Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. TBI Simulation
- -
- Sham-operated animals undergoing anesthesia and surgery preparation without TBI + 70%N2/30% O2 inhalation (SO group), n = 7;
- -
- Control group with TBI + inhalation of 70%N2/30%O2 (TBI group), n = 14;
- -
- Experimental group with TBI + 70%Ar/O230% inhalation (TBI + iAr group), n = 14.
2.3. Argon Exposure
2.4. Neurological Assessment
2.5. MRI Scan
2.6. Histological Examination
2.7. Statistical Analysis
3. Results
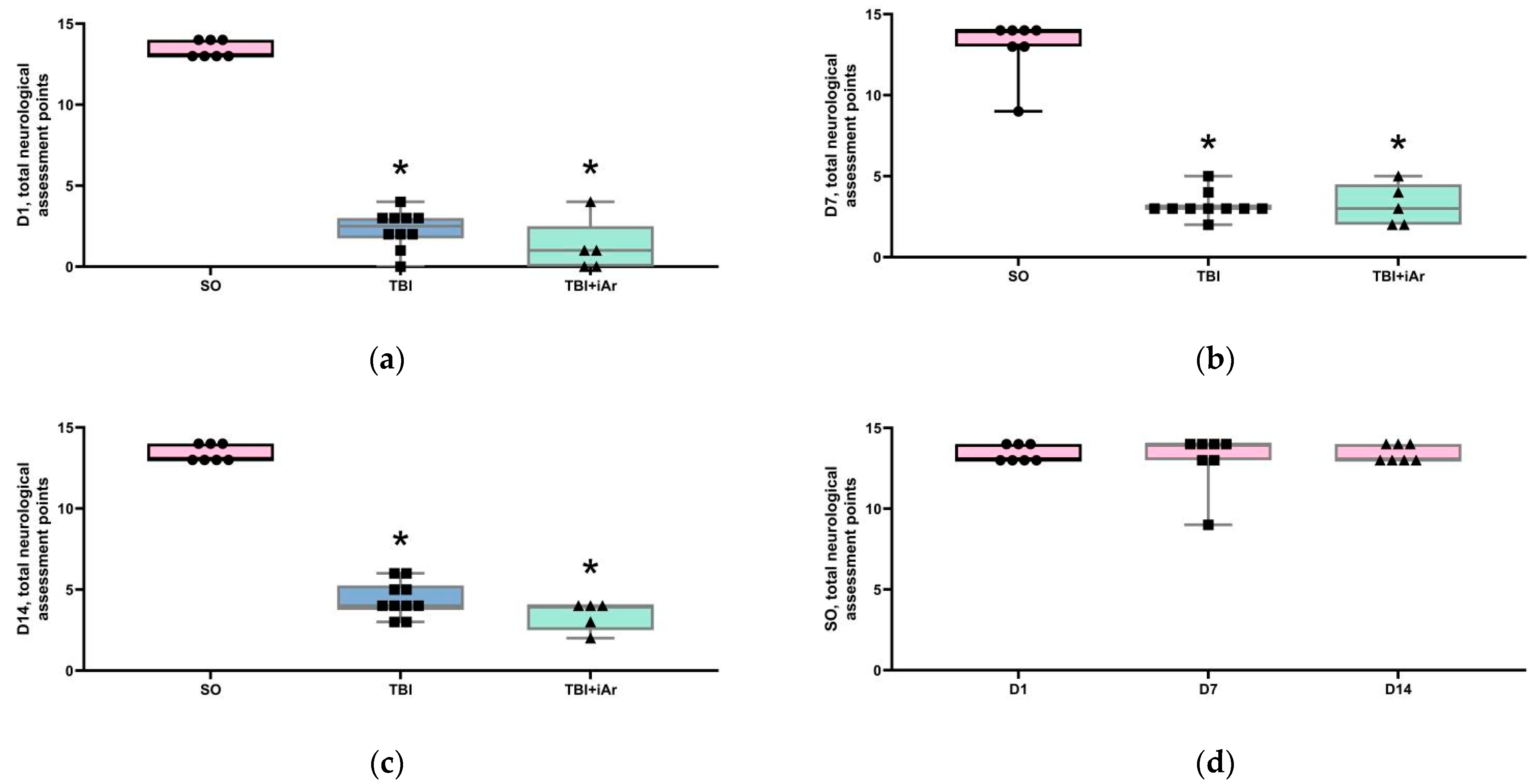
3.1. Neurological Assessment and Body Mass Analysis
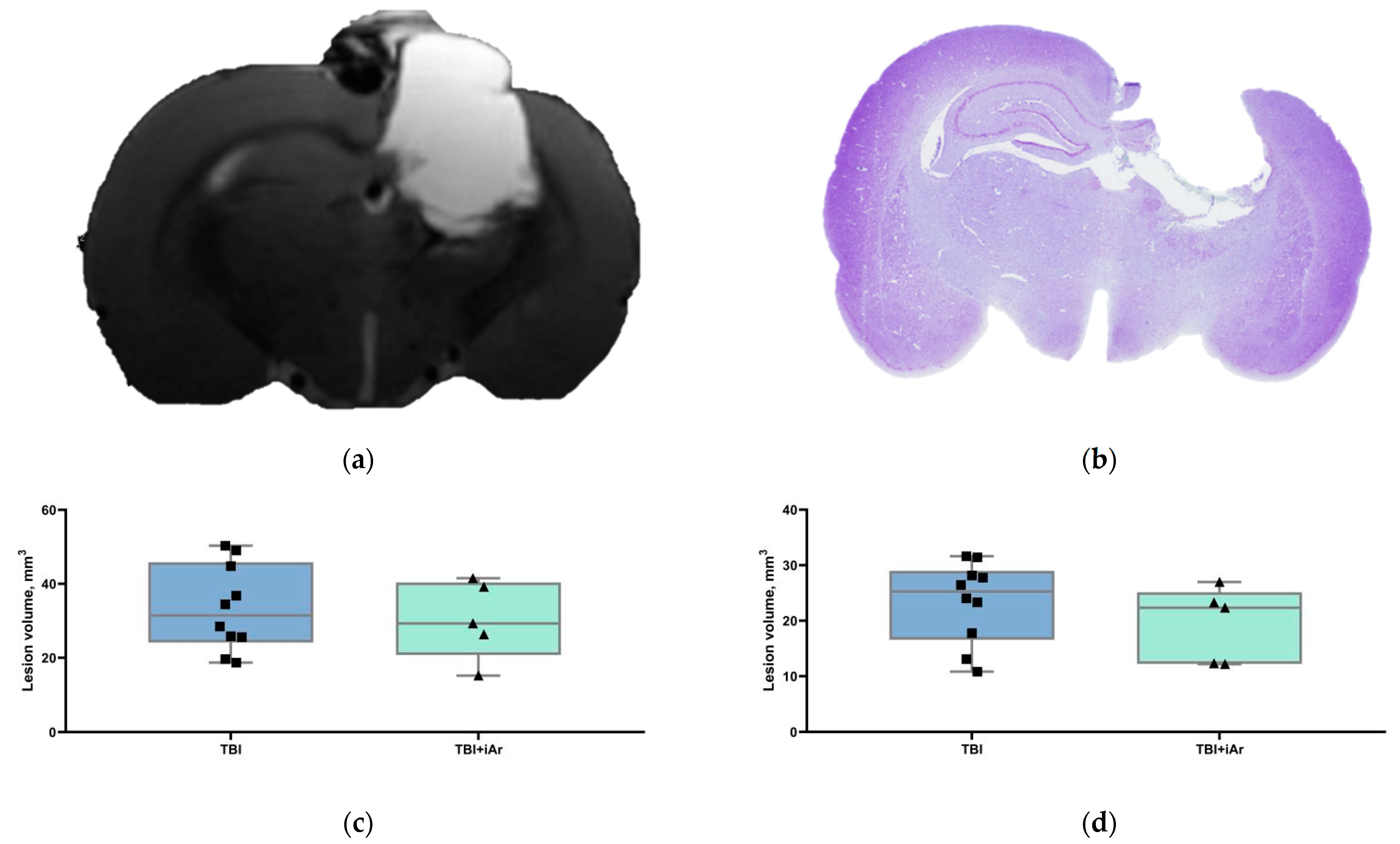
3.2. Brain MRI
3.3. Histological Examination of the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Talypov, A.E.; Grin’, A.A.; Petrikov, S.S.; Krylov, V.V.; Solodov, A.A.; Kordonsky, A.Ю.; Shabanov, A.K.; Barmina, T.G.; Mullagulov, T.R. Intracranial pressure monitoring in patients with severe head injury. Russ. J. Neurosurg. 2021, 22, 14–27. [Google Scholar] [CrossRef]
- Ovsyannikov, D.M.; Chekhonatsky, A.A.; Kolesov, V.N.; Bubashvili, A.I. Social and Epidemiological Aspects of Craniocerebral Trauma (review). Saratov J. Med. Sci. Res. 2012, 8, 777–785. (In Russian) [Google Scholar]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Liu, J.; Nolte, K.; Brook, G.; Liebenstund, L.; Weinandy, A.; Höllig, A.; Veldeman, M.; Willuweit, A.; Langen, K.-J.; Rossaint, R.; et al. Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization: A randomized controlled animal study. Crit. Care 2019, 23, 198. [Google Scholar] [CrossRef] [Green Version]
- Moro, F.; Fossi, F.; Magliocca, A.; Pascente, R.; Sammali, E.; Baldini, F.; Tolomeo, D.; Micotti, E.; Citerio, G.; Stocchetti, N.; et al. Efficacy of acute administration of inhaled argon on traumatic brain injury in mice. Br. J. Anaesth. 2021, 126, 256–264. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Investig. 2021, 131, e143777. [Google Scholar] [CrossRef]
- Anna, R.; Rolf, R.; Mark, C. Update of the organoprotective properties of xenon and argon: From bench to beside. Intensiv. Care Med. Exp. 2020, 8, 11–16. [Google Scholar] [CrossRef]
