Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis
Abstract
1. Introduction
2. Materials and Methods
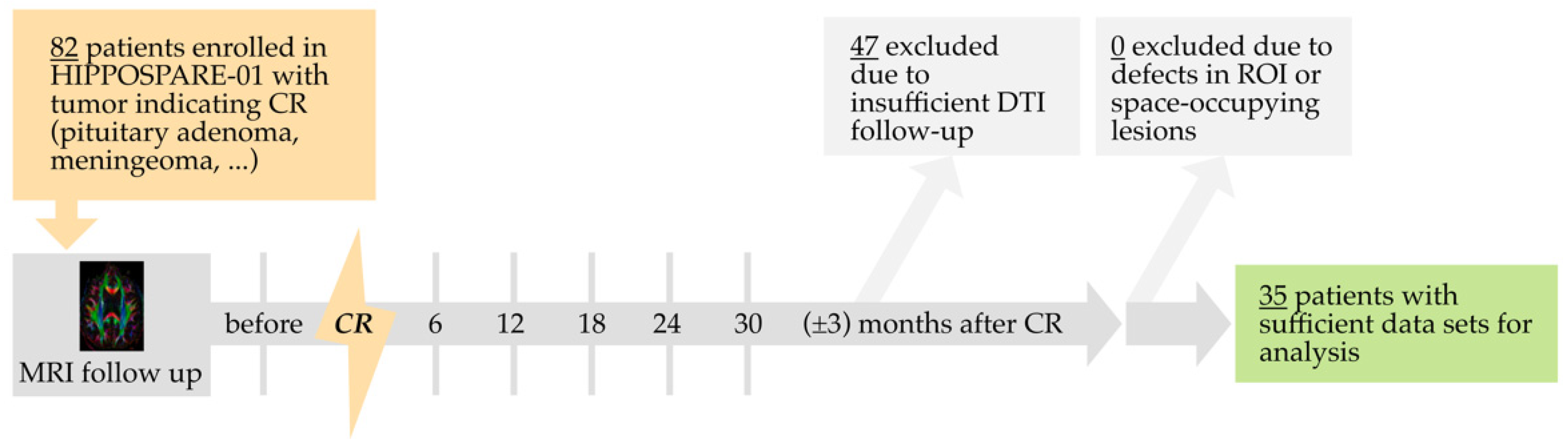
2.1. Study Design
2.2. Follow-Up and Imaging Protocol
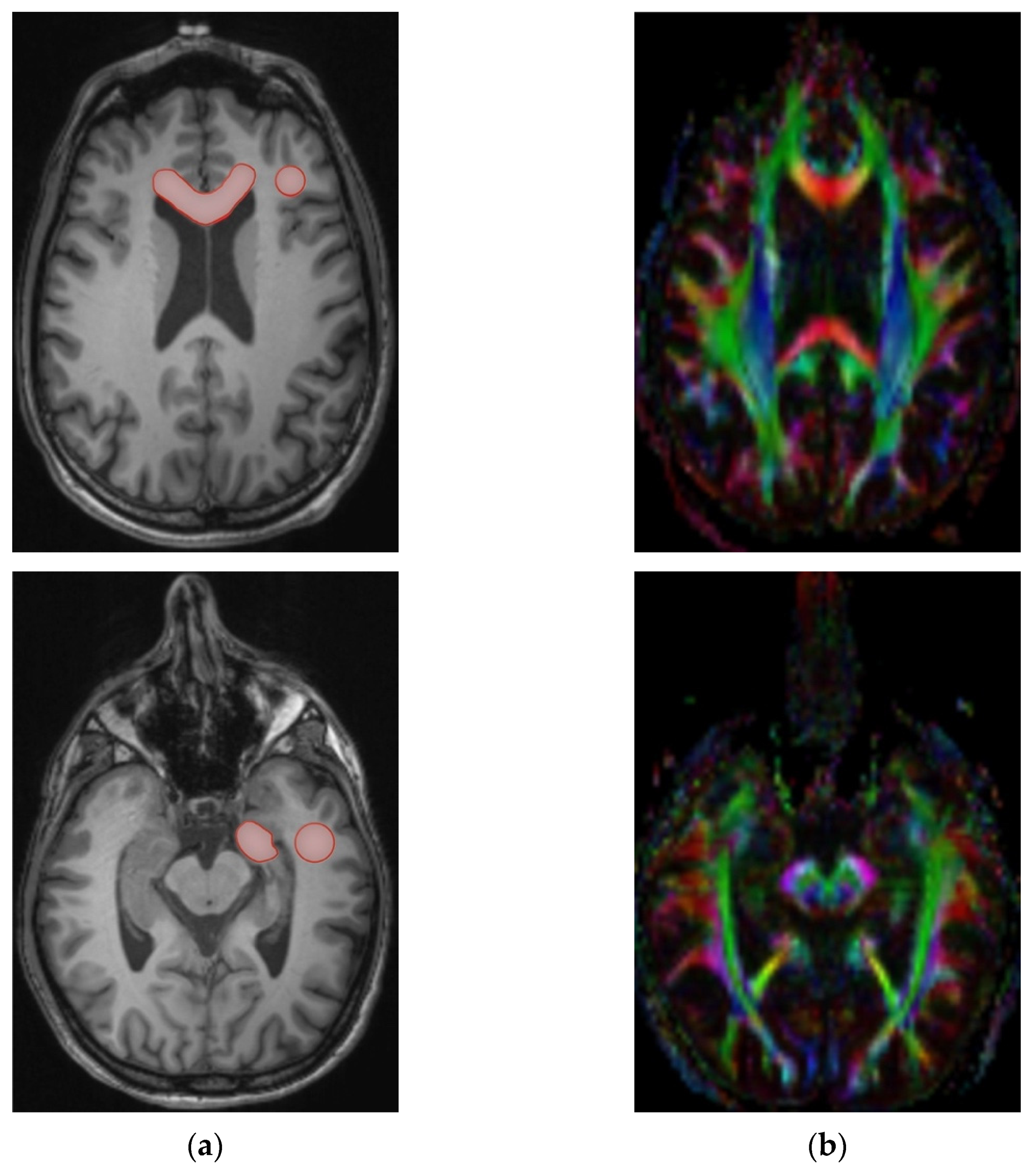
2.3. Processing and Measurement
2.4. Statistics
2.4.1. Analysis of Patient Characteristics
2.4.2. Analysis of DTI Changes after Cranial Radiotherapy
2.4.3. Analysis of the Effects of Mean Hippocampal Dose on DTI Changes
2.4.4. Analysis of the Effects of Hippocampal Sparing on DTI Changes
3. Results
3.1. Patient Characteristics
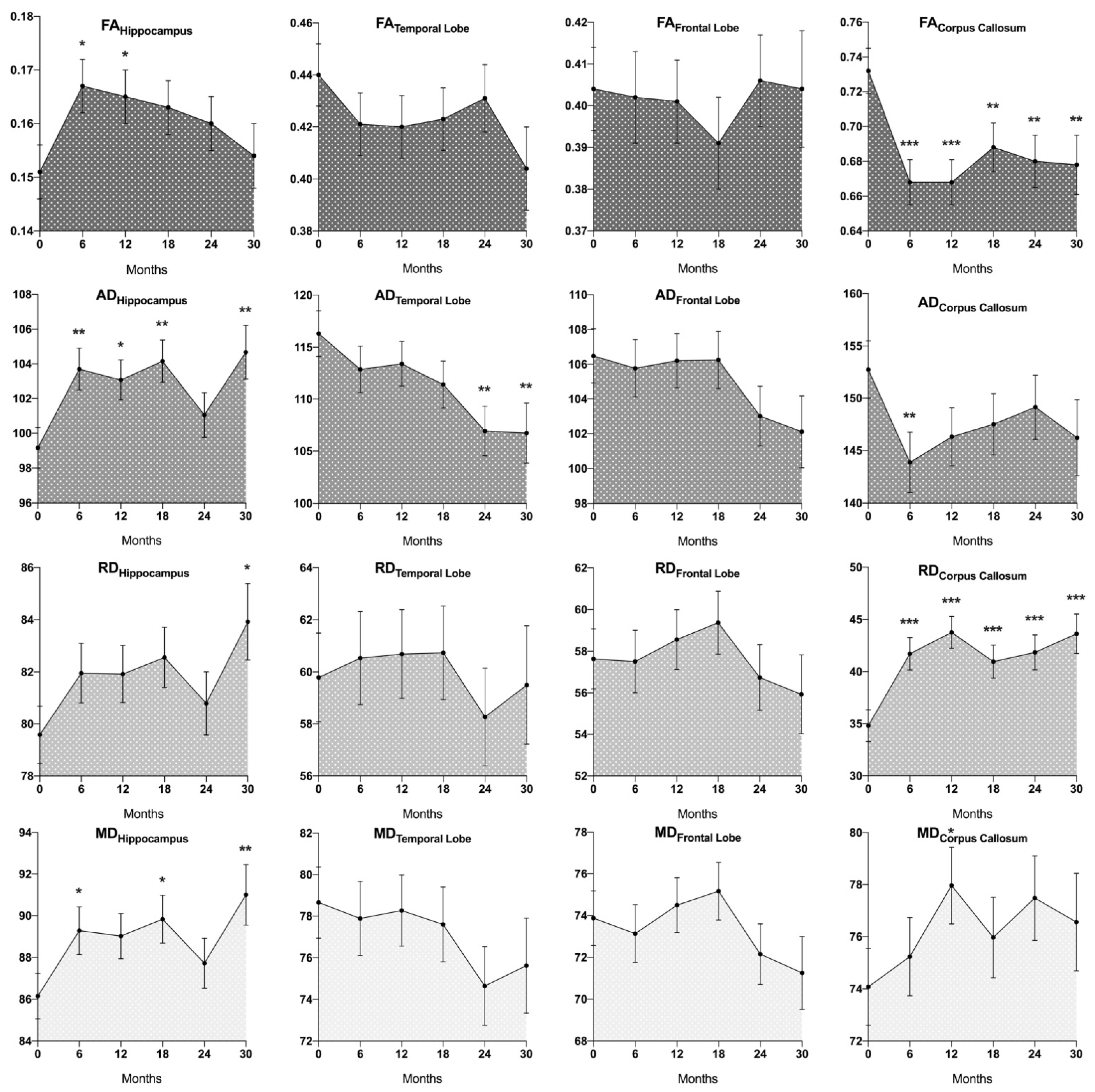
3.2. DTI Changes after Cranial Radiotherapy
3.3. Effects of Mean Hippocampal Dose on DTI Changes
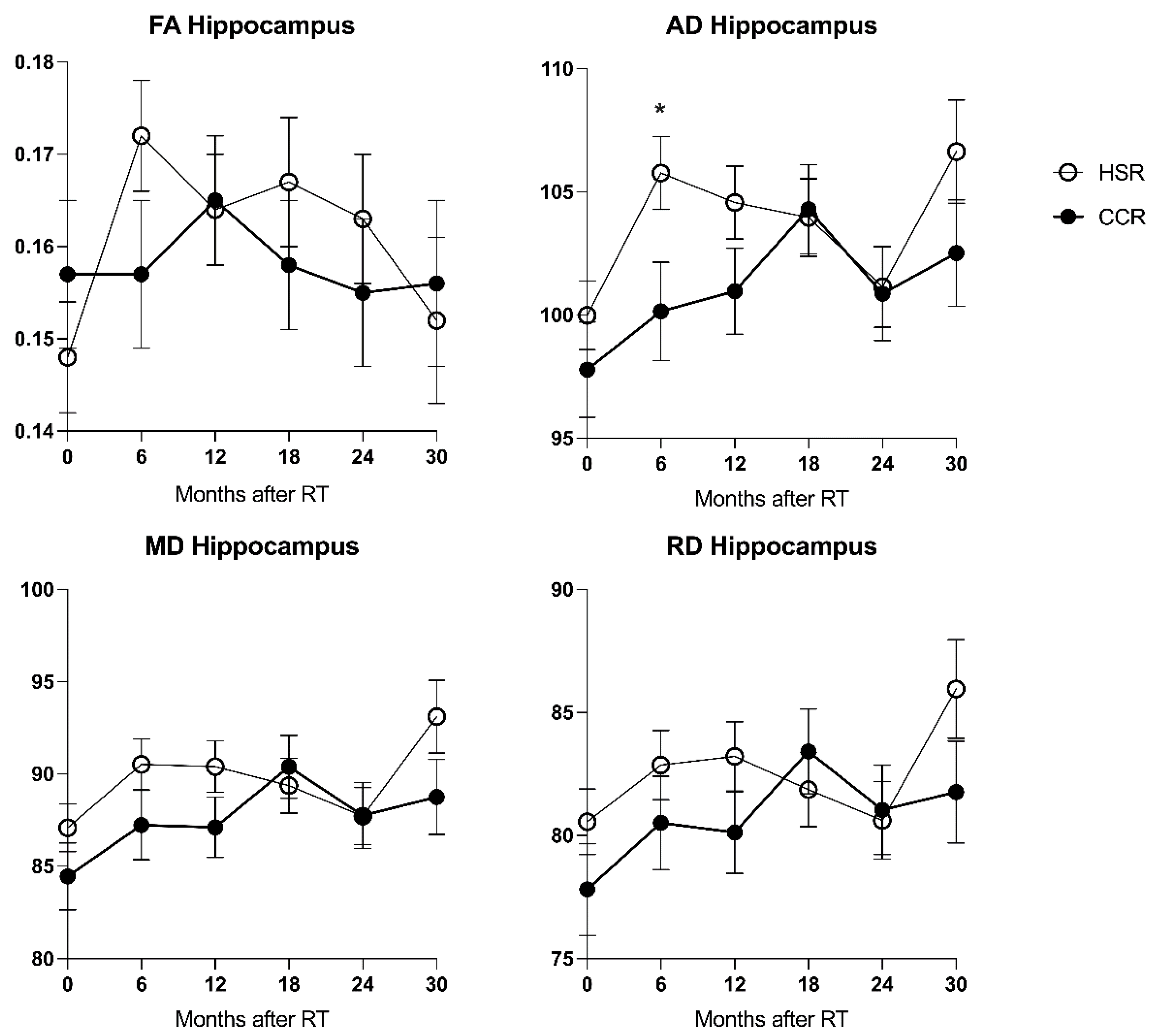
3.4. Effects of Hippocampal Sparing on DTI Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McTyre, E.; Scott, J.; Chinnaiyan, P. Whole brain radiotherapy for brain metastasis. Surg. Neurol. Int. 2013, 4, S236–S244. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.E.; Glantz, M.J. Neurologic complications of radiation therapy. Neurol. Clin. 2003, 21, 249–277. [Google Scholar] [CrossRef]
- Balentova, S.; Adamkov, M. Molecular, cellular and functional effects of radiation-induced brain injury: A review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Connor, M.; Karunamuni, R.; McDonald, C.; White, N.; Pettersson, N.; Moiseenko, V.; Seibert, T.; Marshall, D.; Cervino, L.; Bartsch, H.; et al. Dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2016, 121, 209–216. [Google Scholar] [CrossRef]
- Tsao, M.N.; Xu, W.; Wong, R.K.S.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomised controlled trial. J. Am. Med. Assoc. 2006, 45, 427–434. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Jane, H.; Anderson, S.K.; Carrero, X.W.; Ii, F.G.B.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Aoyama, H.; Tago, M.; Kato, N.; Toyoda, T.; Kenjyo, M.; Hirota, S.; Shioura, H.; Inomata, T.; Kunieda, E.; Hayakawa, K.; et al. Neurocognitive Function of Patients with Brain Metastasis Who Received Either Whole Brain Radiotherapy Plus Stereotactic Radiosurgery or Radiosurgery Alone. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Regine, W.F.; Scott, C.; Murray, K.; Curran, W. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: An analysis from Radiation Therapy Oncology Group Study 91-04. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 711–717. [Google Scholar] [CrossRef]
