The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence
Abstract
1. Introduction
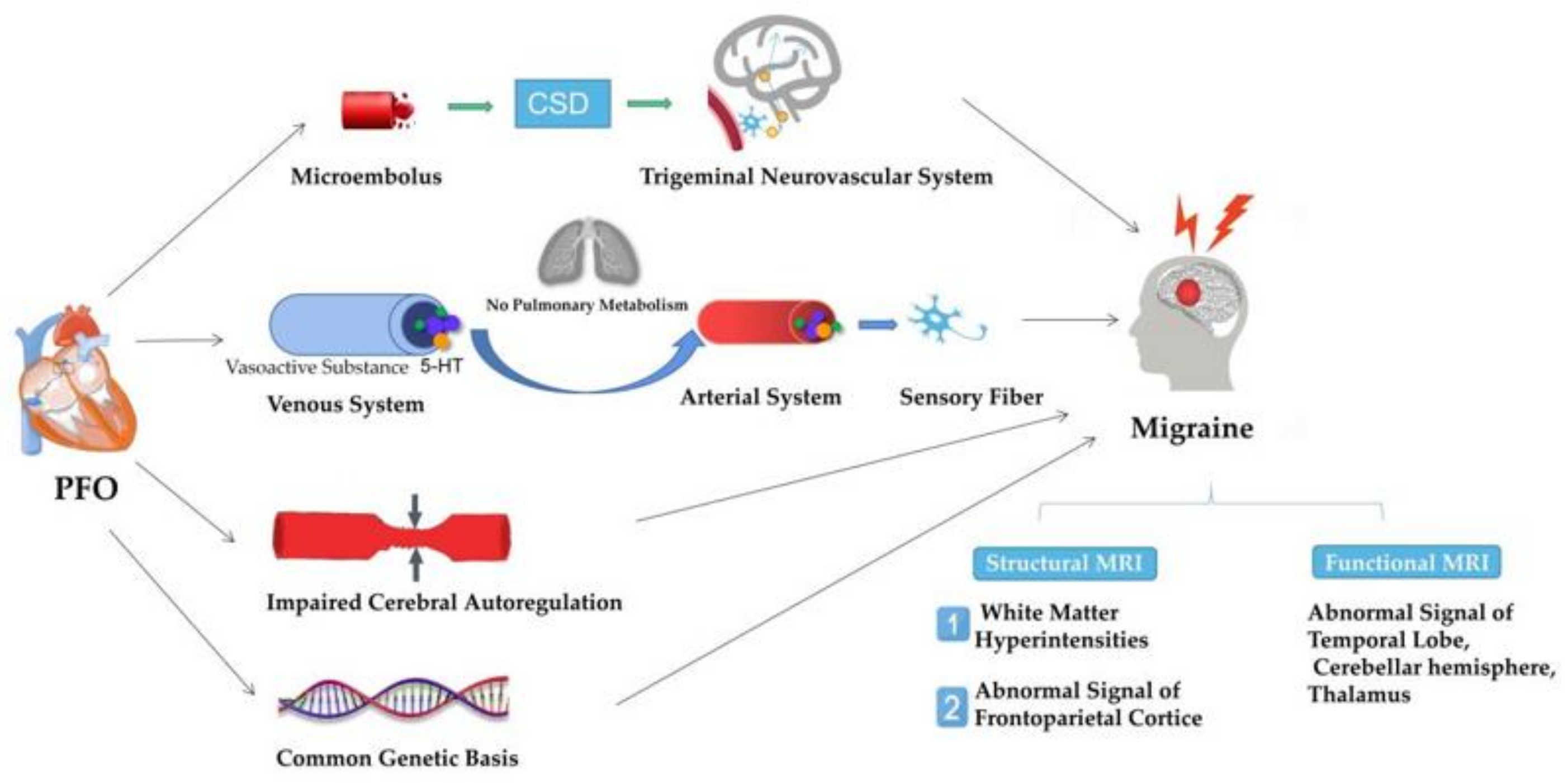
2. PFO and Migraine
3. MRI and Migraine
| Technique | Study | Population | Target Location | Findings | Reference |
|---|---|---|---|---|---|
| VBM | Valfrè et al. | CM | Right superior temporal gyrus, right inferior frontal gyrus, and left precentral gyrus | The significant reduction in gray matter in several cortical areas involved in the pain circuit is related to migraine. | [49] |
| Palm-Meinders et al. | MA and MwoA | Right occipital cortex | Migraine patients show small changes in brain volume in cortical areas involving visual motor processing. | [50] | |
| Chen et al. | EM | Periaqueductal gray (PAG) | PAG volume expansion proves the existence of brain injury and can be used as an imaging biomarker for the diagnosis and evaluation of migraine. | [51] | |
| Zhang et al. | MwoA | Bilateral cerebellar culmen, occipital–temporal cortex, right insula, left postcentral gyrus, superior parietal lobule, right lateral occipital cortex, left rostral middle frontal gyrus | The significant changes in these gray areas may be related to the perception, integration, and processing of pain. | [52] | |
| Qin et al. | MwoA | Cerebellum and brainstem | The microstructure changes in the cerebellum and local brainstem that appeared in MwoA indicate that they are involved in the pathologies of migraine without aura. | [53] | |
| Neeb et al. | CM and EM | Amygdala and putamen, frontal and temporal gyrus, left cuneus | GM changes are associated with migraine frequency, so the increase in gray matter volume may reflect the remodeling of the central nervous system. | [54] | |
| SBM | Schwedt et al. | EM and CM | Temporal pole, anterior cingulate cortex, superior temporal lobe, entorhinal cortex, medial orbital frontal gyrus, and pars triangularis | Compared with EM, the cortical surface area, cortical thickness, and cortical volume in CM showed significant differences, and these differences can be used to accurately distinguish CM and EM. | [55] |
| DTI | Planchuelo-Gómez et al. | EM and CM | Bilateral external capsule | Compared with patients with episodic migraine, patients with chronic migraine may have axonal integrity damage in the first few months of chronic migraine attacks. | [56] |
| Porcaro et al. | MwoA | Hypothalamic | The hypothalamus plays a crucial role in the onset of migraine. | [57] | |
| Tantik Pak et al. | Migraine patients | Corpus callosum | The corpus callosum of migraine patients showed microstructural changes. | [58] | |
| Task-fMRI | Cao et al. | MA and MwoA | Visual stimuli, red nucleus and substantia nigra | The brain stem is activated during migraine attacks. | [59] |
| Yu et al. | VM and MwoA | Vestibular stimulation, parietal lobe, temporal lobe, insular lobe, cingulate gyrus, thalamus, caudate nucleus, optic radiation, precuneus. | The abnormal activation of the thalamus and fusiform gyrus may be involved in the pathophysiological mechanism of VM. | [60] | |