- Gardner, A.; Menon, D. Moving to human trials for argon neuroprotection in neurological injury: A narrative review. Br. J. Anaesth. 2018, 120, 453–468. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, R.; Franks, N.P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit. Care 2010, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Filev, A.; Silachev, D.; Ryzhkov, I.; Lapin, K.; Babkina, A.; Grebenchikov, O.; Pisarev, V. Effect of Xenon Treatment on Gene Expression in Brain Tissue after Traumatic Brain Injury in Rats. Brain Sci. 2021, 11, 889. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, T.; Zhao, H.; Fidalgo, A.R.; Vizcaychipi, M.; Sanders, R.D.; Yu, B.; Takata, M.; Johnson, M.R.; Ma, D. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit. Care Med. 2012, 40, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Coburn, M.; Maze, M.; Franks, N.P. The neuroprotective effects of xenon and helium in an in vitro model of traumatic brain injury. Crit. Care Med. 2008, 36, 588–595. [Google Scholar] [CrossRef]
- Harris, K.; Armstrong, S.P.; Campos-Pires, R.; Kiru, L.; Franks, N.P.; Dickinson, R. Neuroprotection against Traumatic Brain Injury by Xenon, but Not Argon, Is Mediated by Inhibition at the N-Methyl-d-Aspartate Receptor Glycine Site. Anesthesiology 2013, 119, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Campos-Pires, R.; Koziakova, M.; Yonis, A.; Pau, A.; Macdonald, W.; Harris, K.; Edge, C.J.; Franks, N.P.; Mahoney, P.F.; Dickinson, R. Xenon Protects against Blast-Induced Traumatic Brain Injury in an In Vitro Model. J. Neurotrauma 2018, 35, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Campos-Pires, R.; Hirnet, T.; Valeo, F.; Ong, B.E.; Radyushkin, K.; Aldhoun, J.; Saville, J.; Edge, C.J.; Franks, N.P.; Thal, S.C.; et al. Xenon improves long-term cognitive function, reduces neuronal loss and chronic neuroinflammation, and improves survival after traumatic brain injury in mice. Br. J. Anaesth. 2019, 123, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Irani, Y.; Pype, J.; Martin, A.; Chong, C.; Daniel, L.; Gaudart, J.; Ibrahim, Z.; Magalon, G.; Lemaire, M.; Hardwigsen, J. Noble Gas (Argon and Xenon)-Saturated Cold Storage Solutions Reduce Ischemia-Reperfusion Injury in a Rat Model of Renal Transplantation. Nephron Extra 2011, 1, 272–282. [Google Scholar] [CrossRef]