- Lynch, M. Preservation of cognitive function following whole brain radiotherapy in patients with brain metastases: Complications, treatments, and the emerging role of memantine. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2019, 25, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.; Yeap, B.Y.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Dworkin, M.; Fullerton, B.C.; Daartz, J.; Royce, T.J.; Oh, K.S.; et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiother. Oncol. 2019, 137, 95–101. [Google Scholar] [CrossRef]
- Joo, K.M.; Jin, J.; Kang, B.G.; Lee, S.J.; Kim, K.H.; Yang, H.; Lee, Y.A.; Cho, Y.J.; Im, Y.S.; Lee, D.S.; et al. Trans-differentiation of neural stem cells: A therapeutic mechanism against the radiation induced brain damage. PLoS ONE 2012, 7, e25936. [Google Scholar] [CrossRef]
- Peiffer, A.M.; Leyrer, C.M.; Greene-Schloesser, D.M.; Shing, E.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Ip, E.H.; Rapp, S.R.; Robbins, M.E.; et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology 2013, 80, 747–753. [Google Scholar] [CrossRef]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef]
- Rola, R.; Raber, J.; Rizk, A.; Otsuka, S.; VandenBerg, S.R.; Morhardt, D.R.; Fike, J.R. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004, 188, 316–330. [Google Scholar] [CrossRef]
- Monje, M.L.; Vogel, H.; Masek, M.; Ligon, K.L.; Fisher, P.G.; Palmer, T.D. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. 2007, 62, 515–520. [Google Scholar] [CrossRef]
- Belarbi, K.; Jopson, T.; Arellano, C.; Fike, J.R.; Rosi, S. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 2013, 73, 1201–1210. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [PubMed]
- Okoukoni, C.; McTyre, E.R.; Ayala Peacock, D.N.; Peiffer, A.M.; Strowd, R.; Cramer, C.; Hinson, W.H.; Rapp, S.; Metheny-Barlow, L.; Shaw, E.G.; et al. Hippocampal dose volume histogram predicts Hopkins Verbal Learning Test scores after brain irradiation. Adv. Radiat. Oncol. 2017, 2, 624–629. [Google Scholar] [CrossRef]
- Acharya, S.; Wu, S.; Ashford, J.M.; Tinkle, C.L.; Lucas, J.T.; Qaddoumi, I.; Gajjar, A.; Krasin, M.J.; Conklin, H.M.; Merchant, T.E. Association between hippocampal dose and memory in survivors of childhood or adolescent low-grade glioma: A 10-year neurocognitive longitudinal study. Neuro. Oncol. 2019, 21, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.F.; Yang, C.C.; Chuang, C.C.; Huang, T.Y.; Wu, Y.M.; Pai, P.C.; Tseng, C.K.; Wu, T.H.; Shen, Y.L.; Lin, S.Y. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: A prospective study. Radiat. Oncol. 