Long-Term Outcomes of Local Tirofiban Infusion for Intracranial Atherosclerosis-Related Occlusion
Abstract
:1. Introduction
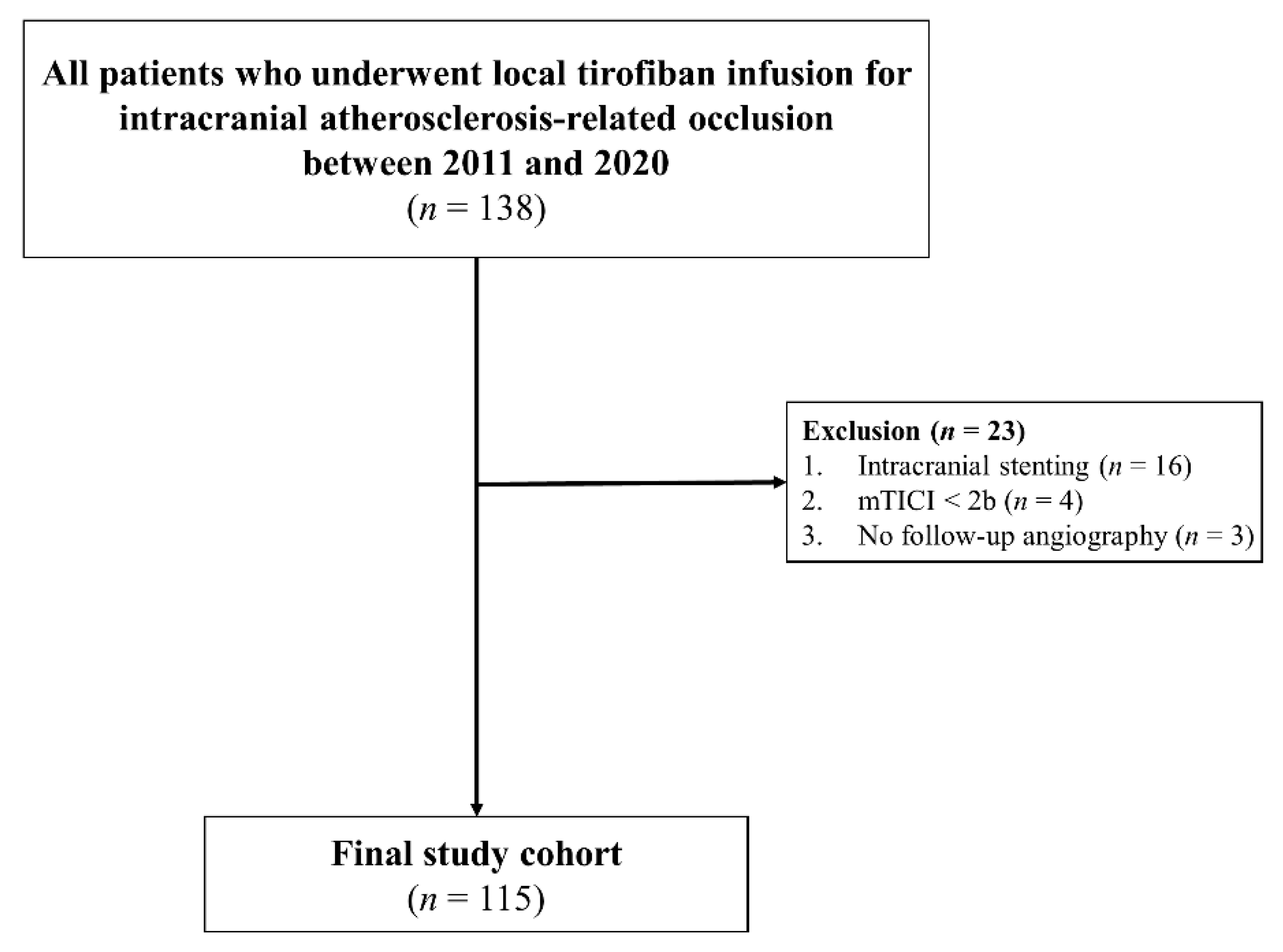
2. Materials and Methods
2.1. Patients
2.2. Endovascular Therapy
2.3. Post-ERT Management and Follow-Up
2.4. Evaluation of Clinical and Radiological Outcomes
2.5. Statistical Analysis
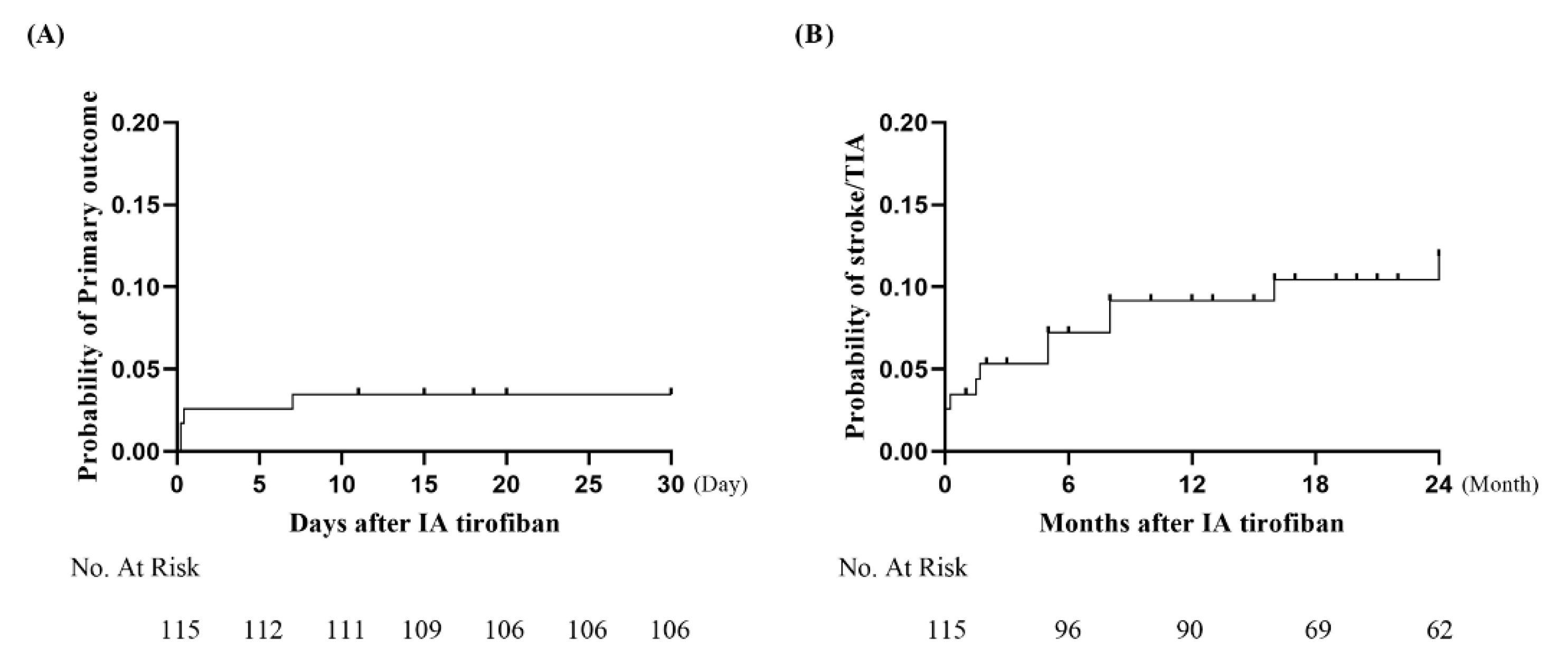
3. Results
3.1. Baseline Characteristics and Outcomes of ERT
3.2. Follow-Up Clinical and Radiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef]
- Kim, J.S.; Bang, O.Y. Medical Treatment of Intracranial Atherosclerosis: An Update. J. Stroke 2017, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M. Causes and Solutions of Endovascular Treatment Failure. J. Stroke 2017, 19, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Sohn, S.I.; Yoo, J.; Hong, J.H.; Kim, C.H.; Kang, D.H.; Kim, Y.S.; Lee, S.J.; Hong, J.M.; Choi, J.W.; et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: Tirofiban ASSIST study. BMC Neurol. 2020, 20, 284. [Google Scholar] [CrossRef]
- King, S.; Short, M.; Harmon, C. Glycoprotein IIb/IIIa inhibitors: The resurgence of tirofiban. Vascul. Pharmacol. 2016, 78, 10–16. [Google Scholar] [CrossRef]
- Pan, X.; Zheng, D.; Zheng, Y.; Chan, P.W.L.; Lin, Y.; Zou, J.; Zhou, J.; Yang, J. Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur. J. Neurol. 2019, 26, 1105–1110. [Google Scholar] [CrossRef]
- Zhao, W.; Che, R.; Shang, S.; Wu, C.; Li, C.; Wu, L.; Chen, J.; Duan, J.; Song, H.; Zhang, H.; et al. Low-Dose Tirofiban Improves Functional Outcome in Acute Ischemic Stroke Patients Treated with Endovascular Thrombectomy. Stroke 2017, 48, 3289–3294. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Zhao, Z.; Chen, X.; Huang, C.; Li, X.; Shan, Y.; Zou, Y.; Liu, Y.; Ibrahim, M.; et al. Safety and Efficacy of Tirofiban Combined With Mechanical Thrombectomy Depend on Ischemic Stroke Etiology. Front. Neurol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Yoo, A.J.; Khatri, P.; Tomsick, T.A.; von Kummer, R.; Saver, J.L.; Marks, M.P.; Prabhakaran, S.; Kallmes, D.F.; Fitzsimmons, B.F.; et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013, 44, 2650–2663. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Ryu, W.S.; Park, S.S.; Kim, Y.S.; Lee, S.H.; Kang, K.; Kim, C.; Sohn, C.H.; Lee, S.H.; Yoon, B.W. Long-term natural history of intracranial arterial stenosis: An MRA follow-up study. Cerebrovasc. Dis. 2014, 38, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Yang, W.J.; Wong, K.S.; Chen, X.Y. Intracranial Atherosclerosis: From Microscopy to High-Resolution Magnetic Resonance Imaging. J. Stroke 2017, 19, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, G. Safety of Glycoprotein IIb-IIIa Inhibitors Used in Stroke-Related Treatment: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620942594. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ko, S.B.; Jung, K.H.; Jang, M.U.; Kim, D.H.; Kim, J.T.; Choi, J.C.; Jeong, H.S.; Kim, C.; Heo, J.H.; et al. 2022 Update of the Korean Clinical Practice Guidelines for Stroke: Antithrombotic Therapy for Patients with Acute Ischemic Stroke or Transient Ischemic Attack. J. Stroke 2022, 24, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kasner, S.E.; Chimowitz, M.I.; Lynn, M.J.; Howlett-Smith, H.; Stern, B.J.; Hertzberg, V.S.; Frankel, M.R.; Levine, S.R.; Chaturvedi, S.; Benesch, C.G.; et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006, 113, 555–563. [Google Scholar] [CrossRef]
- Derdeyn, C.P.; Chimowitz, M.I.; Lynn, M.J.; Fiorella, D.; Turan, T.N.; Janis, L.S.; Montgomery, J.; Nizam, A.; Lane, B.F.; Lutsep, H.L.; et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): The final results of a randomised trial. Lancet 2014, 383, 333–341. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Fitzsimmons, B.F.; Woodward, B.K.; Wang, Z.; Killer-Oberpfalzer, M.; Wakhloo, A.; Gupta, R.; Kirshner, H.; Megerian, J.T.; Lesko, J.; et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: The VISSIT randomized clinical trial. JAMA 2015, 313, 1240–1248. [Google Scholar] [CrossRef]
- Alexander, M.J.; Zauner, A.; Chaloupka, J.C.; Baxter, B.; Callison, R.C.; Gupta, R.; Song, S.S.; Yu, W.; Sites, W.T.; WEAVE Trial Sites and Interventionalists. WEAVE Trial: Final Results in 152 On-Label Patients. Stroke 2019, 50, 889–894. [Google Scholar] [CrossRef]
- Alexander, M.J.; Zauner, A.; Gupta, R.; Alshekhlee, A.; Fraser, J.F.; Toth, G.; Given, C.; Mackenzie, L.; Kott, B.; Hassan, A.E.; et al. The WOVEN trial: Wingspan One-year Vascular Events and Neurologic Outcomes. J. Neurointerv. Surg. 2021, 13, 307–310. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Furukawa, M.; Goldsberry, S.; Movahed, M.R. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review. Exp. Clin. Cardiol. 2008, 13, 192–197. [Google Scholar] [PubMed]