- Loetscher, P.D.; Rossaint, J.; Rossaint, R.; Weis, J.; Fries, M.; Fahlenkamp, A.; Ryang, Y.-M.; Grottke, O.; Coburn, M. Argon: Neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit. Care 2009, 13, R206. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Mitchell, S.; Ciechanowicz, S.; Savage, S.; Wang, T.; Ji, X.; Ma, D. Argon protects against hypoxic-ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid-derived 2)-like 2. Oncotarget 2016, 7, 25640–25651. [Google Scholar] [CrossRef]
- Ma, S.; Chu, D.; Li, L.; Creed, J.A.; Ryang, Y.-M.; Sheng, H.; Yang, W.; Warner, D.S.; Turner, D.A.; Hoffmann, U. Argon Inhalation for 24 Hours After Onset of Permanent Focal Cerebral Ischemia in Rats Provides Neuroprotection and Improves Neurologic Outcome. Crit. Care Med. 2019, 47, e693–e699. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Russo, I.; Tantillo, S.; Zani, D.D.; Locatelli, V.; De Maglie, M.; Novelli, D.; Staszewsky, L.; Vago, T.; et al. Postresuscitation Treatment with Argon Improves Early Neurological Recovery in a Porcine Model of Cardiac Arrest. Shock 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Höllig, A.; Weinandy, A.; Liu, J.; Clusmann, H.; Rossaint, R.; Coburn, M. Beneficial properties of argon after experimental subarachnoid hemorrhage: Early treatment reduces mortality and influences hippocampal protein expression. Crit. Care Med. 2016, 44, e520–e529. [Google Scholar] [CrossRef]
- Creed, J.; Cantillana-Riquelme, V.; Yan, B.H.; Ma, S.; Chu, D.; Wang, H.; Turner, D.A.; Laskowitz, D.T.; Hoffmann, U. Argon Inhalation for 24 h After Closed-Head Injury Does not Improve Recovery, Neuroinflammation, or Neurologic Outcome in Mice. Neurocritical Care 2020, 34, 833–843. [Google Scholar] [CrossRef]
- Cucino, A.; Ruggeri, L.; Olivari, D.; De Giorgio, D.; Latini, R.; Ristagno, G. Safety of ventilation with an argon and oxygen gas mixture. Br. J. Anaesth. 2019, 122, e31–e32. [Google Scholar] [CrossRef] [Green Version]
- Feeney, D.M.; Boyeson, M.G.; Linn, R.T.; Murray, H.M.; Dail, W.G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981, 211, 67–77. [Google Scholar] [CrossRef]
- Isaev, N.K.; Novikova, S.V.; Stelmashook, E.V.; Barskov, I.V.; Silachev, D.N.; Khaspekov, L.G.; Skulachev, V.P.; Zorov, D.B. Mitochondria-targeted plastoquinone antioxidant SkQR1 decreases trauma-induced neurological deficit in rat. Biochemistry 2012, 77, 996–999. [Google Scholar] [CrossRef]