2015, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.M.; Grimm, J.; McIntyre, R.; Anderson-Keightly, H.; Kleinberg, L.R.; Hales, R.K.; Moore, J.; Vannorsdall, T.; Redmond, K.J. A prospective evaluation of hippocampal radiation dose volume effects and memory deficits following cranial irradiation. Radiother. Oncol. 2017, 125, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Vannorsdall, T.D.; Kleinberg, L.R.; Assadi, R.; Moore, J.A.; Hu, C.; Quiñones-Hinojosa, A.; Redmond, K.J. A Prospective Cohort Study of Neural Progenitor Cell-Sparing Radiation Therapy Plus Temozolomide for Newly Diagnosed Patients with Glioblastoma. Neurosurgery 2020, 87, E31–E40. [Google Scholar] [CrossRef]
- Leyrer, C.M.; Peiffer, A.M.; Greene-Schloesser, D.M.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Rapp, S.R.; Robbins, M.E.; Shaw, E.G.; Chan, M.D. Normal Tissue Complication Modeling of the Brain: Dose-volume Histogram Analysis of Neurocognitive Outcomes of Two CCOP Trials. Int. J. Radiat. Oncol. 2011, 81, S184–S185. [Google Scholar] [CrossRef]
- Blomstrand, M.; Holmberg, E.; Åberg, M.A.I.; Lundell, M.; Björk-Eriksson, T.; Karlsson, P.; Blomgren, K. No clinically relevant effect on cognitive outcomes after low-dose radiation to the infant brain: A population-based cohort study in Sweden. Acta Oncol. (Madr.) 2014, 53, 1143–1150. [Google Scholar] [CrossRef]
- Janssen, S.; Mehta, P.; Bartscht, T.; Schmid, S.M.; Fahlbusch, F.B.; Rades, D. Prevalence of metastases within the hypothalamic-pituitary area in patients with brain metastases. Radiat. Oncol. 2019, 14, 152. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Redmond, K.J.; Hales, R.K.; Anderson-Keightly, H.; Zhou, X.C.; Kummerlowe, M.; Sair, H.I.; Duhon, M.; Kleinberg, L.; Rosner, G.L.; Vannorsdall, T. Prospective Study of Hippocampal-Sparing Prophylactic Cranial Irradiation in Limited-Stage Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Robinson, C.G.; Rusthoven, C.G. Hippocampal-Sparing Radiotherapy for Patients With Glioblastoma and Grade II-III Gliomas. JAMA Oncol. 2020, 6, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III trial NRG oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.G.; Hedlund, G.; Salzman, K.L. Osborn’s Brain, 2nd ed.; Elsevier: Salt Lake City, UT, USA, 2017. [Google Scholar]
- MRIcron dcm2nii. Available online: http://www.mccauslandcenter.sc.edu/micro/mricron/dcm2nii.html (accessed on 2 May 2022).
- Andersson, J.L.R.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- FMRIB Software Library. Available online: fsl.fmrib.ox.ac.uk (accessed on 2 May 2022).
- Alexander, A.L.; Hasan, K.M.; Lazar, M.; Tsuruda, J.S.; Parker, D.L. Analysis of partial volume effects in diffusion-tensor MRI. Magn. Reson. Med. 2001, 45, 770–780. [Google Scholar] [CrossRef]