| Stankewitz et al. | Migraine patients | Olfactory stimulation, brain areas, rostral pons | The increased activity in the rostral part of pons indicates that there may be a physiological relationship between olfaction and the trigeminal nociceptive pathway. | [61] | |
| RS-fMRI | Cui et al. | MwoA | Vision-related brain networks | Visual–related brain networks are dysfunctional in migraine patients. | [62] |
| Tu et al. | MwoA | The occipital lobe, the sensorimotor network, part of the medial-cerebellum, the cingulo–opercular network, the default mode network, the frontal–parietal network | The functional connections of 6 regions in patients with migraine without aura have specific changes, which can be used to distinguish migraine patients from healthy controls. | [63] |
4. MRI Evidence in Migraine Patients with PFO
4.1. Structural MRI-Based Evidence
4.1.1. Gray Matter Changes
4.1.2. White Matter Changes
| Literature | Patients | Location of WMH | Findings | Reference |
|---|---|---|---|---|
| Signoriello et al. | Migraine patients | Occipital lesions and juxtacortical seat. | PFO may be associated with white matter lesions in migraine patients, especially those with occipital lesions and visual aura. | [70] |
| Park et al. | Tension-type headache and migraineurs | Deep white matter | In young migraine patients, small deep WMH is associated with RLS. | [26] |
| Yoon et al. | Migraine patients | Juxtacortex and cortico–subcortical junction. | Juxtacortical spots on FLAIR images may be related to the presence of PFO in migraine patients. | [71] |
| Iwasaki et al. | MA and MwoA | Deep or subcortical white matters | RLS may be associated with WMH in Japanese migraine patients. | [72] |
4.2. Functional MRI-Based Evidence
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Pourfathi, H.; Eagan, A.; Mansournia, M.A.; Khodayari, M.T.; Sullman, M.; Kaufman, J.; Collins, G.; Dai, H.; Bragazzi, N.L.; et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 2022, 163, e293–e309. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Buse, D.C.; Fanning, K.M.; Reed, M.L.; Murray, S.; Dumas, P.K.; Adams, A.M.; Lipton, R.B. Life with Migraine: Effects on Relationships, Career, and Finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache 2019, 59, 1286–1299. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Romano, V.; Gallinoro, C.M.; Mottola, R.; Serio, A.; Di Meglio, F.; Castaldo, C.; Sirico, F.; Nurzynska, D. Patent Foramen Ovale—A Not So Innocuous Septal Atrial Defect in Adults. J. Cardiovasc. Dev. Dis. 2021, 8, 60. [Google Scholar] [CrossRef]
- Liu, K.; Wang, B.Z.; Hao, Y.; Song, S.; Pan, M. The Correlation between Migraine and Patent Foramen Ovale. Front. Neurol. 2020, 11, 543485. [Google Scholar] [CrossRef]
- Ailani, J. Migraine and patent foramen ovale. Curr. Neurol. Neurosci. Rep. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Jiang, S.-L.; Zhu, X.-Y. Chinese expert guidelines for the prevention of patent foramen ovale-associated stroke. Chin. Heart J. 2021, 33, 1–10. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Roberts, S.C.; Winoker, J.S.; Romero, J.; Goodman-Meza, D.; Gevorgyan, R.; Tobis, J.M. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: A bivariate meta-analysis of prospective studies. JACC Cardiovasc. Imaging 2014, 7, 236–250. [Google Scholar] [CrossRef]