- De Ryck, M.; Van Reempts, J.; Borgers, M.; Wauquier, A.; Janssen, P.A. Photochemical stroke model: Flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 1989, 20, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Jolkkonen, J.; Puurunen, K.; Rantakömi, S.; Härkönen, A.; Haapalinna, A.; Sivenius, J. Behavioral effects of the α2-adrenoceptor antagonist, atipamezole, after focal cerebral ischemia in rats. Eur. J. Pharmacol. 2000, 400, 211–219. [Google Scholar] [CrossRef]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Stereotaxic Atlas; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Chao, H.; Lin, C.; Zuo, Q.; Liu, Y.; Xiao, M.; Xu, X.; Li, Z.; Bao, Z.; Chen, H.; You, Y.; et al. Cardiolipin-Dependent Mitophagy Guides Outcome after Traumatic Brain Injury. J. Neurosci. 2019, 39, 1930–1943. [Google Scholar] [CrossRef] [Green Version]
- Laitio, R.; Hynninen, M.; Arola, O.; Virtanen, S.; Parkkola, R.; Saunavaara, J.; Roine, R.O.; Grönlund, J.; Ylikoski, E.; Wennervirta, J.; et al. Effect of Inhaled Xenon on Cerebral White matter Damage in Comatose Survivors of Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2016, 315, 1120–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grüne, F.; Kazmaier, S.; Hoeks, S.E.; Stolker, R.J.; Coburn, M.; Weyland, A. Argon does not affect cerebral circulation or metabolism in male humans. PLoS ONE 2017, 12, e0171962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brücken, A.; Kurnaz, P.; Bleilevens, C.; Derwall, M.; Weis, J.; Nolte, K.; Rossaint, R.; Fries, M. Delayed Argon Administration Provides Robust Protection Against Cardiac Arrest-Induced Neurological Damage. Neurocritical Care 2015, 22, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Koziakova, M.; Harris, K.; Edge, C.J.; Franks, N.; White, I.L.; Dickinson, R. Noble gas neuroprotection: Xenon and argon protect against hypoxic–ischaemic injury in rat hippocampus in vitro via distinct mechanisms. Br. J. Anaesth. 2019, 123, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Ryang, Y.-M.; Fahlenkamp, A.V.; Rossaint, R.; Wesp, D.; Loetscher, P.D.; Beyer, C.; Coburn, M. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit. Care Med. 2011, 39, 1448–1453. [Google Scholar] [CrossRef]