- Klein, M.; Heimans, J.J.; Aaronson, N.K.; van der Ploeg, H.M.; Grit, J.; Muller, M.; Postma, T.J.; Mooij, J.J.; Boerman, R.H.; Beute, G.N.; et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: A comparative study. Lancet 2002, 360, 1361–1368. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Yeh, S.A.; Chang, C.C.; Tsai, P.C.; Wu, J.M.; Gau, J.S. Cognitive Function Before and After Intensity-Modulated Radiation Therapy in Patients With Nasopharyngeal Carcinoma: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 722–726. [Google Scholar] [CrossRef]
- Gondi, V.; Paulus, R.; Bruner, D.W.; Meyers, C.A.; Gore, E.M.; Wolfson, A.; Werner-Wasik, M.; Sun, A.Y.; Choy, H.; Movsas, B. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 656–664. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Nagesh, V.; Tsien, C.I.; Chenevert, T.L.; Ross, B.D.; Lawrence, T.S.; Junick, L.; Cao, Y. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: A diffusion tensor imaging study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1002–1010. [Google Scholar] [CrossRef]
- Nazem-Zadeh, M.-R.; Chapman, C.H.; Lawrence, T.L.; Tsien, C.I.; Cao, Y. Radiation therapy effects on white matter fiber tracts of the limbic circuit. Med. Phys. 2012, 39, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.H.; Nazem-Zadeh, M.; Lee, O.E.; Schipper, M.J.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. Regional Variation in Brain White Matter Diffusion Index Changes following Chemoradiotherapy: A Prospective Study Using Tract-Based Spatial Statistics. PLoS ONE 2013, 8, e57768. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.; Karunamuni, R.; McDonald, C.; Seibert, T.; White, N.; Moiseenko, V.; Bartsch, H.; Farid, N.; Kuperman, J.; Krishnan, A.; et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2017, 123, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Makola, M.; Douglas Ris, M.; Mahone, E.M.; Yeates, K.O.; Cecil, K.M. Long-term effects of radiation therapy on white matter of the corpus callosum: A diffusion tensor imaging study in children. Pediatr. Radiol. 2017, 47, 1809–1816. [Google Scholar] [CrossRef]
- Tringale, K.R.; Nguyen, T.; Bahrami, N.; Marshall, D.C.; Leyden, K.M.; Karunamuni, R.; Seibert, T.M.; Kay Gorman, M.; Connor, M.; Burkeen, J.; et al. Identifying early diffusion imaging biomarkers of regional white matter injury as indicators of executive function decline following brain radiotherapy: A prospective clinical trial in primary brain tumor patients. Radiother. Oncol. 2019, 132, 27–33. [Google Scholar] [CrossRef]