- Ter Schiphorst, A.; Charron, S.; Hassen, W.B.; Provost, C.; Naggara, O.; Benzakoun, J.; Seners, P.; Turc, G.; Baron, J.C.; Oppenheim, C. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: A clinical study. J. Cereb. Blood Flow Metab. 2021, 41, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Renu, A.; Millan, M.; San Roman, L.; Blasco, J.; Marti-Fabregas, J.; Terceno, M.; Amaro, S.; Serena, J.; Urra, X.; Laredo, C.; et al. Effect of Intra-arterial Alteplase vs Placebo Following Successful Thrombectomy on Functional Outcomes in Patients With Large Vessel Occlusion Acute Ischemic Stroke: The CHOICE Randomized Clinical Trial. JAMA 2022, 327, 826–835. [Google Scholar] [CrossRef]
- Kim, G.E.; Yoon, W.; Kim, S.K.; Kim, B.C.; Heo, T.W.; Baek, B.H.; Lee, Y.Y.; Yim, N.Y. Incidence and Clinical Significance of Acute Reocclusion after Emergent Angioplasty or Stenting for Underlying Intracranial Stenosis in Patients with Acute Stroke. AJNR Am. J. Neuroradiol. 2016, 37, 1690–1695. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Cotsonis, G.A.; Saver, J.L.; Lynn, M.J.; Cloft, H.J.; Chimowitz, M.I.; Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collateral circulation in symptomatic intracranial atherosclerosis. J. Cereb. Blood Flow Metab. 2011, 31, 1293–1301. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Klucznik, R.; Alexander, M.J.; Chaloupka, J.; Lutsep, H.; Barnwell, S.; Mawad, M.; Lane, B.; Lynn, M.J.; Chimowitz, M.; et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology 2008, 70, 1518–1524. [Google Scholar] [CrossRef]
- Levy, E.I.; Turk, A.S.; Albuquerque, F.C.; Niemann, D.B.; Aagaard-Kienitz, B.; Pride, L.; Purdy, P.; Welch, B.; Woo, H.; Rasmussen, P.A.; et al. Wingspan in-stent restenosis and thrombosis: Incidence, clinical presentation, and management. Neurosurgery 2007, 61, 644–650; discussion 650–651. [Google Scholar] [CrossRef]
- Guo, X.; Ma, N.; Gao, F.; Mo, D.P.; Luo, G.; Miao, Z.R. Long-Term Risk Factors for Intracranial In-Stent Restenosis From a Multicenter Trial of Stenting for Symptomatic Intracranial Artery Stenosis Registry in China. Front. Neurol. 2020, 11, 601199. [Google Scholar] [CrossRef]
