Brain Metabolic Connectivity Patterns in Patients with Prolonged Disorder of Consciousness after Hypoxic-Ischemic Injury: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. FDG PET Scanning
2.3. Image Preprocessing
2.4. Metabolic Connectivity Matrix
2.5. Statistical Analysis
3. Results
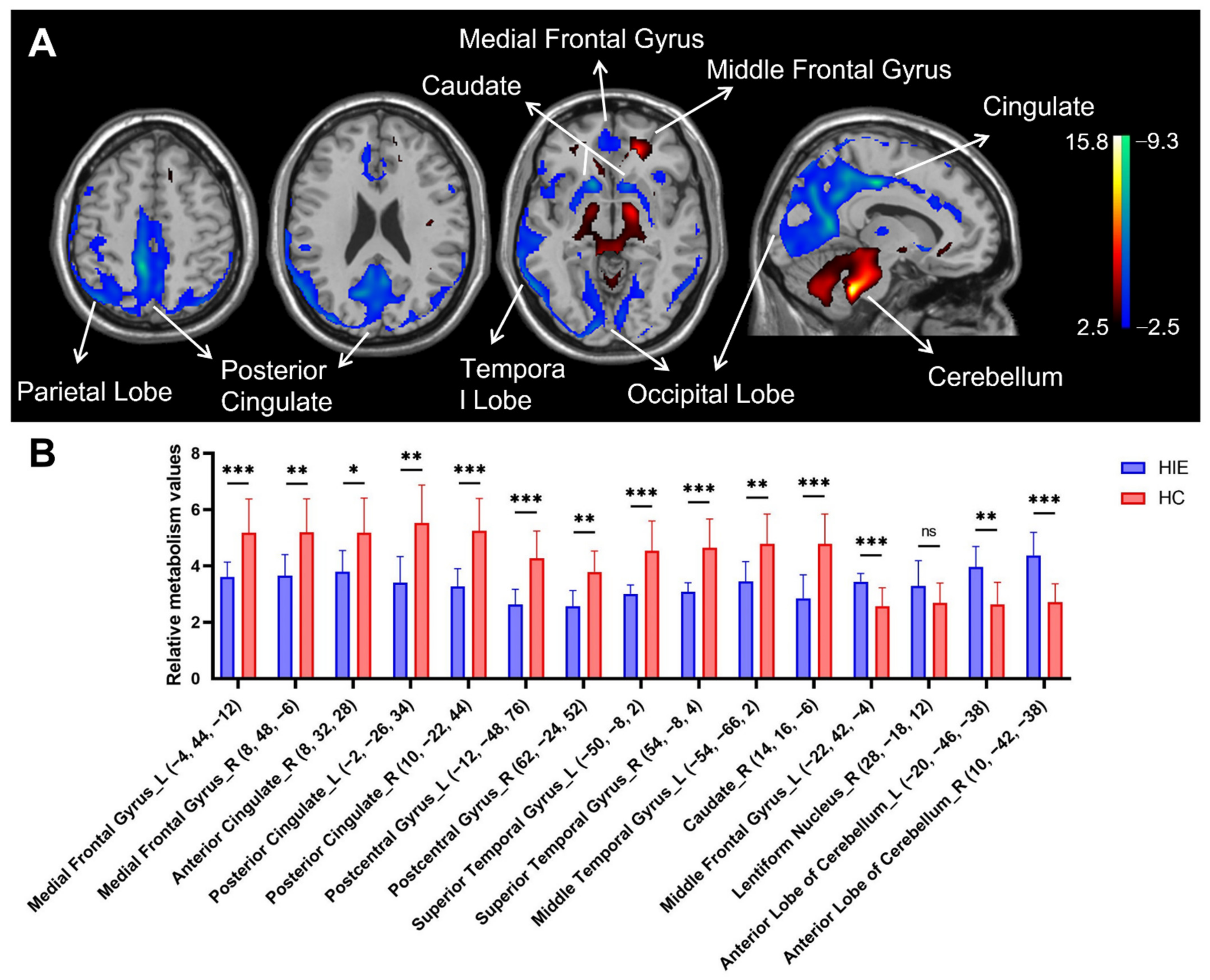
3.1. Characteristics of Relative Brain Activity in Patients with HIE
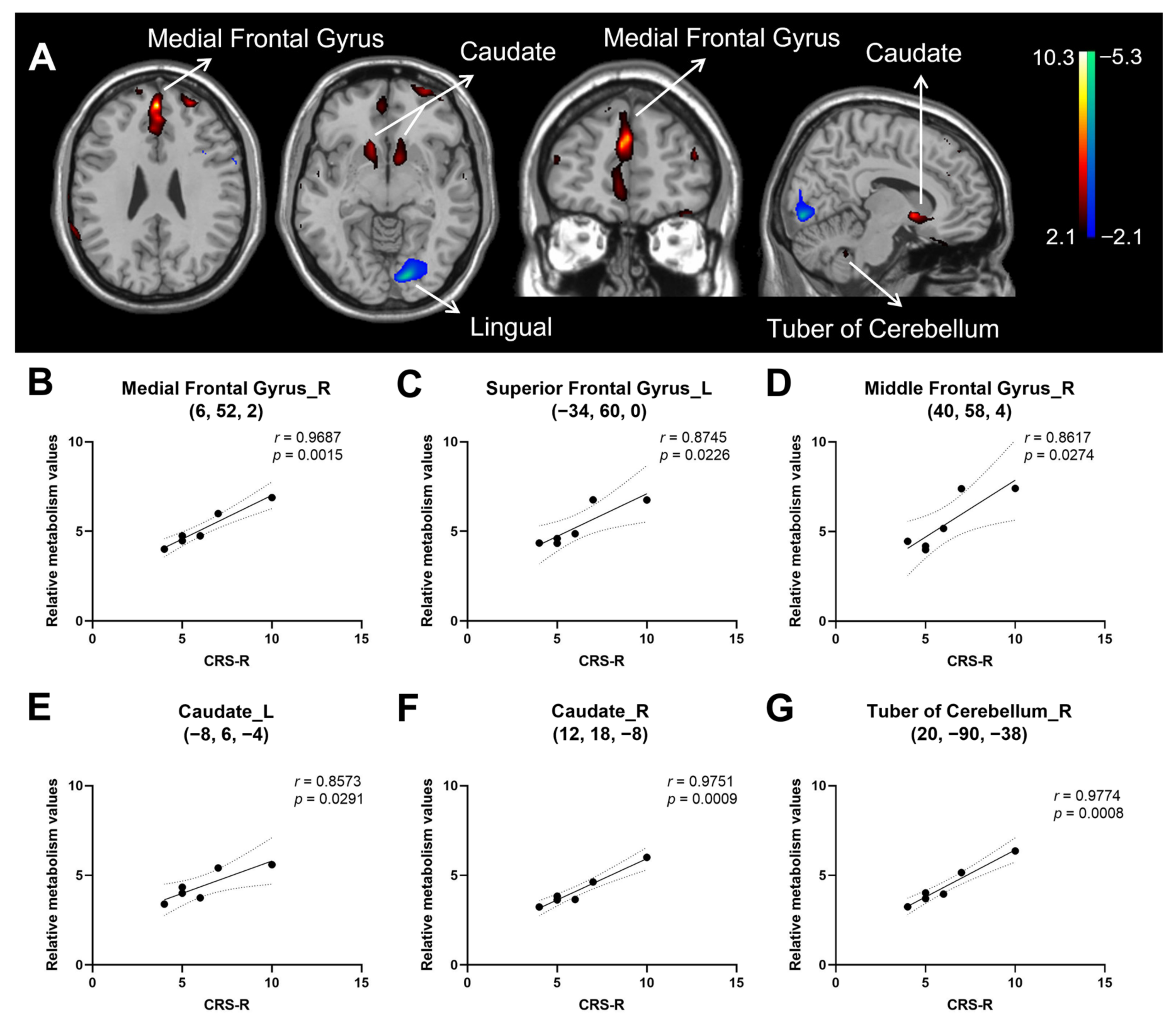
3.2. Correlation of Consciousness Level with Relative Brain Activity in Patients with HIE
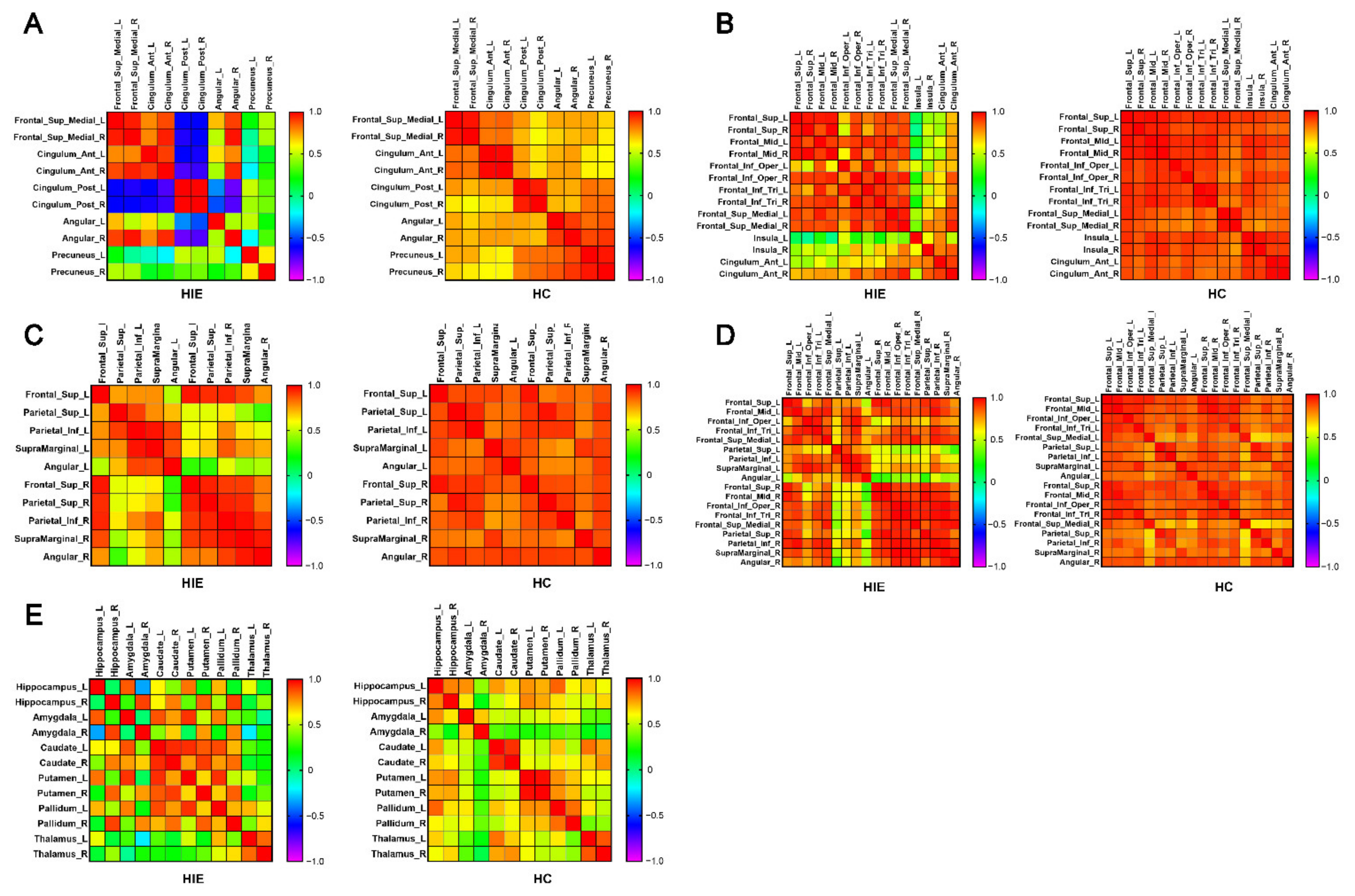
3.3. Metabolic Connectivity
3.4. Brain Network Metabolic Connectivity Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.; Zafonte, R.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting awareness in the vegetative state. Science 2006, 313, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Panda, R.; Annen, J.; Sanz, L.; Naccache, L.; Martial, C.; Chatelle, C.; Aubinet, C.; Bonin, E.; Barra, A.; et al. Preservation of Brain Activity in Unresponsive Patients Identifies MCS Star. Ann. Neurol. 2021, 90, 89–100. [Google Scholar] [CrossRef]
- Bodart, O.; Gosseries, O.; Wannez, S.; Thibaut, A.; Annen, J.; Boly, M.; Rosanova, M.; Casali, A.G.; Casarotto, S.; Tononi, G.; et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin. 2017, 14, 354–362. [Google Scholar] [CrossRef]
- Massimini, M.; Ferrarelli, F.; Sarasso, S.; Tononi, G. Cortical mechanisms of loss of consciousness: Insight from TMS/EEG studies. Arch. Ital. Biol. 2012, 150, 44–55. [Google Scholar] [CrossRef]
- Zeman, A. Consciousness. Brain 2001, 124, 1263–1289. [Google Scholar] [CrossRef] [Green Version]
- Hannawi, Y.; Lindquist, M.A.; Caffo, B.S.; Sair, H.I.; Stevens, R.D. Resting brain activity in disorders of consciousness: A systematic review and meta-analysis. Neurology 2015, 84, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.H.; Yuan, R.; Huang, Y.C.; Yeh, M.Y.; Lin, C.P.; Biswal, B.B. Disruption of brain connectivity in acute stroke patients with early impairment in consciousness. Front. Psychol. 2014, 4, 956. [Google Scholar] [CrossRef] [Green Version]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.; Bruno, M.A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Norton, L.; Hutchison, R.M.; Young, G.B.; Lee, D.H.; Sharpe, M.D.; Mirsattari, S.M. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology 2012, 78, 175–181. [Google Scholar] [CrossRef]
