Brain Tissue Oxygenation-Guided Therapy and Outcome in Traumatic Brain Injury: A Single-Center Matched Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
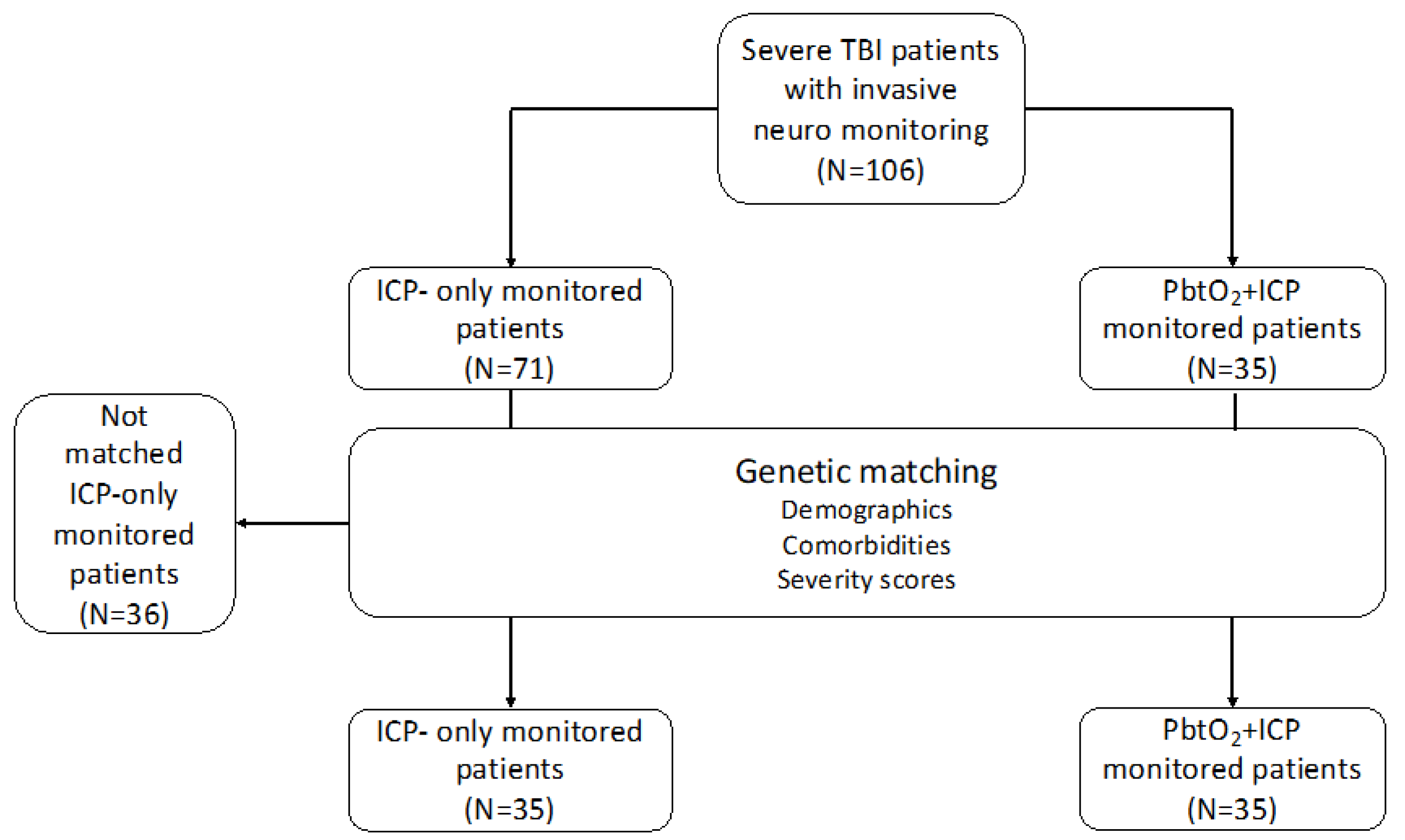
3.1. Genetic Matched Cohort
3.2. Entire Cohort Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Injury, G.B.D.T.B.; Spinal Cord Injury, C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Badri, S.; Chen, J.; Barber, J.; Temkin, N.R.; Dikmen, S.S.; Chesnut, R.M.; Deem, S.; Yanez, N.D.; Treggiari, M.M. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012, 38, 1800–1809. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Cruz, J. On-line monitoring of global cerebral hypoxia in acute brain injury. Relationship to intracranial hypertension. J. Neurosurg. 1993, 79, 228–233. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Marshall, S.B.; Piek, J.; Blunt, B.A.; Klauber, M.R.; Marshall, L.F. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. Suppl. 1993, 59, 121–125. [Google Scholar] [CrossRef]
- Marmarou, A.; Anderson, R.L.; Ward, J.D.; Choi, S.C.; Young, H.F.; Eisenberg, H.M.; Foulkes, M.A.; Marshall, L.F.; Jane, J.A. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg. 1991, 75, S59–S66. [Google Scholar] [CrossRef]
- Bouma, G.J.; Muizelaar, J.P.; Choi, S.C.; Newlon, P.G.; Young, H.F. Cerebral circulation and metabolism after severe traumatic brain injury: The elusive role of ischemia. J. Neurosurg. 1991, 75, 685–693. [Google Scholar] [CrossRef]
- Bouzat, P.; Sala, N.; Payen, J.F.; Oddo, M. Beyond intracranial pressure: Optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann. Intensive Care 2013, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Nangunoori, R.; Maloney-Wilensky, E.; Stiefel, M.; Park, S.; Andrew Kofke, W.; Levine, J.M.; Yang, W.; Le Roux, P.D. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: A systematic literature review. Neurocrit. Care 2012, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wu, H.B.; Yan, Y.F.; Liu, M.; Wang, E.S. Mortality and Outcome Comparison Between Brain Tissue Oxygen Combined with Intracranial Pressure/Cerebral Perfusion Pressure-Guided Therapy and Intracranial Pressure/Cerebral Perfusion Pressure-Guided Therapy in Traumatic Brain Injury: A Meta-Analysis. World Neurosurg. 2017, 100, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, M.C.; Huang, S.J.; Chang, C.K.; Chao, D.P.; Lui, T.N.; Ma, H.I.; Liu, M.Y.; Chung, W.Y.; Shih, Y.H.; et al. A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. Biomed. Res. Int. 2015, 2015, 529580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiotta, A.M.; Stiefel, M.F.; Gracias, V.H.; Garuffe, A.M.; Kofke, W.A.; Maloney-Wilensky, E.; Troxel, A.B.; Levine, J.M.; Le Roux, P.D. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J. Neurosurg. 2010, 113, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Narotam, P.K.; Morrison, J.F.; Nathoo, N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: Outcome analysis of a brain tissue oxygen-directed therapy. J. Neurosurg. 2009, 111, 672–682. [Google Scholar] [CrossRef]
- Stiefel, M.F.; Spiotta, A.; Gracias, V.H.; Garuffe, A.M.; Guillamondegui, O.; Maloney-Wilensky, E.; Bloom, S.; Grady, M.S.; LeRoux, P.D. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 2005, 103, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Adamides, A.A.; Cooper, D.J.; Rosenfeldt, F.L.; Bailey, M.J.; Pratt, N.; Tippett, N.; Vallance, S.; Rosenfeld, J.V. Focal cerebral oxygenation and neurological outcome with or without brain tissue oxygen-guided therapy in patients with traumatic brain injury. Acta Neurochir. 2009, 151, 1399–1409. [Google Scholar] [CrossRef]
- Meixensberger, J.; Jaeger, M.; Vath, A.; Dings, J.; Kunze, E.; Roosen, K. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2003, 74, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Martini, R.P.; Deem, S.; Yanez, N.D.; Chesnut, R.M.; Weiss, N.S.; Daniel, S.; Souter, M.; Treggiari, M.M. Management guided by brain tissue oxygen monitoring and outcome following severe traumatic brain injury. J. Neurosurg. 2009, 111, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Green, J.A.; Pellegrini, D.C.; Vanderkolk, W.E.; Figueroa, B.E.; Eriksson, E.A. Goal directed brain tissue oxygen monitoring versus conventional management in traumatic brain injury: An analysis of in hospital recovery. Neurocrit. Care 2013, 18, 20–25. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Moncrief, H.; Sands, J.M.; Markert, R.J.; Hall, L.C.; Wenker, I.C.; Anderson, H.L., 3rd; Ekeh, A.P.; Walusimbi, M.S.; Woods, R.J.; et al. Neurologic outcomes with cerebral oxygen monitoring in traumatic brain injury. Surgery 2009, 146, 585–590; discussion 590–581. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Clark, M.v.B.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991, 75, S14–S20. [Google Scholar] [CrossRef] [Green Version]