- Mosimann, P.J.; Kaesmacher, J.; Gautschi, D.; Bellwald, S.; Panos, L.; Piechowiak, E.; Dobrocky, T.; Zibold, F.; Mordasini, P.; El-Koussy, M.; et al. Predictors of Unexpected Early Reocclusion After Successful Mechanical Thrombectomy in Acute Ischemic Stroke Patients. Stroke 2018, 49, 2643–2651. [Google Scholar] [CrossRef]
- Li, W.; Ding, J.; Sui, X.; Qi, Z.; Wu, L.; Sun, C.; Ji, K.; Ma, Q.; Ji, X.; Liu, K.J. Prognosis and risk factors for reocclusion after mechanical thrombectomy. Ann. Clin. Transl. Neurol. 2020, 7, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.; Remollo, S.; Quesada, H.; Renu, A.; Tomasello, A.; Minhas, P.; Perez de la Ossa, N.; Rubiera, M.; Llull, L.; Cardona, P.; et al. Vessel Patency at 24 Hours and Its Relationship with Clinical Outcomes and Infarct Volume in REVASCAT Trial (Randomized Trial of Revascularization with Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset). Stroke 2017, 48, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.P.; Strambo, D.; Hajdu, S.D.; Eskandari, A.; Nannoni, S.; Sirimarco, G.; Bartolini, B.; Puccinelli, F.; Maeder, P.; Saliou, G.; et al. Twenty-Four-Hour Reocclusion After Successful Mechanical Thrombectomy: Associated Factors and Long-Term Prognosis. Stroke 2019, 50, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.Y.; Wong, E.H.; Wong, A.; Mok, V.C.; Leung, T.W.; Wong, K.S. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc. Dis. 2012, 33, 517–524. [Google Scholar] [CrossRef]
- Fanari, Z.; Malodiya, A.; Weiss, S.A.; Hammami, S.; Kolm, P.; Weintraub, W.S. Long-term use of dual antiplatelet therapy for the secondary prevention of atherothrombotic events: Meta-analysis of randomized controlled trials. Cardiovasc. Revascula. 2017, 18, 10–15. [Google Scholar] [CrossRef]
| Variables | All Patients (n = 115) |
|---|---|
| Age, years | 67.0 (59.0–75.0) |
| Sex, male | 77 (67.0%) |
| Admission NIHSS | 13.0 (9.0–19.0) |
| Intravenous thrombolysis | 36 (31.3%) |
| Occlusion site | |
| MCA M1 | 71 (61.7%) |
| MCA M2 | 7 (6.1%) |
| VBA | 21 (18.3%) |
| ICA | 16 (13.9%) |
| Risk factor | |
| Hypertension | 76 (66.1%) |
| Diabetes | 35 (30.4%) |
| Hyperlipidemia | 55 (47.8%) |
| Smoking | 57 (49.6%) |
| Atrial fibrillation | 9 (7.8%) |
| Coronary artery disease | 9 (7.8%) |
| History of stroke or TIA | 22 (19.1%) |