- Demertzi, A.; Gomez, F.; Crone, J.S.; Vanhaudenhuyse, A.; Tshibanda, L.; Noirhomme, Q.; Thonnard, M.; Charland-Verville, V.; Kirsch, M.; Laureys, S.; et al. Multiple fMRI system-level baseline connectivity is disrupted in patients with consciousness alterations. Cortex 2014, 52, 35–46. [Google Scholar] [CrossRef]
- Silva, S.; de Pasquale, F.; Vuillaume, C.; Riu, B.; Loubinoux, I.; Geeraerts, T.; Seguin, T.; Bounes, V.; Fourcade, O.; Demonet, J.F.; et al. Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology 2015, 85, 2036–2044. [Google Scholar] [CrossRef] [Green Version]
- Afrasiabi, M.; Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Raz, A.; Haun, A.M.; Saalmann, Y.B. Consciousness depends on integration between parietal cortex, striatum, and thalamus. Cell Syst. 2021, 12, 363–373. [Google Scholar] [CrossRef]
- Chen, L.; Rao, B.; Li, S.; Gao, L.; Xie, Y.; Dai, X.; Fu, K.; Peng, X.Z.; Xu, H. Altered Effective Connectivity Measured by Resting-State Functional Magnetic Resonance Imaging in Posterior Parietal-Frontal-Striatum Circuit in Patients with Disorder of Consciousness. Front. Neurosci. 2021, 15, 766633. [Google Scholar] [CrossRef]
- Laureys, S.; Faymonville, M.E.; Luxen, A.; Lamy, M.; Franck, G.; Maquet, P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 2000, 355, 1790–1791. [Google Scholar] [CrossRef] [Green Version]
- Barra, A.; Rosenfelder, M.; Mortaheb, S.; Carriere, M.; Martens, G.; Bodien, Y.G.; Morales-Quezada, L.; Bender, A.; Laureys, S.; Thibaut, A.; et al. Transcranial Pulsed-Current Stimulation versus Transcranial Direct Current Stimulation in Patients with Disorders of Consciousness: A Pilot, Sham-Controlled Cross-Over Double-Blind Study. Brain Sci. 2022, 12, 429. [Google Scholar] [CrossRef]
- Cain, J.A.; Spivak, N.M.; Coetzee, J.P.; Crone, J.S.; Johnson, M.A.; Lutkenhoff, E.S.; Real, C.; Buitrago-Blanco, M.; Vespa, P.M.; Schnakers, C.; et al. Ultrasonic Deep Brain Neuromodulation in Acute Disorders of Consciousness: A Proof-of-Concept. Brain Sci. 2022, 12, 428. [Google Scholar] [CrossRef]