- Qiu, D.; Kwong, D.L.W.; Chan, G.C.F.; Leung, L.H.T.; Khong, P.L. Diffusion Tensor Magnetic Resonance Imaging Finding of Discrepant Fractional Anisotropy Between the Frontal and Parietal Lobes After Whole-Brain Irradiation in Childhood Medulloblastoma Survivors: Reflection of Regional White Matter Radiosensitivity? Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 846–851. [Google Scholar] [CrossRef]
- Dellani, P.R.; Eder, S.; Gawehn, J.; Vucurevic, G.; Fellgiebel, A.; Müller, M.J.; Schmidberger, H.; Stoeter, P.; Gutjahr, P. Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. J. Magn. Reson. Imaging 2008, 27, 1250–1255. [Google Scholar] [CrossRef]
- Stricker, N.H.; Schweinsburg, B.C.; Delano-Wood, L.; Wierenga, C.E.; Bangen, K.J.; Haaland, K.Y.; Frank, L.R.; Salmon, D.P.; Bondi, M.W. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage 2009, 45, 10–16. [Google Scholar] [CrossRef]
- Chawla, S.; Wang, S.; Kim, S.; Sheriff, S.; Lee, P.; Rengan, R.; Lin, A.; Melhem, E.; Maudsley, A.; Poptani, H. Radiation Injury to the Normal Brain Measured by 3D-Echo-Planar Spectroscopic Imaging and Diffusion Tensor Imaging: Initial Experience. J. Neuroimaging 2015, 25, 97–104. [Google Scholar] [CrossRef]
- Bian, Y.; Meng, L.; Peng, J.; Li, J.; Wei, R.; Huo, L.; Yang, H.; Wang, Y.; Fu, J.; Shen, L.; et al. Effect of radiochemotherapy on the cognitive function and diffusion tensor and perfusion weighted imaging for high-grade gliomas: A prospective study. Sci. Rep. 2019, 9, 5967. [Google Scholar] [CrossRef]
- Tringale, K.R.; Karunamuni, R.; Nguyen, T.; Seibert, T.M.; Leyden, K.; Uttarwar, V.; Murzin, V.; Marshall, D.C.; Simpson, D.R.; Sanghvi, P.; et al. Prospective Trial of Quantitative Neuroimaging Correlates of Verbal and Nonverbal Memory Decline. Int. J. Radiat. Oncol. 2017, 99, S101. [Google Scholar] [CrossRef]
- Constanzo, J.; Dumont, M.; Lebel, R.; Tremblay, L.; Whittingstall, K.; Masson-Côté, L.; Geha, S.; Sarret, P.; Lepage, M.; Paquette, B.; et al. Diffusion MRI monitoring of specific structures in the irradiated rat brain. Magn. Reson. Med. 2018, 80, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Watve, A.; Gupta, M.; Khushu, S.; Rana, P. Longitudinal changes in gray matter regions after cranial radiation and comparative analysis with whole body radiation: A DTI study. Int. J. Radiat. Biol. 2018, 94, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Haridas, S.; Trivedi, R.; Khushu, S.; Manda, K. Early cognitive changes due to whole body γ-irradiation: A behavioral and diffusion tensor imaging study in mice. Exp. Neurol. 