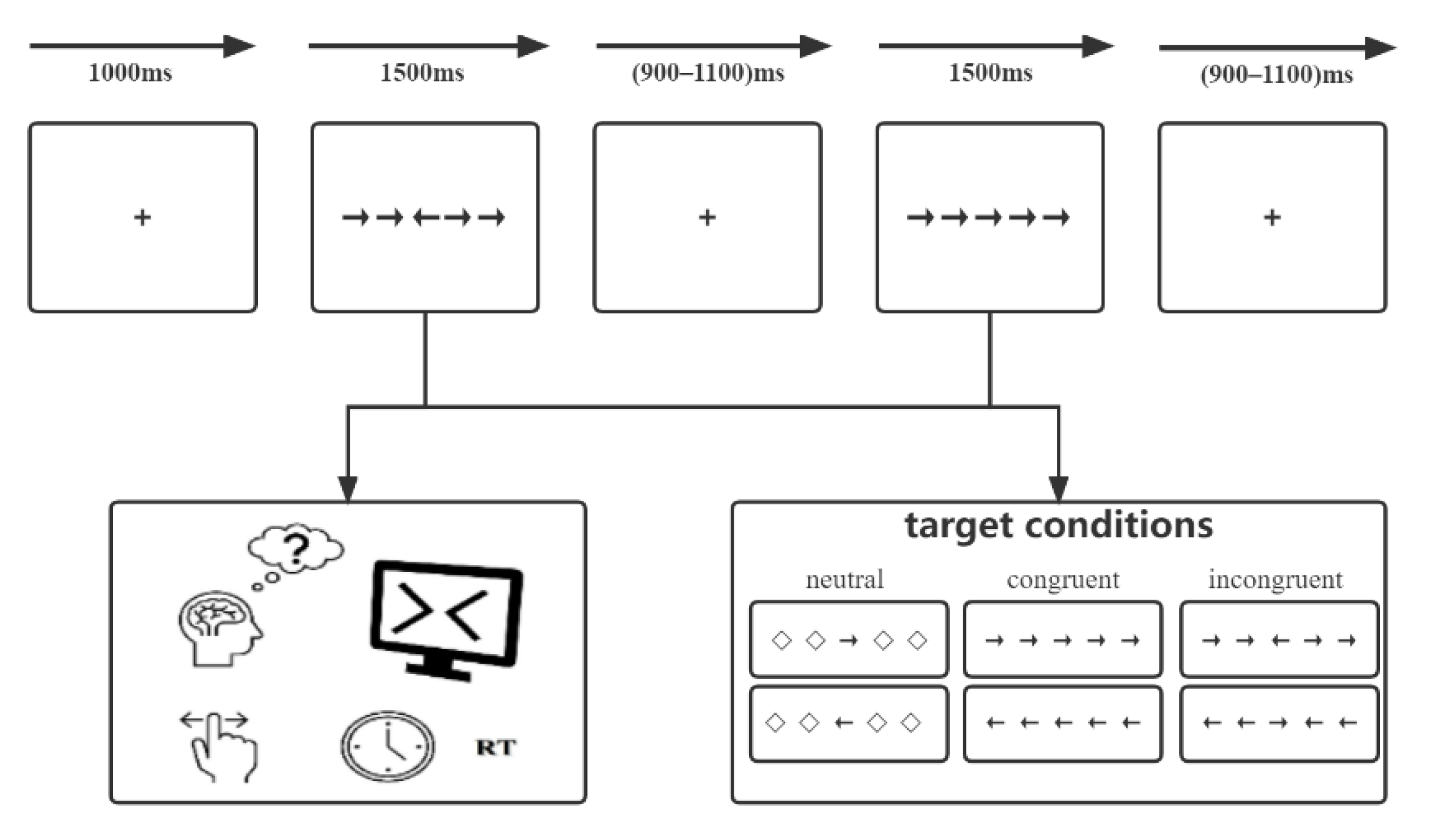
2.2. Stimuli and Procedure
2.3. EEG Acquisition
2.4. Behavioral Data Analysis
2.5. Electroencephalography Data Analysis
3. Results
3.1. Behavioral Results
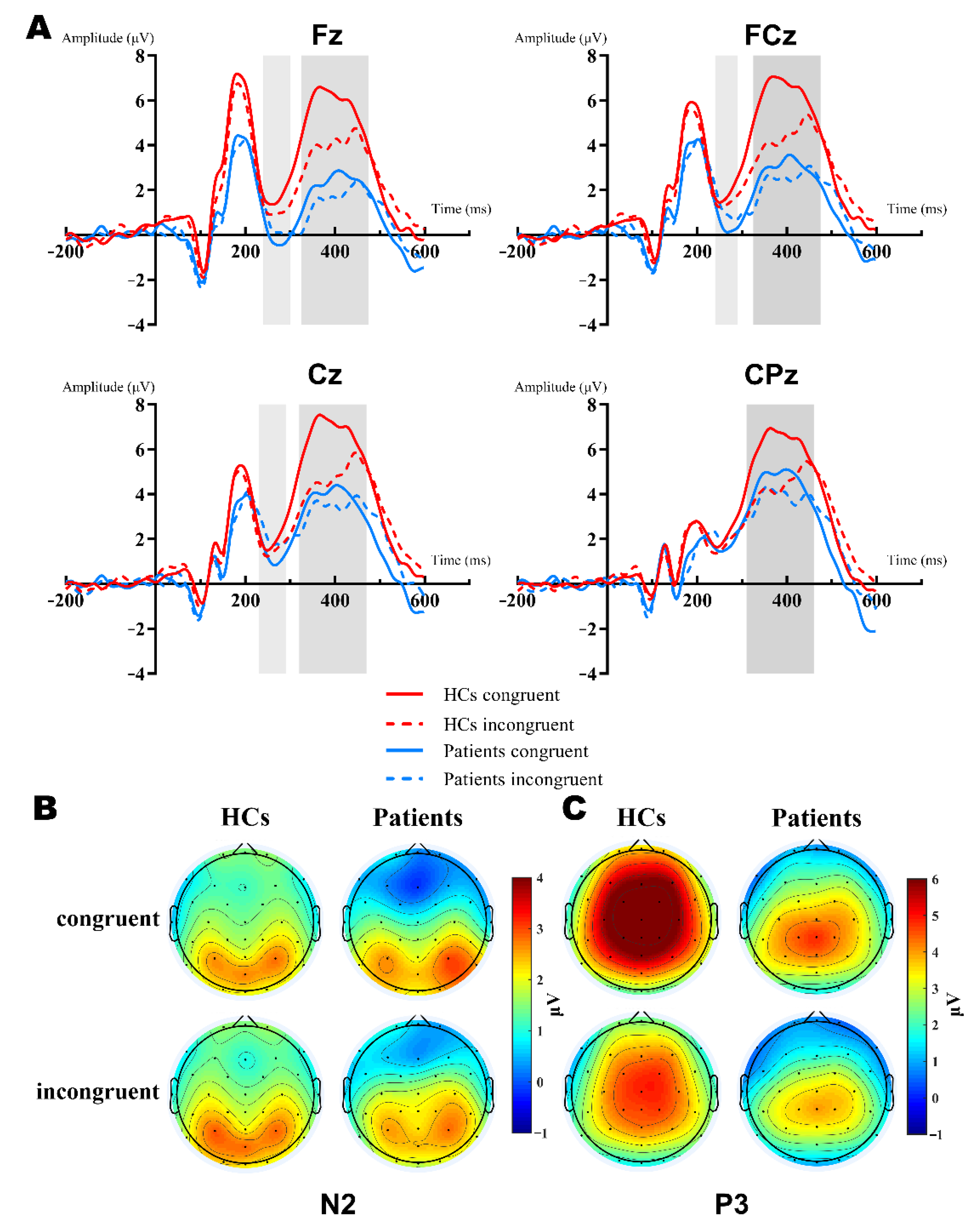
3.2. Electrophysiology Results
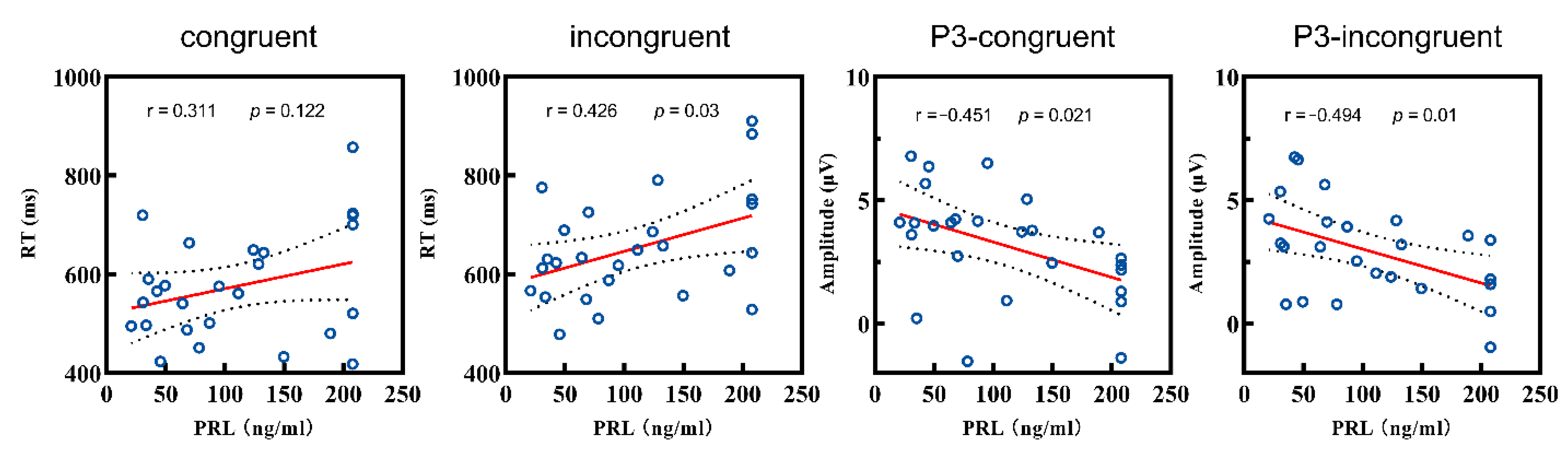
3.3. Correlation Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishioka, H.; Inoshita, N. New WHO classification of pituitary adenomas (4th edition): Assessment of pituitary transcription factors and the prognostic histological factors. Brain Tumor Pathol. 2018, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Beckers, A. The Epidemiology of Pituitary Adenomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Łojek, E.; Marchel, A. Cognitive functioning of patients with a PRL-secreting pituitary adenoma: A preliminary report. Neurology 2016, 86, 731–734. [Google Scholar] [CrossRef]
- Song, J.; Cao, C.; Yang, M.; Yao, S.; Yan, Y.; Peng, G.; Ma, P.; Huang, C.; Ding, H.; Xu, G. The dysfunction of processing task-irrelevant emotional faces in pituitary patients: An evidence from expression-related visual mismatch negativity. Neuroreport 2018, 29, 328–333. [Google Scholar] [CrossRef]
- Tooze, A.; Gittoes, N.J.; Jones, C.A.; Toogood, A.A. Neurocognitive consequences of surgery and radiotherapy for tumours of the pituitary. Clin. Endocrinol. 2009, 70, 503–511. [Google Scholar] [CrossRef]
- Cao, C.; Huang, Y.; Chen, A.; Xu, G.; Song, J. Improvement in Attention Processing After Surgical Treatment in Functional Pituitary Adenomas: Evidence From ERP Study. Front. Neurol. 2021, 12, 656255. [Google Scholar] [CrossRef]
- Yang, C.J.; Huang, G.S.; Xiao, F.R.; Lou, M.F. Symptom distress and quality of life after stereotactic radiosurgery in patients with pituitary tumors: A questionnaire survey. PLoS ONE 2014, 9, e88460. [Google Scholar] [CrossRef][Green Version]
- Grattan-Smith, P.J.; Morris, J.G.; Shores, E.A.; Batchelor, J.; Sparks, R.S. Neuropsychological abnormalities in patients with pituitary tumours. Acta Neurol. Scand. 1992, 86, 626–631. [Google Scholar] [CrossRef]
- Meyers, C.A. Neurobehavioral functioning of adults with pituitary disease. Psychother. Psychosom. 1998, 67, 168–172. [Google Scholar] [CrossRef]