- Maset, A.L.; Marmarou, A.; Ward, J.D.; Choi, S.; Lutz, H.A.; Brooks, D.; Moulton, R.J.; DeSalles, A.; Muizelaar, J.P.; Turner, H.; et al. Pressure-volume index in head injury. J. Neurosurg. 1987, 67, 832–840. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Chen, H.I.; Stiefel, M.F.; Oddo, M.; Milby, A.H.; Maloney-Wilensky, E.; Frangos, S.; Levine, J.M.; Kofke, W.A.; LeRoux, P.D. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery 2011, 69, 53–63; discussion 63. [Google Scholar] [CrossRef] [Green Version]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef] [Green Version]
- Taccone, F.S.; De Oliveira Manoel, A.L.; Robba, C.; Vincent, J.L. Use a “GHOST-CAP” in acute brain injury. Crit. Care 2020, 24, 89. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A.; Sekhon, J.S. Genetic Matching for Estimating Causal Effects: A General Multivariate Matching Method for Achieving Balance in Observational Studies. Rev. Econ. Stat. 2013, 95, 932–945. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.K.; Coles, J.P.; Gupta, A.K.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Aigbirhio, F.; Skepper, J.N.; Minhas, P.S.; Hutchinson, P.J.; et al. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004, 32, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Levine, J.M.; Mackenzie, L.; Frangos, S.; Feihl, F.; Kasner, S.E.; Katsnelson, M.; Pukenas, B.; Macmurtrie, E.; Maloney-Wilensky, E.; et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery 2011, 69, 1037–1045; discussion 1045. [Google Scholar] [CrossRef]
- Eriksson, E.A.; Barletta, J.F.; Figueroa, B.E.; Bonnell, B.W.; Vanderkolk, W.E.; McAllen, K.J.; Ott, M.M. Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin. Neurophysiol. 2012, 123, 1255–1260. [Google Scholar] [CrossRef]
- Treggiari, M.M.; Schutz, N.; Yanez, N.D.; Romand, J.A. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: A systematic review. Neurocrit. Care 2007, 6, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payen, J.F.; Richard, M.; Francony, G.; Audibert, G.; Barbier, E.L.; Bruder, N.; Dahyot-Fizelier, C.; Geeraerts, T.; Gergele, L.; Puybasset, L.; et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: The multicentre randomised controlled OXY-TC trial study protocol. BMJ Open 2020, 10, e040550. [Google Scholar] [CrossRef]
- Shutter, L.D.-A.R.; Barsan, W.; Yeatts, S. Brain Oxygen Optimization in Severe Tbi (Boost3): A Comparative Effectiveness Study to Test the Efficacy of a Prescribed Treatment Protocol Based on Monitoring the Partial Pressure of Brain Tissue Oxygen. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03754114 (accessed on 4 March 2022).