2013, 248, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Rana, P.; Trivedi, R.; Kumar, B.S.H.; Khan, A.R.; Soni, R.; Rathore, R.K.S.; Khushu, S. Comparative evaluation of brain neurometabolites and DTI indices following whole body and cranial irradiation: A magnetic resonance imaging and spectroscopy study. NMR Biomed. 2013, 26, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Creer, R.M.; Linville, C.; Olson, J.; Kulkarni, P.; Brown, J.A.; Riddle, D.R.; Robbins, M.E.; Brunso-Bechtold, J.E. Radiation-induced cognitive impairment and altered diffusion tensor imaging in a juvenile rat model of cranial radiotherapy. Int. J. Radiat. Biol. 2014, 90, 799–806. [Google Scholar] [CrossRef][Green Version]
- Tofilon, P.J.; Fike, J.R. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000, 153, 357–370. [Google Scholar] [CrossRef]
- Montero-Menei, C.N.; Sindji, L.; Garcion, E.; Mege, M.; Couez, D.; Gamelin, E.; Darcy, F. Early events of the inflammatory reaction induced in rat brain by lipopolysaccharide intracerebral injection: Relative contribution of peripheral monocytes and activated microglia. Brain Res. 1996, 724, 55–66. [Google Scholar] [CrossRef]
- Robbins, M.E.C.; Zhao, W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004, 80, 251–259. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Chiang, C.S.; McBride, W.H.; Withers, H.R. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1993, 29, 60–68. [Google Scholar] [CrossRef]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Ringstad, G.; Vatnehol, S.A.S.; Eide, P.K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017, 140, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Sepehrband, F.; Cabeen, R.P.; Choupan, J.; Barisano, G.; Law, M.; Toga, A.W. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage 2019, 197, 243–254. [Google Scholar] [CrossRef]
- Taoka, T.; Masutani, Y.; Kawai, H.; Nakane, T.; Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Naganawa, S. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 2017, 35, 172–178. [Google Scholar] [CrossRef]
- Thomas, C.; Sadeghi, N.; Nayak, A.; Trefler, A.; Sarlls, J.; Baker, C.I.; Pierpaoli, C. Impact of time-of-day on diffusivity measures of brain tissue derived from diffusion tensor imaging. Neuroimage 2018, 173, 25–34. [Google Scholar] [CrossRef]
- Andrews, R.N.; Caudell, D.L.; Metheny-Barlow, L.J.; Peiffer, A.M.; Tooze, J.A.; Bourland, J.D.; Hampson, R.E.; Deadwyler, S.A.; Cline, J.M. Fibronectin Produced by Cerebral Endothelial and Vascular Smooth Muscle Cells Contributes to Perivascular Extracellular Matrix in Late-Delayed Radiation-Induced Brain Injury. Radiat. Res. 2018, 190, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Mathias, J.L.; Rose, S.E. Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: A meta-analysis. Dev. Neuropsychol. 2014, 39, 600–637. [Google Scholar] [CrossRef] [PubMed]