- David, H.N.; Haelewyn, B.; Degoulet, M.; Colomb Jr, D.G.; Risso, J.J.; Abraini, J.H. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS ONE 2012, 7, e30934. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Mitchell, S.; Koumpa, S.; Cui, Y.T.; Lian, Q.; Hagberg, H.; Johnson, M.R.; Takata, M.; Ma, D. Heme oxygenase-1 mediates neuroprotection conferred by argon in combination with hypothermia in neonatal hypoxia–ischemia brain injury. Anesthesiology 2016, 125, 180–192. [Google Scholar] [CrossRef]
- Ulbrich, F.; Kaufmann, K.B.; Coburn, M.; Lagrèze, W.A.; Roesslein, M.; Biermann, J.; Buerkle, H.; Loop, T.; Goebel, U. Neuroprotective effects of Argon are mediated via an ERK-1/2 dependent regulation of heme-oxygenase-1 in retinal ganglion cells. J. Neurochem. 2015, 134, 717–727. [Google Scholar] [CrossRef]
- Fahlenkamp, A.V.; Rossaint, R.; Haase, H.; Al Kassam, H.; Ryang, Y.-M.; Beyer, C.; Coburn, M. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur. J. Pharmacol. 2011, 674, 104–111. [Google Scholar] [CrossRef]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef]
- Schneider, F.I.; Krieg, S.M.; Lindauer, U.; Stoffel, M.; Ryang, Y.-M. Neuroprotective Effects of the Inert Gas Argon on Experimental Traumatic Brain Injury In Vivo with the Controlled Cortical Impact Model in Mice. Biology 2022, 11, 158. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, V.V.; Silachev, D.N.; Ryzhkov, I.A.; Lapin, K.N.; Kalabushev, S.N.; Ostrova, I.V.; Varnakova, L.A.; Grebenchikov, O.A. Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats. Brain Sci. 2022, 12, 920. https://doi.org/10.3390/brainsci12070920
Antonova VV, Silachev DN, Ryzhkov IA, Lapin KN, Kalabushev SN, Ostrova IV, Varnakova LA, Grebenchikov OA. Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats. Brain Sciences. 2022; 12(7):920. https://doi.org/10.3390/brainsci12070920
Chicago/Turabian StyleAntonova, Viktoriya V., Denis N. Silachev, Ivan A. Ryzhkov, Konstantin N. Lapin, Sergey N. Kalabushev, Irina V. Ostrova, Lydia A. Varnakova, and Oleg A. Grebenchikov. 2022. "Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats" Brain Sciences 12, no. 7: 920. https://doi.org/10.3390/brainsci12070920
APA StyleAntonova, V. V., Silachev, D. N., Ryzhkov, I. A., Lapin, K. N., Kalabushev, S. N., Ostrova, I. V., Varnakova, L. A., & Grebenchikov, O. A. (2022). Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats. Brain Sciences, 12(7), 920. https://doi.org/10.3390/brainsci12070920