- Pertichetti, M.; Serioli, S.; Belotti, F.; Mattavelli, D.; Schreiber, A.; Cappelli, C.; Padovani, A.; Gasparotti, R.; Nicolai, P.; Fontanella, M.M.; et al. Pituitary adenomas and neuropsychological status: A systematic literature review. Neurosurg. Rev. 2020, 43, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Athanasoulia, A.P.; Ising, M.; Pfister, H.; Mantzoros, C.S.; Stalla, G.K.; Sievers, C. Distinct dopaminergic personality patterns in patients with prolactinomas: A comparison with nonfunctioning pituitary adenoma patients and age- and gender-matched controls. Neuroendocrinology 2012, 96, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cesar de Oliveira Naliato, E.; Dutra Violante, A.H.; Caldas, D.; Lamounier Filho, A.; Rezende Loureiro, C.; Fontes, R.; Schrank, Y.; Gomes de Souza, R.; Vaisman, M.; Guerra, E.; et al. Quality of life in women with microprolactinoma treated with dopamine agonists. Pituitary 2008, 11, 247–254. [Google Scholar] [CrossRef]
- Kars, M.; van der Klaauw, A.A.; Onstein, C.S.; Pereira, A.M.; Romijn, J.A. Quality of life is decreased in female patients treated for microprolactinoma. Eur. J. Endocrinol. 2007, 157, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Andela, C.D.; van Haalen, F.M.; Ragnarsson, O.; Papakokkinou, E.; Johannsson, G.; Santos, A.; Webb, S.M.; Biermasz, N.R.; van der Wee, N.J.; Pereira, A.M. Mechanisms in Endocrinology: Cushing’s syndrome causes irreversible effects on the human brain: A systematic review of structural and functional magnetic resonance imaging studies. Eur. J. Endocrinol. 2015, 173, R1–R14. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J.F.; Madrazo-Atutxa, A.; Venegas-Moreno, E.; Benito-López, P.; Gálvez, M.; Cano, D.A.; Tinahones, F.J.; Torres-Vela, E.; Soto-Moreno, A.; Leal-Cerro, A. Neurocognitive function in acromegaly after surgical resection of GH-secreting adenoma versus naïve acromegaly. PLoS ONE 2013, 8, e60041. [Google Scholar] [CrossRef] [PubMed]
- Brisman, M.H.; Fetell, M.R.; Post, K.D. Reversible dementia due to macroprolactinoma. Case report. J. Neurosurg. 1993, 79, 135–137. [Google Scholar] [CrossRef]
- Butterbrod, E.; Gehring, K.; Voormolen, E.H.; Depauw, P.; Nieuwlaat, W.A.; Rutten, G.M.; Sitskoorn, M.M. Cognitive functioning in patients with nonfunctioning pituitary adenoma before and after endoscopic endonasal transsphenoidal surgery. J. Neurosurg. 2019, 133, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tong, X.; Zou, Y.; Tian, X.; Mao, Z.; Sun, Z. The impact on cognitive functions of patients with pituitary adenoma before and after surgery. Neurol. Sci. 2017, 38, 1315–1321. [Google Scholar] [CrossRef]