- The BONANZA Trial—A Randomised Controlled Trial That Is Testing Whether a Management Strategy Guided by Early Brain Tissue Oxygen Monitoring in Patients in with Severe Traumatic Brain Injury Improves Long Term Neurological and Functional Outcomes. ACTRN12619001328167.2019. 2020 Issue 02. Available online: http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001328167 (accessed on 29 February 2020).
| All Patients (N = 106) | ICP-Group (N = 71) | ICP/PbtO2-Group (N = 35) | p-Value | |
|---|---|---|---|---|
| Age, years | 51 (±19) | 54 (±19) | 45 (±17) | 0.02 |
| Male gender, n (%) | 63 (59) | 39 (55) | 24 (69) | 0.21 |
| Arterial hypertension, n (%) | 27 (26) | 20 (28) | 7 (20) | 0.48 |
| Diabetes mellitus, n (%) | 7 (7) | 5 (7) | 2 (6) | 0.99 |
| Heart disease, n (%) | 11 (10) | 9 (13) | 2 (6) | 0.33 |
| Previous neurological disease, n (%) | 4 (4) | 4 (6) | 2 (6) | 0.30 |
| Alcohol use, n (%) | 36 (34) | 26 (37) | 10 (29) | 0.51 |
| Smoking, n (%) | 19 (18) | 14 (20) | 5 (14) | 0.60 |
| COPD, n (%) | 3 (3) | 3 (4) | 0 | 0.55 |
| Liver cirrhosis, n (%) | 5 (5) | 5 (6) | 1 (3) | 0.99 |
| Cancer, n (%) | 2 (2) | 1 (1) | 1 (3) | 0.99 |
| Chronic kidney disease, n (%) | 3 (3) | 3 (4) | 0 | 0.55 |
| On admission | ||||
| APACHE score | 18 (15–21) | 18 (15–21) | 20 (17–23) | 0.03 |
| SOFA score | 8 (4–10) | 6 (4–10) | 8 (8–10) | 0.01 |
| GCS on admission | 5 (3–8) | 5 (3–9) | 5 (3–7) | 0.72 |
| Marshall score, n (%) | 0.02 | |||
| 1 | 0 | 0 | 0 | |
| 2 | 5 (5) | 3 (4) | 2 (6) | |
| 3 | 3 (3) | 2 (3) | 1 (3) | |
| 4 | 27 (26) | 25 (35) | 2 (6) | |
| 5 | 69 (65) | 39 (55) | 30 (86) | |
| 6 | 2 (2) | 2 (3) | 0 | |
| Reacting pupils, n (%) | 79 (75) | 53 (75) | 26 (74) | 0.99 |
| Traumatic SAH, n (%) | 95 (90) | 67 (94) | 28 (80) | 0.04 |
| Epidural Hematoma, n (%) | 56 (53) | 26 (37) | 30 (86) | 0.001 |
| Hypotension, n (%) | 40 (0.29) | 24 (34) | 16 (46) | 0.29 |
| Hypoxemia, n (%) | 54 (51) | 33 (47) | 21 (60) | 0.22 |
| Sodium, mmol/L | 138 (135–141) | 138 (136–141) | 137 (135–140) | 0.19 |