- Jauss, M.; Zanette, E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc. Dis. 2000, 10, 490–496. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Zaman, M.O.; Elgendy, I.Y.; Mahmoud, A.N.; Patel, N.K.; Agarwal, N.; Tobis, J.M.; Meier, B. Cryptogenic Stroke and Patent Foramen Ovale. J. Am. Coll. Cardiol. 2018, 71, 1035–1043. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Gevorgyan, R.; Tobis, J.M. A comparison of methods to detect and quantitate PFO: TCD, TTE, ICE and TEE. In Patent Foramen Ovale; Springer: London, UK, 2015; pp. 55–65. [Google Scholar] [CrossRef]
- Martín, M.; Secades, S.; Campos, A.G.; Corros, C.; Rodríguez, M.L.; De La Hera, J.M. Patent foramen ovale and stroke: Rethinking the need for systematic transesophageal echocardiography. Minerva Med. 2012, 103, 413–414. [Google Scholar]
- Rodrigues, A.C.; Picard, M.H.; Carbone, A.; Arruda, A.L.; Flores, T.; Klohn, J.; Furtado, M.; Lira-Filho, E.B.; Cerri, G.G.; Andrade, J.L. Importance of adequately performed Valsalva maneuver to detect patent foramen ovale during transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 1337–1343. [Google Scholar] [CrossRef]
- Del Sette, M.; Angeli, S.; Leandri, M.; Ferriero, G.; Bruzzone, G.L.; Finocchi, C.; Gandolfo, C. Migraine with aura and right-to-left shunt on transcranial Doppler: A case-control study. Cerebrovasc. Dis. 1998, 8, 327–330. [Google Scholar] [CrossRef]
- Lip, P.Z.; Lip, G.Y. Patent foramen ovale and migraine attacks: A systematic review. Am. J. Med. 2014, 127, 411–420. [Google Scholar] [CrossRef]
- Kijima, Y.; Miller, N.; Noureddin, N.; Gevorgyan, R.; Tobis, J. TCT-738 the Degree of Right-to-Left Shunt Is Associated with Visual Aura due to Migraine. J. Am. Coll. Cardiol. 2015, 66, B301. [Google Scholar] [CrossRef][Green Version]
- Altamura, C.; Paolucci, M.; Costa, C.M.; Brunelli, N.; Cascio Rizzo, A.; Cecchi, G.; Vernieri, F. Right-to-Left Shunt and the Clinical Features of Migraine with Aura: Earlier but Not More. Cerebrovasc. Dis. 2019, 47, 268–274. [Google Scholar] [CrossRef]
- Tian, D.-C.; Wang, H.; Chen, W.; Tian, Q.; Zhang, L.-J.; Hui, X.; Wang, X.-J. Meta-analysis of white matter lesions and patent foramen ovale in migraine. Neural Inj. Funct. Reconstr. 2019, 14, 236–240, 252. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, R.; Zhou, J.; Dong, Z.; Chen, Y. Prevalence and grade of RLS in migraine: A prospective study of 251 migraineurs by synchronous test of c-TTE and c-TCD. Medicine 2021, 100, e24175. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, A.; Peng, B.; He, S.; Zhao, X.; Zhu, Y.; Lai, W.; Song, T.; Chen, L. Association between patent foramen ovale and migraine without aura: A community-based cross-sectional study in China. BMJ Open 2022, 12, e056937. [Google Scholar] [CrossRef]