- Psaras, T.; Milian, M.; Hattermann, V.; Gerlach, C.; Honegger, J. Executive functions recover earlier than episodic memory after microsurgical transsphenoidal resection of pituitary tumors in adult patients--a longitudinal study. J. Clin. Neurosci. 2011, 18, 1340–1345. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef] [PubMed]
- Sinai, J.; Wong, A.H. Craniopharyngeoma presenting as psychosis, disinhibition and personality change without neurological signs. Acta Neuropsychiatr. 2003, 15, 94–96. [Google Scholar] [CrossRef]
- Gratton, G.; Cooper, P.; Fabiani, M.; Carter, C.S.; Karayanidis, F. Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology 2018, 55, e13016. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C.; Shum, D.; Toulopoulou, T.; Chen, E.Y. Assessment of executive functions: Review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008, 23, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Song, J.; Gao, J.; Lin, P.; Yang, M.; Zahid, K.R.; Yan, Y.; Cao, C.; Ma, P.; Zhang, H.; et al. Cognitive Function and Serum Hormone Levels Are Associated with Gray Matter Volume Decline in Female Patients with Prolactinomas. Front. Neurol. 2017, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Luck, S. An Introduction to The Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Pires, L.; Leitão, J.; Guerrini, C.; Simões, M.R. Event-Related Brain Potentials in the Study of Inhibition: Cognitive Control, Source Localization and Age-Related Modulations. Neuropsychol. Rev. 2014, 24, 461–490. [Google Scholar] [CrossRef]
- Chang, S.; Yang, L.; Wang, Y.; Faraone, S.V. Shared polygenic risk for ADHD, executive dysfunction and other psychiatric disorders. Transl. Psychiatry 2020, 10, 182. [Google Scholar] [CrossRef]
- Clawson, A.; Clayson, P.E.; Larson, M.J. Cognitive control adjustments and conflict adaptation in major depressive disorder. Psychophysiology 2013, 50, 711–721. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Kok, A. Cognitive and biological determinants of P300: An integrative review. Biol. Psychol. 1995, 41, 103–146. [Google Scholar] [CrossRef]
- Neuhaus, A.H.; Urbanek, C.; Opgen-Rhein, C.; Hahn, E.; Ta, T.M.; Koehler, S.; Gross, M.; Dettling, M. Event-related potentials associated with Attention Network Test. Int. J. Psychophysiol. 2010, 76, 72–79. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.C.; Tartar, J.L.; Widmayer, S.; Rosselli, M. Negative attention bias and processing deficits during the cognitive reappraisal of unpleasant emotions in HIV+ women. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e32–e39. [Google Scholar] [CrossRef]