| Previous antiplatelet | 22 (19.1%) |
| Previous oral anticoagulant | 2 (1.7%) |
| Onset to groin puncture time, min | 402.0 (244.0–825.0) |
| Puncture to final reperfusion time, min | 71.0 (52.0–90.0) |
| Post-ERT intracranial hemorrhage | 6 (5.2%) |
| HI type 1 | 4 (3.5%) |
| HI type 2 | 2 (1.7%) |
| PH type 1 | 0 (0.0%) |
| PH type 2 | 0 (0.0%) |
| Post-ERT symptomatic ICH | 0 (0.0%) |
| Post-ERT subarachnoid hemorrhage | 1 (0.9%) |
| mRS score 0–2 at 3 months | 66 (57.4%) |
| Mortality at 3 months | 8 (7.0%) |
| Primary Outcome | Patients (n = 115) |
|---|---|
| Ischemic stroke within 30 days | 3 (2.6%) |
| Transient ischemic attack within 30 days | 0 (0.0%) |
| Stroke-related death | 1 (0.9%) |
| Secondary Outcome | |
| Ischemic stroke beyond 30 days | 6 (5.2%) |
| Transient ischemic attack beyond 30 days | 2 (1.8%) |
| Any stroke outside of treated artery | 0 (0.0%) |
| Symptomatic brain hemorrhage | 0 (0.0%) |
| Non-stroke-related death | 11 (9.6%) |
| Radiological Outcome | |
| Change in stenosis within 7 days | Patients (n = 115) |
| Stationary | 99 (86.1%) |
| Progression including reocclusion | 16 (13.9%) |
| Reocclusion | 14 (12.2%) |
| Long-term change in stenosis | Patients (n = 56) |
| Stationary | 42 (75.0%) |
| Progression including reocclusion | 14 (25.0%) |
| Reocclusion | 3 (5.4%) |
| Variables | Non-Early Reocclusion (n = 101) | Early Reocclusion (n = 14) | p Value | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Sex, male | 70 (69.3%) | 7 (50.0%) | 0.158 | 1.802 (0.567–13.539) | 0.567 |
| Age, years | 67.0 (59.0–75.0) | 67.5 (58.8–75.2) | 0.952 | ||
| Admission NIHSS | 13.0 (9.0–19.0) | 14 (8.0–20.3) | 0.906 | ||
| Intravenous thrombolysis | 31 (30.7%) | 5 (35.7%) | 0.705 | ||
| Occlusion site | 0.545 | ||||
| MCA | 70 (69.3%) | 8 (57.1%) | |||
| VBA | 17 (16.8%) | 4 (28.6%) | |||
| ICA | 14 (13.9%) | 2 (14.3%) | |||
| Hypertension | 69 (68.3%) | 7 (50.0%) | 0.182 | 0.69 (0.149–3.204) | 0.636 |
| Diabetes | 32 (31.7%) | 3 (21.4%) | 0.439 | ||
| Hyperlipidemia | 50 (49.5%) | 5 (35.7%) | 0.338 | ||
| Smoking | 53 (52.5%) | 4 (28.6%) | 0.104 | 2.844 (0.369–21.915) | 0.316 |
| Atrial fibrillation | 9 (8.9%) | 0 (0.0%) | 0.999 | ||
| Coronary artery disease | 8 (7.9%) | 1 (7.1%) | 0.919 | ||
| History of stroke or TIA | 19 (18.8%) | 3 (21.4%) | 0.816 | ||