- Blaumanis, O.R.; Rennels, M.L.; Grady, P.A. Focal cerebral edema impedes convective fluid/tracer movement through paravascular pathways in cat brain. Adv. Neurol. 1990, 52, 385–389. [Google Scholar]
- Chenevert, T.L.; Ross, B.D. Diffusion Imaging for Therapy Response Assessment of Brain Tumor. Neuroimaging Clin. N. Am. 2009, 19, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.E.; Hatzigeorgiou, X.; Strudwick, M.W.; Durbridge, G.; Davies, P.S.W.; Colditz, P.B. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn. Reson. Med. 2008, 60, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.D.; Janes, L.; Gold, E.; Turtzo, L.C.; Frank, J.A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain 2011, 134, 2248–2260. [Google Scholar] [CrossRef]
- Raschke, F.; Wesemann, T.; Wahl, H.; Appold, S.; Krause, M.; Linn, J.; Troost, E.G.C. Reduced diffusion in normal appearing white matter of glioma patients following radio(chemo)therapy. Radiother. Oncol. 2019, 140, 110–115. [Google Scholar] [CrossRef]
- Chapman, C.H.; Nagesh, V.; Sundgren, P.C.; Buchtel, H.; Chenevert, T.L.; Junck, L.; Lawrence, T.S.; Tsien, C.I.; Cao, Y. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2033–2040. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Reddick, W.E.; Luxton, J.; Glass, J.O.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro. Oncol. 2012, 14, iv25–iv36. [Google Scholar] [CrossRef]
- Chapman, C.H.; Zhu, T.; Nazem-Zadeh, M.; Tao, Y.; Buchtel, H.A.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother. Oncol. 2016, 120, 234–240. [Google Scholar] [CrossRef]
| All Patients (n = 35) (SD) | [%] | Patients in CCR Arm (n = 15) | [%] | Patients in HSR Arm (n = 20) | [%] | |
|---|---|---|---|---|---|---|
| Age in years | ||||||
| Median | 56 (±12) | 52 (±8) | 59 (±14) | |||
| Range | 29–77 | 43–75 | 29–77 | |||
| Sex | ||||||
| Female | 20 | [57] | 8 | [53] | 12 | [60] |
| Male | 15 | [43] | 7 | [47] | 8 | [40] |
| Tumor Entity | ||||||
| Meningioma | 18 | [51] | 10 | [67] | 8 | [40] |
| Pituitary Adenoma | 12 | [34] | 5 | [33] | 7 | [35] |
| Meningioma and Pituitary Adenoma | 1 | [3] | 0 | [0] | 1 | [5] |
| Craniopharyngioma | 2 | [6] | 0 | [0] | 2 | [10] |
| Small Cell Lung Cancer | 1 | [3] | 0 | [0] | 1 | [5] |
| Large Cell Neuroendocrine Carcinoma | 1 | [3] | 0 | [0] | 1 | [5] |
| Radiotherapy | ||||||