- Wilmshurst, P.T.; Pearson, M.J.; Nightingale, S.; Walsh, K.P.; Morrison, W.L. Inheritance of persistent foramen ovale and atrial septal defects and the relation to familial migraine with aura. Heart 2004, 90, 1315–1320. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, S.Y.; Kim, S.E.; Yun, C.H.; Kim, S.H. Small deep white matter lesions are associated with right-to-left shunts in migraineurs. J. Neurol. 2011, 258, 427–433. [Google Scholar] [CrossRef]
- Jia, Z.Y. Recognition and Cognitive Risk Assessment of Brain Dysfunction in Patients with Migraine by Resting-State Functional Magnetic Resonance Imaging. Master’s Thesis, Jilin University, Changchun, China, 2021. [Google Scholar] [CrossRef]
- Bridges, N.D.; Hellenbrand, W.; Latson, L.; Filiano, J.; Newburger, J.W.; Lock, J.E. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992, 86, 1902–1908. [Google Scholar] [CrossRef]
- Wilmshurst, P.T.; Nightingale, S.; Walsh, K.P.; Morrison, W.L. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet 2000, 356, 1648–1651. [Google Scholar] [CrossRef]
- Dowson, A.; Mullen, M.J.; Peatfield, R.; Muir, K.; Khan, A.A.; Wells, C.; Lipscombe, S.L.; Rees, T.; De Giovanni, J.V.; Morrison, W.L.; et al. Migraine Intervention with STARFlex Technology (MIST) trial: A prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008, 117, 1397–1404. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Kumar, P.; Mahmoud, A.N.; Elgendy, I.Y.; Shapiro, H.; West, B.; Charles, A.C.; Mattle, H.P.; Sorensen, S.; Meier, B.; et al. Pooled Analysis of PFO Occluder Device Trials in Patients with PFO and Migraine. J. Am. Coll. Cardiol. 2021, 77, 667–676. [Google Scholar] [CrossRef]
- Tobis, J.M.; Charles, A.; Silberstein, S.D.; Sorensen, S.; Maini, B.; Horwitz, P.A.; Gurley, J.C. Percutaneous Closure of Patent Foramen Ovale in Patients with Migraine: The PREMIUM Trial. J. Am. Coll. Cardiol. 2017, 70, 2766–2774. [Google Scholar] [CrossRef]
- Mattle, H.P.; Evers, S.; Hildick-Smith, D.; Becker, W.J.; Baumgartner, H.; Chataway, J.; Gawel, M.; Göbel, H.; Heinze, A.; Horlick, E.; et al. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur. Heart J. 2016, 37, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Tamim, I.; Chung, D.Y.; de Morais, A.L.; Loonen, I.; Qin, T.; Misra, A.; Schlunk, F.; Endres, M.; Schiff, S.J.; Ayata, C. Spreading depression as an innate antiseizure mechanism. Nat. Commun. 2021, 12, 2206. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Woitzik, J.; Hecht, N.; Pinczolits, A.; Sandow, N.; Major, S.; Winkler, M.K.; Weber-Carstens, S.; Dohmen, C.; Graf, R.; Strong, A.J.; et al. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 2013, 80, 1095–1102. [Google Scholar] [CrossRef]
- Nozari, A.; Dilekoz, E.; Sukhotinsky, I.; Stein, T.; Eikermann-Haerter, K.; Liu, C.; Wang, Y.; Frosch, M.P.; Waeber, C.; Ayata, C.; et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann. Neurol. 2010, 67, 221–229. [Google Scholar] [CrossRef]
- Petrusic, I.; Podgorac, A.; Zidverc-Trajkovic, J.; Radojicic, A.; Jovanovic, Z.; Sternic, N. Do interictal microembolic signals play a role in higher cortical dysfunction during migraine aura? Cephalalgia 2016, 36, 561–567. [Google Scholar] [CrossRef]