| Glucose, mg/dL | 136 (124–170) | 141 (126–176) | 131 (122–167) | 0.62 |
| Hemoglobin, g/dL | 12.1 (10.5–13.6) | 11.9 (10.7–13.2) | 12.2 (10.3–14.5) | 0.63 |
| During ICU stay | ||||
| Mechanical ventilation, n (%) | 106 (100) | 71 (100) | 35 (100) | - |
| Vasopressors, n (%) | 78 (74) | 45 (63) | 33 (94) | 0.001 |
| Inotropes, n (%) | 10 (9) | 4 (6) | 6 (17) | 0.08 |
| RRT, n (%) | 1 (1) | 0 | 1 (3) | 0.33 |
| EVD, n (%) | 93 (88) | 66 (93) | 27 (77) | 0.55 |
| Complications | ||||
| Intracranial hypertension, n (%) | 73 (69) | 57 (80) | 16 (46) | 0.001 |
| Brain tissue hypoxia, n (%) | NA | NA | 24 (69) | - |
| Seizures, n (%) | 25 (24) | 17 (24) | 8 (23) | 0.99 |
| Treatments | ||||
| TIL score basic | 0.13 | |||
| 1 | 20 (20) | 11 (17) | 9 (26) | |
| 2 | 35 (35) | 28 (43) | 7 (20) | |
| 3 | 16 (16) | 10 (15) | 6 (17) | |
| 4 | 29 (29) | 16 (25) | 13 (37) | |
| Osmotic therapy, n (%) | 61 (58) | 43 (61) | 18 (51) | 0.41 |
| Hypothermia, n (%) | 23 (22) | 15 (21) | 8 (23) | 0.99 |
| Barbiturates, n (%) | 19 (18) | 15 (21) | 4 (11) | 0.29 |
| Decompressive craniectomy, n (%) | 25 (24) | 14 (20) | 11 (31) | 0.23 |
| Outcomes | ||||
| ICU LOS, days | 9 (4–17) | 7 (3–14) | 16 (9–25) | 0.001 |
| Hospital LOS, days | 17 (5–42) | 10 (4–38) | 30 (14–66) | 0.006 |
| GCS at ICU discharge | 6 (3–13) | 3 (3–12) | 10 (3–14) | 0.19 |
| ICU death, n (%) | 50 (47) | 37 (52) | 13 (37) | 0.16 |
| Hospital death, n (%) | 51 (48) | 38 (54) | 13 (37) | 0.15 |
| GOS at 3 months | 2 (1–4) | 1 (1–4) | 3 (1–4) | 0.15 |
| ICP-Group N = 35 | ICP/PbtO2 Group N = 35 | SMD | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male gender, n (%) | 22 (63) | 24 (69) | 0.12 | 0.80 |
| Age, years | 47 (34–66) | 44 (35–59) | 0.24 | 0.32 |
| Comorbidities | ||||
| Arterial Hypertension, n (%) | 7 (20) | 7 (20) | 0.00 | 1 |
| Diabetes mellitus, n (%) | 0 | 2 (6) | 0.35 | 0.49 |
| Heart disease, n (%) | 2 (6) | 2 (6) | 0.00 | 1.0 |
| Previous neurological disease, n (%) | 0 | 0 | - | - |
| Alcohol, n (%) | 10 (29) | 10 (29) | 0.00 | 1.0 |
| Smoking, n (%) | 0 | 0 | - | - |