- Olson, R.L.; Brush, C.J.; Ehmann, P.J.; Buckman, J.F.; Alderman, B.L. A history of sport-related concussion is associated with sustained deficits in conflict and error monitoring. Int. J. Psychophysiol. 2018, 132, 145–154. [Google Scholar] [CrossRef]
- Liu, Y.; Hanna, G.L.; Hanna, B.S.; Rough, H.E.; Arnold, P.D.; Gehring, W.J. Behavioral and Electrophysiological Correlates of Performance Monitoring and Development in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Brain Sci. 2020, 10, 79. [Google Scholar] [CrossRef]
- Kałamała, P.; Szewczyk, J.; Senderecka, M.; Wodniecka, Z. Flanker task with equiprobable congruent and incongruent conditions does not elicit the conflict N2. Psychophysiology 2018, 55, e12980. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Q.; Chen, A.; Li, H.; Wang, Q.; Zhuang, Z.; Jia, S. Are we sensitive to valence differences in emotionally negative stimuli? Electrophysiological evidence from an ERP study. Neuropsychologia 2007, 45, 2764–2771. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, X.; Pei, G.; Xu, Z. The Hazard Perception for the Surrounding Shape of Warning Signs: Evidence From an Event-Related Potentials Study. Front. Neurosci. 2018, 12, 824. [Google Scholar] [CrossRef]
- Daffner, K.R.; Alperin, B.R.; Mott, K.K.; Tusch, E.S.; Holcomb, P.J. Age-related differences in early novelty processing: Using PCA to parse the overlapping anterior P2 and N2 components. Biol. Psychol. 2015, 105, 83–94. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M.; Brown, C.R.; Heaven, P.C. Event-related potentials in adults with Attention-Deficit/Hyperactivity Disorder: An investigation using an inter-modal auditory/visual oddball task. Int. J. Psychophysiol. 2009, 71, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, F.; Silveira, C.; Almeida, P.R.; Palha, A.; Barbosa, F.; Marques-Teixeira, J. The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clin. Neurophysiol. 2012, 123, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Grondin, S.; Blanchet, S. Event-related potentials of episodic encoding after traumatic brain injury in older adults. Brain Res. 2021, 1766, 147504. [Google Scholar] [CrossRef] [PubMed]