- Caputi, L.; Usai, S.; Carriero, M.R.; Grazzi, L.; D’Amico, D.; Falcone, C.; Anzola, G.P.; Del Sette, M.; Parati, E.; Bussone, G. Microembolic air load during contrast-transcranial Doppler: A trigger for migraine with aura? Headache 2010, 50, 1320–1327. [Google Scholar] [CrossRef]
- Wilmshurst, P.; Nightingale, S. The role of cardiac and pulmonary pathology in migraine: A hypothesis. Headache 2006, 46, 429–434. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef]
- Borgdorff, P.; Tangelder, G.J. Migraine: Possible role of shear-induced platelet aggregation with serotonin release. Headache 2012, 52, 1298–1318. [Google Scholar] [CrossRef]
- Villalón, C.M.; VanDenBrink, A.M. The Role of 5-Hydroxytryptamine in the Pathophysiology of Migraine and its Relevance to the Design of Novel Treatments. Mini Rev. Med. Chem. 2017, 17, 928–938. [Google Scholar] [CrossRef]
- Wang, H.L. The Clinical Curative Effect and Safety of Percutaneous Closure of Patent Foramen Ovale. Master’s Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]
- Paulson, O.B.; Strandgaard, S.; Edvinsson, L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 1990, 2, 161–192. [Google Scholar]
- Guo, Z.N.; Xing, Y.; Liu, J.; Wang, S.; Yan, S.; Jin, H.; Yang, Y. Compromised dynamic cerebral autoregulation in patients with a right-to-left shunt: A potential mechanism of migraine and cryptogenic stroke. PLoS ONE 2014, 9, e104849. [Google Scholar] [CrossRef]
- Schürks, M.; Rist, P.M.; Kurth, T. MTHFR 677C>T and ACE D/I polymorphisms in migraine: A systematic review and meta-analysis. Headache 2010, 50, 588–599. [Google Scholar] [CrossRef]
- Szczygioł, D.; Motta, E.; Gołba, A.; Stęposz, A.; Witecka, J.; Dębski, M.; Błaszkiewicz, D.; Sieroń, A. Frequency of the C677T variant of the methylenetetrahydrofolate reductase (MTHFR) gene in patients with migraine with or without aura—A preliminary report. Neurol. Neurochir. Pol. 2012, 46, 443–449. [Google Scholar] [CrossRef]
- Valfrè, W.; Rainero, I.; Bergui, M.; Pinessi, L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008, 48, 109–117. [Google Scholar] [CrossRef]
- Palm-Meinders, I.H.; Arkink, E.B.; Koppen, H.; Amlal, S.; Terwindt, G.M.; Launer, L.J.; van Buchem, M.A.; Ferrari, M.D.; Kruit, M.C. Volumetric brain changes in migraineurs from the general population. Neurology 2017, 89, 2066–2074. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Liu, M.; Liu, S.; Ma, L.; Yu, S. Volume expansion of periaqueductal gray in episodic migraine: A pilot MRI structural imaging study. J. Headache Pain 2017, 18, 83. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.L.; Su, J.; Yao, Q.; Wang, M.; Li, G.F.; Zhao, R.; Shi, Y.H.; Zhao, Y.; Zhang, Q.; et al. Assessment of gray and white matter structural alterations in migraineurs without aura. J. Headache Pain 2017, 18, 74. [Google Scholar] [CrossRef]
- Qin, Z.; He, X.W.; Zhang, J.; Xu, S.; Li, G.F.; Su, J.; Shi, Y.H.; Ban, S.; Hu, Y.; Liu, Y.S.; et al. Structural changes of cerebellum and brainstem in migraine without aura. J. Headache Pain 2019, 20, 93. [Google Scholar] [CrossRef]