| COPD, n (%) | 0 | 0 | - | - |
| Liver Cirrhosis, n (%) | 1 (3) | 1 (3) | 0.00 | 1.0 |
| Cancer, n (%) | 1 (3) | 1 (3) | 0.00 | 1.0 |
| Chronic kidney disease, n (%) | 1 (3) | 0 | 0.24 | 0.99 |
| On ICU admission | ||||
| APACHE score | 18 (17–21) | 20 (17–23) | −0.35 | 0.32 |
| GCS score | 4 (3–7) | 10 (3–14) | −0.07 | 0.51 |
| Hemoglobin, g/dL | 12.1 (10.3–13.4) | 12.2 (10.3–14.5) | −0.05 | 0.67 |
| Glucose, mg/dL | 129 (115–156) | 131 (122–167) | −0.34 | 0.31 |
| Sodium, mmol/L | 138 (137–141) | 137 (135–140) | 0.38 | 0.26 |
| Reacting pupils, n (%) | 26 (74) | 26 (74) | 0 | 0.99 |
| Traumatic SAH, n (%) | 15 (43) | 16 (46) | 0.44 | 0.15 |
| Epidural hematoma, n (%) | 15 (43) | 30 (86) | 1.00 | 0.001 |
| Hypotension, n (%) | 15 (43) | 16 (46) | 0.05 | 0.99 |
| Hypoxemia, n (%) | 21 (60) | 21 (60) | 0.34 | 1.0 |
| Marshall CT score | 0.53 | 0.34 | ||
| 1 | 0 | 0 | ||
| 2 | 1 (3) | 2 (6) | ||
| 3 | 1 (3) | 1 (3) | ||
| 4 | 7 (20) | 2 (6) | ||
| 5 | 25 (71) | 30 (86) | ||
| 6 | 1 (3) | 0 | ||
| During ICU stay | ||||
| Mechanical ventilation, n (%) | 35 (100) | 35 (100) | - | - |
| Vasopressors, n (%) | 26 (74) | 33 (94) | 0.57 | 0.05 |
| Inotropic agents, n (%) | 2 (6) | 6 (17) | 0.37 | 0.26 |
| RRT, n (%) | 0 | 1 (3) | 0.24 | 0.99 |
| ECMO, n (%) | 0 | 0 | - | - |
| EVD placement, n (%) | 34 (97) | 27 (77) | 0.63 | 0.03 |
| Intracranial Hypertension, n (%) | 27 (77) | 16 (46) | 0.68 | 0.01 |
| Seizures, n (%) | 10 (29) | 8 (23) | 0.13 | 0.79 |
| TIL score | 0.65 | 0.09 | ||
| 1 | 5 (14) | 1 (34) | ||
| 2 | 13 (37) | 7 (20) | ||
| 3 | 2 (6) | 5(14) | ||
| 4 | 15(43) | 11(31) | ||
| Brain tissue hypoxia, n (%) | - | 24 (69) | - | - |
| Osmotic therapy, n (%) | 21 (60) | 18 (51) | 0.17 | 0.63 |
| Barbiturates, n (%) | 10 (29) | 4 (11) | 0.43 | 0.13 |
| Hypothermia, n (%) | 8 (23) | 10 (29) | 0.13 | 0.79 |
| Decompressive craniectomy, n (%) | 11 (31) | 11 (31) | 0 | 1.0 |
| Outcomes | ||||
| ICU length of stay, days | 7 (3–14) | 16 (9–25) | −0.77 | 0.001 |
| Hospital length of stay, days | 14 (3–41) | 30 (14–66) | −0.39 | 0.03 |