- Rezaei, H.A.; Lythe, V.; Green, A.L. Deep Brain Stimulation for Recovery of Consciousness in Minimally Conscious Patients After Traumatic Brain Injury: A Systematic Review. Neuromodulation 2019, 22, 373–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Schnakers, C.; He, M.; Luo, H.; Cheng, L.; Wang, F.; Nie, Y.; Huang, W.; Hu, X.; et al. Validation of the Chinese version of the Coma Recovery Scale-Revised (CRS-R). Brain Inj. 2019, 33, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; He, M.; Zhang, Y.; Cheng, L.; Wang, F.; Nie, Y.; Huang, W.; Laureys, S.; Schnakers, C. Chinese translation of the Coma Recovery Scale-Revised. Brain Inj. 2017, 31, 363–365. [Google Scholar] [CrossRef]
- Paszkiel, S.; Szpulak, P. Methods of Acquisition, Archiving and Biomedical Data Analysis of Brain Functioning. In Proceedings of the BCI 2018, Opole, Poland, 13–14 March 2018; Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2018; Volume 720, pp. 158–171. [Google Scholar] [CrossRef]
- Mortensen, K.N.; Gjedde, A.; Thompson, G.J.; Herman, P.; Parent, M.J.; Rothman, D.L.; Kupers, R.; Ptito, M.; Stender, J.; Laureys, S.; et al. Impact of Global Mean Normalization on Regional Glucose Metabolism in the Human Brain. Neural Plast. 2018, 2018, 6120925. [Google Scholar] [CrossRef] [Green Version]
- Laureys, S.; Owen, A.M.; Schiff, N.D. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004, 3, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Fridman, E.A.; Osborne, J.R.; Mozley, P.D.; Victor, J.D.; Schiff, N.D. Presynaptic dopamine deficit in minimally conscious state patients following traumatic brain injury. Brain 2019, 142, 1887–1893. [Google Scholar] [CrossRef]
- Ge, J.; Wu, P.; Peng, S.; Yu, H.; Zhang, H.; Guan, Y.; Eidelberg, D.; Zuo, C.; Ma, Y.; Wang, J. Assessing cerebral glucose metabolism in patients with idiopathic rapid eye movement sleep behavior disorder. J. Cereb. Blood Flow Metab. 2015, 35, 2062–2069. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Margulies, D.S.; Breakspear, M.; Zalesky, A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 2020, 23, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Hermann, B.; Stender, J.; Habert, M.O.; Kas, A.; Denis-Valente, M.; Raimondo, F.; Perez, P.; Rohaut, B.; Sitt, J.D.; Naccache, L. Multimodal FDG-PET and EEG assessment improves diagnosis and prognostication of disorders of consciousness. Neuroimage Clin. 2021, 30, 102601. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Du, J.; Huo, S.; Li, R.; Song, W. Neural correlates of different behavioral response to transcranial direct current stimulation between patients in the unresponsive wakefulness syndrome and minimally conscious state. Neurol. Sci. 2020, 41, 75–82. [Google Scholar] [CrossRef]
- Rosazza, C.; Andronache, A.; Sattin, D.; Bruzzone, M.G.; Marotta, G.; Nigri, A.; Ferraro, S.; Rossi, S.D.; Porcu, L.; Bersano, A.; et al. Multimodal study of default-mode network integrity in disorders of consciousness. Ann. Neurol. 2016, 79, 841–853. [Google Scholar] [CrossRef]
- Stender, J.; Gosseries, O.; Bruno, M.A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 384, 514–522. [Google Scholar] [CrossRef]
- Edlow, B.L.; Chatelle, C.; Spencer, C.A.; Chu, C.J.; Bodien, Y.G.; O’Connor, K.L.; Hirschberg, R.E.; Hochberg, L.R.; Giacino, J.T.; Rosenthal, E.S.; et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017, 140, 2399–2414. [Google Scholar] [CrossRef]
- Lant, N.D.; Gonzalez-Lara, L.E.; Owen, A.M.; Fernandez-Espejo, D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin. 2016, 10, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Qi, Z.; Wu, X.; Zhang, J.; Wu, C.; Huang, Z.; Zang, D.; Fogel, S.; Tanabe, S.; Hudetz, A.G.; et al. Anterior precuneus related to the recovery of consciousness. Neuroimage Clin. 2022, 33, 102951. [Google Scholar] [CrossRef]
- Lemaire, J.J.; Pontier, B.; Chaix, R.; El, O.Y.; Khalil, T.; Sinardet, D.; Achim, V.; Postelnicu, A.; Coste, J.; Germain, V.; et al. Neural correlates of consciousness and related disorders: From phenotypic descriptors of behavioral and relative consciousness to cortico-subcortical circuitry. Neurochirurgie 2022, 68, 212–222. [Google Scholar] [CrossRef]