- Neeb, L.; Bastian, K.; Villringer, K.; Israel, H.; Reuter, U.; Fiebach, J.B. Structural Gray Matter Alterations in Chronic Migraine: Implications for a Progressive Disease? Headache 2017, 57, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Schwedt, T.J.; Chong, C.D.; Wu, T.; Gaw, N.; Fu, Y.; Li, J. Accurate Classification of Chronic Migraine via Brain Magnetic Resonance Imaging. Headache 2015, 55, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Planchuelo-Gómez, Á.; García-Azorín, D.; Guerrero, Á.L.; Aja-Fernández, S.; Rodríguez, M.; de Luis-García, R. White matter changes in chronic and episodic migraine: A diffusion tensor imaging study. J. Headache Pain 2020, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, C.; Di Renzo, A.; Tinelli, E.; Di Lorenzo, G.; Seri, S.; Di Lorenzo, C.; Parisi, V.; Caramia, F.; Fiorelli, M.; Di Piero, V.; et al. Hypothalamic structural integrity and temporal complexity of cortical information processing at rest in migraine without aura patients between attacks. Sci. Rep. 2021, 11, 18701. [Google Scholar] [CrossRef]
- Tantik Pak, A.; Nacar Dogan, S.; Sengul, Y. Structural integrity of corpus callosum in patients with migraine: A diffusion tensor imaging study. Acta Neurol. Belg. 2022, 122, 1–6. [Google Scholar] [CrossRef]
- Cao, Y.; Aurora, S.K.; Nagesh, V.; Patel, S.C.; Welch, K.M. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology 2002, 59, 72–78. [Google Scholar] [CrossRef]
- Yu, H.X.; Li, H.Y.; Yin, Z.X.; Zhang, J.L.; Liu, G. The fMRI research of vestibular migraine neural pathways. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 906–909. [Google Scholar] [CrossRef]
- Stankewitz, A.; May, A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 2011, 77, 476–482. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Xu, F.; Zhi, H.; Li, H.; Li, B.; Zhang, S.; Peng, W.; Wu, H. MRI Evaluation of the Relationship between Abnormalities in Vision-Related Brain Networks and Quality of Life in Patients with Migraine without Aura. Neuropsychiatr. Dis. Treat. 2021, 17, 3569–3579. [Google Scholar] [CrossRef]
- Tu, Y.; Zeng, F.; Lan, L.; Li, Z.; Maleki, N.; Liu, B.; Chen, J.; Wang, C.; Park, J.; Lang, C.; et al. An fMRI-based neural marker for migraine without aura. Neurology 2020, 94, e741–e751. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Cheng, H.M.; Chen, S.P.; Chung, C.P.; Lin, Y.Y.; Hu, H.H.; Chen, C.H.; Wang, S.J. White matter hyperintensities in migraine: Clinical significance and central pulsatile hemodynamic correlates. Cephalalgia 2018, 38, 1225–1236. [Google Scholar] [CrossRef]
- Rossato, G.; Adami, A.; Thijs, V.N.; Cerini, R.; Pozzi-Mucelli, R.; Mazzucco, S.; Anzola, G.P.; Del Sette, M.; Dinia, L.; Meneghetti, G.; et al. Cerebral distribution of white matter lesions in migraine with aura patients. Cephalalgia 2010, 30, 855–859. [Google Scholar] [CrossRef]
- Chen, D.; Yang, J.; Zeng, W.; Xu, Y.; Jiao, L.; Wang, N. Brain Functional Connectivity in Patients with Migraine Based on Complex Networks Analysis. Chin. J. Med. Imaging 2015, 23, 418–422. [Google Scholar] [CrossRef]