| GCS at ICU discharge | 3 (3–12) | 10 (3–14) | −0.20 | 0.46 |
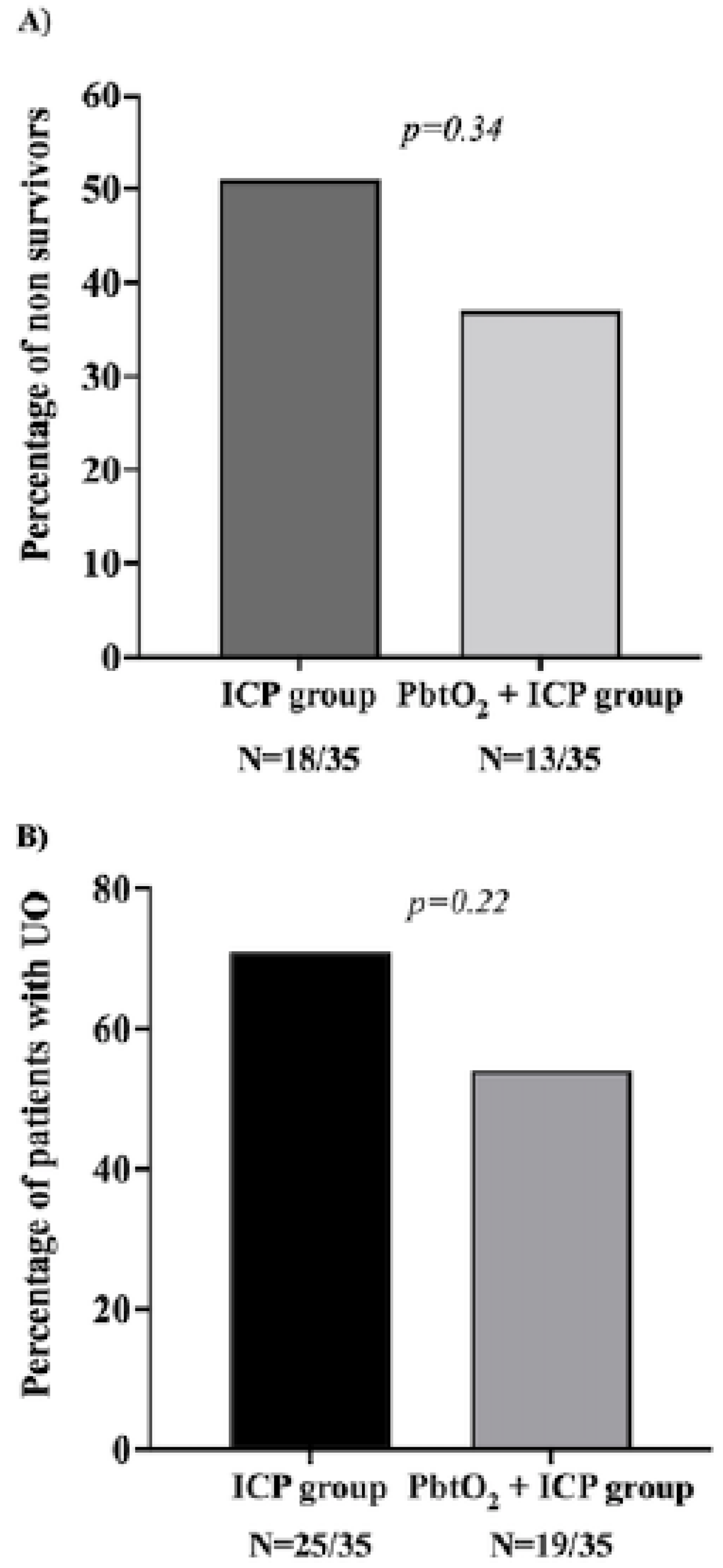
| Deaths at the ICU, n (%) | 18 (51) | 13 (37) | 0.29 | 0.34 |
| Deaths at the hospital, (%) | 18 (51) | 13 (37) | 0.29 | 0.34 |
| 3-month GOS | 1 (1–4) | 3 (1–4) | −0.37 | 0.35 |
| 3-month UO, n (%) | 25 (71) | 19 (54) | 0.36 | 0.22 |
| Univariable Analysis OR (95% CI) | Multivariable Analysis OR (95% CI) | |
|---|---|---|
| Marshall score | 1.37 (0.83–2.26) | 1.54 (0.84–2.82) |
| GCS on admission | 0.96 (0.86–1.07) | 1.00 (0.88–1.14) |
| Reactive pupils | 0.30 (0.09–0.95) | 0.28 (0.08–1.06) |
| PbtO2 monitoring | 0.21 (0.09–0.50) | 0.16 (0.06–0.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrit, S.; Al Barajraji, M.; El Hadweh, S.; Dewitte, O.; Torcida, N.; Andre, J.; Taccone, F.S.; Schuind, S.; Gouvêa Bogossian, E. Brain Tissue Oxygenation-Guided Therapy and Outcome in Traumatic Brain Injury: A Single-Center Matched Cohort Study. Brain Sci. 2022, 12, 887. https://doi.org/10.3390/brainsci12070887
Barrit S, Al Barajraji M, El Hadweh S, Dewitte O, Torcida N, Andre J, Taccone FS, Schuind S, Gouvêa Bogossian E. Brain Tissue Oxygenation-Guided Therapy and Outcome in Traumatic Brain Injury: A Single-Center Matched Cohort Study. Brain Sciences. 2022; 12(7):887. https://doi.org/10.3390/brainsci12070887
Chicago/Turabian StyleBarrit, Sami, Mejdeddine Al Barajraji, Salim El Hadweh, Olivier Dewitte, Nathan Torcida, Joachim Andre, Fabio Silvio Taccone, Sophie Schuind, and Elisa Gouvêa Bogossian. 2022. "Brain Tissue Oxygenation-Guided Therapy and Outcome in Traumatic Brain Injury: A Single-Center Matched Cohort Study" Brain Sciences 12, no. 7: 887. https://doi.org/10.3390/brainsci12070887
APA StyleBarrit, S., Al Barajraji, M., El Hadweh, S., Dewitte, O., Torcida, N., Andre, J., Taccone, F. S., Schuind, S., & Gouvêa Bogossian, E. (2022). Brain Tissue Oxygenation-Guided Therapy and Outcome in Traumatic Brain Injury: A Single-Center Matched Cohort Study. Brain Sciences, 12(7), 887. https://doi.org/10.3390/brainsci12070887