- Mencarelli, L.; Biagi, M.C.; Salvador, R.; Romanella, S.; Ruffini, G.; Rossi, S.; Santarnecchi, E. Network Mapping of Connectivity Alterations in Disorder of Consciousness: Towards Targeted Neuromodulation. J. Clin. Med. 2020, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Wu, X.; Wu, C.; Wu, H.; Zhang, J.; Huang, Z.; Weng, X.; Zang, D.; Qi, Z.; Tang, W.; et al. Higher-order sensorimotor circuit of the brain’s global network supports human consciousness. Neuroimage 2021, 231, 117850. [Google Scholar] [CrossRef]
- Ihalainen, R.; Gosseries, O.; de Steen, F.V.; Raimondo, F.; Panda, R.; Bonhomme, V.; Marinazzo, D.; Bowman, H.; Laureys, S.; Chennu, S. How hot is the hot zone? Computational modelling clarifies the role of parietal and frontoparietal connectivity during anaesthetic-induced loss of consciousness. Neuroimage 2021, 231, 117841. [Google Scholar] [CrossRef]
- He, R.H.; Wang, H.J.; Zhou, Z.; Fan, J.Z.; Zhang, S.Q.; Zhong, Y.H. The influence of high-frequency repetitive transcranial magnetic stimulation on endogenous estrogen in patients with disorders of consciousness. Brain Stimul. 2021, 14, 461–466. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Huang, Z.; Tarnal, V.; Vlisides, P.E.; Janke, E.L.; McKinney, A.M.; Picton, P.; Mashour, G.A.; Hudetz, A.G. Anterior insula regulates brain network transitions that gate conscious access. Cell Rep. 2021, 35, 109081. [Google Scholar] [CrossRef]
- Fridman, E.A.; Beattie, B.J.; Broft, A.; Laureys, S.; Schiff, N.D. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc. Natl. Acad. Sci. USA 2014, 111, 6473–6478. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xie, Q.; Wu, X.; Huang, H.; Lv, W.; Chen, L.; Guo, Y.; Zhang, S.; Hu, H.; Wang, Y.; et al. Abnormal Effective Connectivity of the Anterior Forebrain Regions in Disorders of Consciousness. Neurosci. Bull. 2018, 34, 647–658. [Google Scholar] [CrossRef]
- Slagter, H.A.; Mazaheri, A.; Reteig, L.C.; Smolders, R.; Figee, M.; Mantione, M.; Schuurman, P.R.; Denys, D. Contributions of the Ventral Striatum to Conscious Perception: An Intracranial EEG Study of the Attentional Blink. J. Neurosci. 2017, 37, 1081–1089. [Google Scholar] [CrossRef] [Green Version]
- Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Andryk, S.; Dooley, G.L.; Afrasiabi, M.; Raz, A.; Saalmann, Y.B. Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron 2020, 106, 66–75. [Google Scholar] [CrossRef]
- Tasserie, J.; Uhrig, L.; Sitt, J.D.; Manasova, D.; Dupont, M.; Dehaene, S.; Jarraya, B. Deep brain stimulation of the thalamus restores signatures of consciousness in a nonhuman primate model. Sci. Adv. 2022, 8, l5547. [Google Scholar] [CrossRef]

| Patient No. | Gender | Age (yrs) | Time Since HIE Onset (Weeks) | Cause of HIE | Level of DOC | Total CRS-R Score |
|---|---|---|---|---|---|---|
| 1 | M | 17 | 29 | Cardiac arrest | VS | 6 |
| 2 | F | 50 | 13 | Anesthesia accident | VS | 5 |
| 3 | F | 39 | 16 | Anesthesia accident | VS | 4 |
| 4 | M | 57 | 27 | Massive hemorrhage a | VS | 7 |
| 5 | M | 63 | 32 | Chocking | VS | 5 |
| 6 | F | 49 | 8 | Cardiac arrest | MCS | 10 |
| HIE (n = 6) | HC (n = 18) | p Value | |
|---|---|---|---|
| Gender (M/F) | 3/3 | 9/9 | p > 0.05 |
| Age (yrs) | 45.83 ± 16.23 | 52.67 ± 2.28 | p > 0.05 |
| Handedness (R/L) | 6/0 | 18/0 | p > 0.05 |
| Structure | Broadmann Area | L/R | MNI Coordinates a | Zmax | Cluster Size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Decreased metabolism b | |||||||
| Medial Frontal Gyrus | 11 | L | −4 | 44 | −12 | 3.8 | 150 |
| 10 | R | 8 | 48 | −6 | 3.39 | ||
| Anterior Cingulate | 32 | R | 8 | 32 | 28 | 3.75 | 122 |
| Posterior Cingulate c | 23 | L | −2 | −26 | 34 | 5.63 | 10,079 |
| (Extending to parietal-occipital lobe) | 24 | R | 10 | −22 | 44 | 5.88 | |
| Postcentral Gyrus c | 5 | L | −12 | −48 | 76 | 5.19 | 118 |
| 2 | R | 62 | −24 | 52 | 3.84 | 125 | |
| Superior Temporal Gyrus | 22 | L | −50 | −8 | 2 | 4.31 | 401 |
| (Extending to insula) | 22 | R | 54 | −8 | 4 | 4.22 | 630 |
| Middle Temporal Gyrus | 37 | L | −54 | −66 | 2 | 4.09 | 832 |
| Caudate c | Caudate Head | R | 14 | 16 | −6 | 4.88 | 284 |
| Increased metabolism b | |||||||
| Middle Frontal Gyrus c | 11 | L | −22 | 42 | −4 | 6.08 | 1000 |
| Lentiform Nucleus | Putamen | R | 28 | −18 | 12 | 4.03 | 181 |
| Anterior Lobe c | Cerebellum | L | −20 | −46 | −38 | 6.21 | 8305 |
| Cerebellum | R | 10 | −42 | −38 | 7.36 | ||