- Golonka, K.; Mojsa-Kaja, J.; Marek, T.; Gawlowska, M. Stimulus, response and feedback processing in burnout—An EEG study. Int. J. Psychophysiol. 2018, 134, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Stoerig, P.; Falkenstein, M. ERP—Correlates of response selection in a response conflict paradigm. Brain Res. 2008, 1189, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.H.; Yoon, J.; Jung, K.Y. Influence of task difficulty on the features of event-related potential during visual oddball task. Neurosci. Lett. 2008, 445, 179–183. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Kutas, M. Electrophysiology of cognitive processing. Annu. Rev. Psychol. 1983, 34, 33–61. [Google Scholar] [CrossRef]
- Clayson, P.E.; Larson, M.J. Psychometric properties of conflict monitoring and conflict adaptation indices: Response time and conflict N2 event-related potentials. Psychophysiology 2013, 50, 1209–1219. [Google Scholar] [CrossRef]
- Danielmeier, C.; Wessel, J.R.; Steinhauser, M.; Ullsperger, M. Modulation of the error-related negativity by response conflict. Psychophysiology 2009, 46, 1288–1298. [Google Scholar] [CrossRef]
- Righi, S.; Mecacci, L.; Viggiano, M.P. Anxiety, cognitive self-evaluation and performance: ERP correlates. J. Anxiety Disord. 2009, 23, 1132–1138. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; Galvao-Carmona, A.; González-Rosa, J.J.; Hidalgo-Muñoz, A.R.; Borges, M.; Ruiz-Peña, J.L.; Izquierdo, G. Neural correlates of alerting and orienting impairment in multiple sclerosis patients. PLoS ONE 2014, 9, e97226. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.G.; Schmidt, S.; Inacker, E.; Markowiak, M.; Hinterberger, T. Meditation and attention: A controlled study on long-term meditators in behavioral performance and event-related potentials of attentional control. Int. J. Psychophysiol. 2016, 99, 33–39. [Google Scholar] [CrossRef]
- Parks, A.C.; Moore, R.D.; Wu, C.T.; Broglio, S.P.; Covassin, T.; Hillman, C.H.; Pontifex, M.B. The association between a history of concussion and variability in behavioral and neuroelectric indices of cognition. Int. J. Psychophysiol. 2015, 98, 426–434. [Google Scholar] [CrossRef]