- Nie, W.; Zeng, W.; Yang, J.; Shi, Y.; Zhao, L.; Li, Y.; Chen, D.; Deng, J.; Wang, N. Extraction and Analysis of Dynamic Functional Connectome Patterns in Migraine Sufferers: A Resting-State fMRI Study. Comput. Math. Methods Med. 2021, 2021, 6614520. [Google Scholar] [CrossRef]
- Dai, Z.; Zhong, J.; Xiao, P.; Zhu, Y.; Chen, F.; Pan, P.; Shi, H. Gray matter correlates of migraine and gender effect: A meta-analysis of voxel-based morphometry studies. Neuroscience 2015, 299, 88–96. [Google Scholar] [CrossRef]
- Kang, K.W.; Kim, J.T.; Chang, J.; Choi, W.H.; Lim, D.; Bang, D.H.; Choi, Y.J. Transient sulcal hyperintensities on fluid-attenuated inversion recovery in migraine with aura: Transient sulcal hyperintensities in migraine. Headache 2012, 52, 1430–1433. [Google Scholar] [CrossRef]
- Signoriello, E.; Cirillo, M.; Puoti, G.; Signoriello, G.; Negro, A.; Koci, E.; Melone, M.; Rapacciuolo, A.; Maresca, G.; Lus, G. Migraine as possible red flag of PFO presence in suspected demyelinating disease. J. Neurol. Sci. 2018, 390, 222–226. [Google Scholar] [CrossRef]
- Yoon, G.J.; Kim, J.T.; Chang, J.; Kim, D.E.; Cho, B.H.; Lee, J.H.; Jung, H.J.; Lee, S.H.; Choi, S.M.; Park, M.S.; et al. Right-to-left shunts as a cause of juxtacortical spots in patients with migraine. Eur. J. Neurol. 2012, 19, 1086–1092. [Google Scholar] [CrossRef]
- Iwasaki, A.; Suzuki, K.; Takekawa, H.; Takashima, R.; Suzuki, A.; Suzuki, S.; Hirata, K. The relationship between right-to-left shunt and brain white matter lesions in Japanese patients with migraine: A single center study. J. Headache Pain 2017, 18, 1–6. [Google Scholar] [CrossRef]
- Ter Laan, M.; van Dijk, J.M.; Elting, J.W.; Staal, M.J.; Absalom, A.R. Sympathetic regulation of cerebral blood flow in humans: A review. Br. J. Anaesth. 2013, 111, 361–367. [Google Scholar] [CrossRef]
- Hayashida, K.; Fukuchi, K.; Inubushi, M.; Fukushima, K.; Imakita, S.; Kimura, K. Embolic distribution through patent foramen ovale demonstrated by (99m)Tc-MAA brain SPECT after Valsalva radionuclide venography. J. Nucl. Med. 2001, 42, 859–863. [Google Scholar]
- Xie, Q.Q.; Chen, X.; Tian, Y.; Fang, L.; Zhao, H. Compromised cerebrovascular reactivity in migraineurs with right-to-left shunts: A potential mechanism of white matter hyperintensities. Neurol. Res. 2022, 44, 1–7. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, P.; He, H.; Liu, Y.; Liu, S.; Zhu, M.; Niu, Z.; Yan, B. Analysis of white matter damage and influencing factors in patients with migraine. J. Clin. Psychosom. Dis. 2022, 28, 21–23, 42. [Google Scholar]
- Jiang, X.H.; Wang, S.B.; Tian, Q.; Zhong, C.; Zhang, G.L.; Li, Y.J.; Lin, P.; You, Y.; Guo, R.; Cui, Y.H.; et al. Right-to-left shunt and subclinical ischemic brain lesions in Chinese migraineurs: A multicentre MRI study. BMC Neurol. 2018, 18, 18. [Google Scholar] [CrossRef]
- Del Sette, M.; Dinia, L.; Bonzano, L.; Roccatagliata, L.; Finocchi, C.; Parodi, R.C.; Sivori, G.; Gandolfo, C. White matter lesions in migraine and right-to-left shunt: A conventional and diffusion MRI study. Cephalalgia 2008, 28, 376–382. [Google Scholar] [CrossRef]