| Structure | Broadmann Area | L/R | MNI Coordinates a | Zmax | Cluster Size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive b | |||||||
| Medial Frontal Gyrus c | 9 | L | 0 | 50 | 26 | 3.48 | 104 |
| Anterior Cingulate | 32 | L | 0 | 40 | 26 | 2.79 | |
| Medial Frontal Gyrus | 9 | R | 6 | 52 | 2 | 2.43 | 5 |
| Anterior Cingulate | 32 | R | 4 | 32 | 26 | 2.44 | 3 |
| Superior Frontal Gyrus | 10 | L | −34 | 60 | 0 | 2.84 | 30 |
| Middle Frontal Gyrus | 10 | L | −26 | 52 | 26 | 2.7 | 12 |
| Middle Frontal Gyrus | 10 | R | 40 | 58 | 4 | 2.47 | 6 |
| Inferior Frontal Gyrus c | 47 | L | −30 | 32 | −20 | 3.39 | 82 |
| Caudate | Caudate Head | L | −8 | 6 | −4 | 2.93 | 22 |
| Caudate | Caudate Head | R | 12 | 18 | −8 | 2.45 | 11 |
| Tuber | Cerebellum | R | 20 | −90 | −38 | 2.83 | 42 |
| Negative b | |||||||
| Lingual Gyrus | 17 | L | −14 | −86 | −6 | 2.75 | 113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Lu, R.; Guan, Y.; Wu, Y.; Ge, J.; Liu, G.; Chen, Y.; Xie, H.; Wu, J.; Jia, J. Brain Metabolic Connectivity Patterns in Patients with Prolonged Disorder of Consciousness after Hypoxic-Ischemic Injury: A Preliminary Study. Brain Sci. 2022, 12, 892. https://doi.org/10.3390/brainsci12070892
He Z, Lu R, Guan Y, Wu Y, Ge J, Liu G, Chen Y, Xie H, Wu J, Jia J. Brain Metabolic Connectivity Patterns in Patients with Prolonged Disorder of Consciousness after Hypoxic-Ischemic Injury: A Preliminary Study. Brain Sciences. 2022; 12(7):892. https://doi.org/10.3390/brainsci12070892
Chicago/Turabian StyleHe, Zhijie, Rongrong Lu, Yihui Guan, Yi Wu, Jingjie Ge, Gang Liu, Ying Chen, Hongyu Xie, Junfa Wu, and Jie Jia. 2022. "Brain Metabolic Connectivity Patterns in Patients with Prolonged Disorder of Consciousness after Hypoxic-Ischemic Injury: A Preliminary Study" Brain Sciences 12, no. 7: 892. https://doi.org/10.3390/brainsci12070892
APA StyleHe, Z., Lu, R., Guan, Y., Wu, Y., Ge, J., Liu, G., Chen, Y., Xie, H., Wu, J., & Jia, J. (2022). Brain Metabolic Connectivity Patterns in Patients with Prolonged Disorder of Consciousness after Hypoxic-Ischemic Injury: A Preliminary Study. Brain Sciences, 12(7), 892. https://doi.org/10.3390/brainsci12070892