| Previous antiplatelet | 20 (19.8%) | 2 (14.3%) | 0.625 | ||
| Previous oral anticoagulant | 2 (2.0%) | 0 | 0.999 | ||
| No neurologic improvement | 17 (16.8%) | 11 (78.6%) | 0.001 | 17.907 (3.423–93.694) | 0.001 |
| Admission homocysteine, umol/L | 11.7 (8.8–14.4) | 9.5 (6.5–12.5) | 0.055 | 0.869 (0.702–1.074) | 0.194 |
| Trial | Treatment Method | 30-Day Event Rate | 1-Year Event Rate | Progression of Stenosis |
| Current study (n = 115) | IA tirofiban, aspirin, clopidogrel, atorvastatin | 3.5% (95% Cl, 3.5–24.9%) | 9.2% (95% Cl, 8.0–18.6%) | 25.0% (14/56) |
| WASID (≥70% ICAS, n = 206) [16], ‡ | aspirin, statin | N/A | 23% (95% CI, 15–30%) | N/A |
| SAMMPRIS [17], § (medical arm, n = 227) | aspirin, clopidogrel, rosuvastatin | 5.8% (95% CI, 3.4–9.7%) | 12.2% (95% CI, 8.4–17.6%) | N/A |
| VISSIT [18], || (medical arm, n = 53) | aspirin, clopidogrel, atorvastatin | 9.4% (95% CI, 3.0–20.7%) | 15.1% (95% CI, 6.7–27.6%) | N/A |
| SAMMPRIS [17], § (WS arm, n = 224) | Wingspan stenting, aspirin, clopidogrel, rosuvastatin | 14.7% (95% CI, 10.7–20.1%) | 20.0% (95% CI, 15.2–26.0%) | N/A |
| VISSIT [18], || (WS arm, n = 58) | Wingspan stenting, aspirin, clopidogrel, atorvastatin | 24.1% (95% CI, 13.9–37.2%) | 36.2% (95% CI, 24.0–49.9%) | 29.4% (10/34) |
| WEAVE [19], ¶ (n = 159) /WOVEN [20], ** (n = 129) | Wingspan stenting, aspirin, clopidogrel, statin | 2.6% (WEAVE) | 8.5% (WOVEN) | 17.6% (18/102, WOVEN) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Hwang, Y.-H.; Kim, Y.-W. Long-Term Outcomes of Local Tirofiban Infusion for Intracranial Atherosclerosis-Related Occlusion. Brain Sci. 2022, 12, 1089. https://doi.org/10.3390/brainsci12081089
Choi W, Hwang Y-H, Kim Y-W. Long-Term Outcomes of Local Tirofiban Infusion for Intracranial Atherosclerosis-Related Occlusion. Brain Sciences. 2022; 12(8):1089. https://doi.org/10.3390/brainsci12081089
Chicago/Turabian StyleChoi, Woochan, Yang-Ha Hwang, and Yong-Won Kim. 2022. "Long-Term Outcomes of Local Tirofiban Infusion for Intracranial Atherosclerosis-Related Occlusion" Brain Sciences 12, no. 8: 1089. https://doi.org/10.3390/brainsci12081089
APA StyleChoi, W., Hwang, Y.-H., & Kim, Y.-W. (2022). Long-Term Outcomes of Local Tirofiban Infusion for Intracranial Atherosclerosis-Related Occlusion. Brain Sciences, 12(8), 1089. https://doi.org/10.3390/brainsci12081089