- Candee, M.S.; McCandless, R.T.; Moore, K.R.; Arrington, C.B.; Minich, L.L.; Bale, J.F., Jr. White matter lesions in children and adolescents with migraine. Pediatr. Neurol. 2013, 49, 393–396. [Google Scholar] [CrossRef]
- Tedeschi, G.; Russo, A.; Conte, F.; Salemi, F.; Tessitore, A. The role of BOLD-fMRI in elucidating migraine pathophysiology. Neurol. Sci. 2013, 34 (Suppl. S1), 47–50. [Google Scholar] [CrossRef][Green Version]
- Garg, P.; Servoss, S.J.; Wu, J.C.; Bajwa, Z.H.; Selim, M.H.; Dineen, A.; Kuntz, R.E.; Cook, E.F.; Mauri, L. Lack of association between migraine headache and patent foramen ovale: Results of a case-control study. Circulation 2010, 121, 1406–1412. [Google Scholar] [CrossRef]
- Kahya Eren, N.; Bülbül, N.G.; Yakar Tülüce, S.; Nazlı, C.; Beckmann, Y. To Be or Not to Be Patent: The Relationship between Migraine and Patent Foramen Ovale. Headache 2015, 55, 934–942. [Google Scholar] [CrossRef]
- Koppen, H.; Palm-Meinders, I.H.; Mess, W.H.; Keunen, R.W.; Terwindt, G.M.; Launer, L.J.; van Buchem, M.A.; Kruit, M.C.; Ferrari, M.D. Systemic right-to-left shunts, ischemic brain lesions, and persistent migraine activity. Neurology 2016, 86, 1668–1675. [Google Scholar] [CrossRef]
- Bian, Y.-T.; Xie, H.; Jian, Z.-J.; Li, J.-J.; Ding, N.-N.; Niu, G.; Luo, G.-G.; Yang, J. A comparative study of patent foramen ovale and white matter lesions in migraine without aura. J. Xi’an Jiaotong Univ. 2018, 39, 185–189, 209. [Google Scholar] [CrossRef]
- Larrosa-Campo, D.; Meilán-Martínez, A.; Ramón-Carbajo, C.; Santamarta-Liébana, E.; Saiz-Ayala, A.; Martínez-Camblor, P.; Cernuda-Morollón, E.; Pascual, J. Is there a relationship between white matter lesions associated with migraine and patent foramen ovale? Analysis of a series of patients with chronic migraine. Rev. Neurol. 2020, 70, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Paolucci, M.; Brunelli, N.; Cascio Rizzo, A.; Cecchi, G.; Assenza, F.; Silvestrini, M.; Vernieri, F. Right-to-left shunts and hormonal therapy influence cerebral vasomotor reactivity in patients with migraine with aura. PLoS ONE 2019, 14, e0220637. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Shen, Y.; Zhong, J.; Chen, Z.; Wang, N.; Yang, J. The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence. Brain Sci. 2022, 12, 941. https://doi.org/10.3390/brainsci12070941
Cao W, Shen Y, Zhong J, Chen Z, Wang N, Yang J. The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence. Brain Sciences. 2022; 12(7):941. https://doi.org/10.3390/brainsci12070941
Chicago/Turabian StyleCao, Wenfei, Yinbo Shen, Jiaqi Zhong, Zhenhong Chen, Nizhuan Wang, and Jiajun Yang. 2022. "The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence" Brain Sciences 12, no. 7: 941. https://doi.org/10.3390/brainsci12070941
APA StyleCao, W., Shen, Y., Zhong, J., Chen, Z., Wang, N., & Yang, J. (2022). The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence. Brain Sciences, 12(7), 941. https://doi.org/10.3390/brainsci12070941