- Shenhav, A.; Botvinick, M.M.; Cohen, J.D. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 2013, 79, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.F.; Ysrraelit, M.C. Role of prolactin in B cell regulation in multiple sclerosis. J. Neuroimmunol. 2014, 269, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Rodriguez-Oroz, M.; Antonini, A.; Brotchie, J.M.; Ray Chaudhuri, K.; Brown, R.G.; Galpern, W.R.; Nirenberg, M.J.; Okun, M.S.; Lang, A.E. Management of impulse control disorders in Parkinson’s disease: Controversies and future approaches. Mov. Disord. 2015, 30, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.S.; Muhammed, K.; Baig, F.; Kelly, M.; Saleh, Y.; Sarangmat, N.; Okai, D.; Hu, M.; Manohar, S.; Husain, M. Dopamine and reward hypersensitivity in Parkinson’s disease with impulse control disorder. Brain 2020, 143, 2502–2518. [Google Scholar] [CrossRef]

| HCs Group | Patients Group | p | |
|---|---|---|---|
| N | 26 | 27 | / |
| Females/Males | 16/10 | 14/13 | 0.477 a |
| Age (years) (M ± SD) | 33.36 ± 11.97 | 36.08 ± 11.21 | 0.535 b |
| Education (years) (M ± SD) | 12.80 ± 2.32 | 11.21 ± 3.37 | 0.740 b |
| Serum PRL (ng/mL) (M ± SD) | / | 107.41 ± 68.70 Range (21.02–208) | / |
| Dependent Variables | Hyperprolactinemia-Related Decrease | No Difference | Hyperprolactinemia-Related Increase |
|---|---|---|---|
| Behavioral | |||
| Reaction Time | √ | ||
| Accuracy | √ | ||
| ERP | |||
| P2 Amplitude | √ | ||
| N2 Amplitude | √ | ||
| P3 Amplitude | √ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Cao, C.; Liu, B.; Wang, S.; Wu, S.; Xu, G.; Song, J. Hyperprolactinemia Associated with Attentional Processing and Interference Control Impairments in Patients with Prolactinomas. Brain Sci. 2022, 12, 1091. https://doi.org/10.3390/brainsci12081091
Chen A, Cao C, Liu B, Wang S, Wu S, Xu G, Song J. Hyperprolactinemia Associated with Attentional Processing and Interference Control Impairments in Patients with Prolactinomas. Brain Sciences. 2022; 12(8):1091. https://doi.org/10.3390/brainsci12081091
Chicago/Turabian StyleChen, Aobo, Chenglong Cao, Bangxin Liu, Shuochen Wang, Shukai Wu, Guozheng Xu, and Jian Song. 2022. "Hyperprolactinemia Associated with Attentional Processing and Interference Control Impairments in Patients with Prolactinomas" Brain Sciences 12, no. 8: 1091. https://doi.org/10.3390/brainsci12081091
APA StyleChen, A., Cao, C., Liu, B., Wang, S., Wu, S., Xu, G., & Song, J. (2022). Hyperprolactinemia Associated with Attentional Processing and Interference Control Impairments in Patients with Prolactinomas. Brain Sciences, 12(8), 1091. https://doi.org/10.3390/brainsci12081091