| Definitive Stereotactic | 23 | [66] | 12 | [80] | 11 | [55] |
| Postoperative Stereotactic | 10 | [29] | 3 | [20] | 7 | [35] |
| Definitive Whole Brain Radiotherapy | 1 | [3] | 0 | [0] | 1 | [5] |
| Postoperative Whole Brain Radiotherapy | 1 | [3] | 0 | [0] | 1 | [5] |
| Total Dose in Gy | ||||||
| Mean (±SD) | 49.3 (±3.4) | 50.0 (±2.2) | 48.7 (±4.0) | |||
| Range | 38.0–54.0 | 45.0–52.2 | 38.0–54.0 | |||
| Mean number of fractions | 27 (±2) | 28 (±1) | 27 (±3) | |||
| Mean dose per fraction (Gy) | 1.8 | 1.8 | 1.8 | |||
| Duration (days) | 41 (6) | 42 (3) | 41 (8) | |||
| Duration Range | 24–66 | 37–48 | 24–66 | |||
| Hippocampal Dose in Gy | ||||||
| Mean (SD) | 10.9 (9.0) | 16.2 (10.9) | 6.9 (4.3) | |||
| Range | 0.1–39.2 | 2.0–39.2 | 0.1–14.5 | |||
| Chemotherapy | ||||||
| yes | 4 | [11] | 1 | [7] | 3 | [15] |
| none | 31 | [89] | 14 | [93] | 17 | [85] |
| Cerebral Surgery | ||||||
| yes | 32 | [91] | 14 | [93] | 18 | [90] |
| none | 3 | [9] | 1 | [7] | 2 | [10] |
| Cerebral Biopsy | ||||||
| yes | 3 | [9] | 0 | [0] | 3 | [15] |
| none | 32 | [91] | 15 | [100] | 17 | [85] |
| Mean Hippocampal Baseline DTI Values | ||||||
| Fractional Anisotropy (±SD) | 0.15 (±0.01) | 0.16 (±0.01) | 0.15 (±0.01) | |||
| Mean Diffusivity (±SD) | 86.1 (±1.1) | 84.5 (±1.8) | 90.5 (±1.4) | |||
| Axial Diffusivity (±SD) | 99.1 (±1.2) | 97.8 (±1.9) | 100.0 (±1.4) | |||
| Radial Diffusivity (±SD) | 79.6 (±1.1) | 77.8 (±1.9) | 80.6 (±1.3) | |||
| Availability of Complete MRI Data Sets | ||||||
| before CR | 29 | 10 | 19 | |||
| 6 ± 3 months after CR | 24 | 8 | 16 | |||
| 12 ± 3 months after CR | 27 | 11 | 16 | |||
| 18 ± 3 months after CR | 24 | 10 | 14 | |||
| 24 ± 3 months after CR | 22 | 9 | 13 | |||
| 30 ± 3 months after CR | 15 | 7 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinkel, J.G.; Lahmer, G.; Mennecke, A.; Hock, S.W.; Richter-Schmidinger, T.; Fietkau, R.; Distel, L.; Putz, F.; Dörfler, A.; Schmidt, M.A. Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis. Brain Sci. 2022, 12, 879. https://doi.org/10.3390/brainsci12070879
Dinkel JG, Lahmer G, Mennecke A, Hock SW, Richter-Schmidinger T, Fietkau R, Distel L, Putz F, Dörfler A, Schmidt MA. Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis. Brain Sciences. 2022; 12(7):879. https://doi.org/10.3390/brainsci12070879
Chicago/Turabian StyleDinkel, Johannes G., Godehard Lahmer, Angelika Mennecke, Stefan W. Hock, Tanja Richter-Schmidinger, Rainer Fietkau, Luitpold Distel, Florian Putz, Arnd Dörfler, and Manuel A. Schmidt. 2022. "Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis" Brain Sciences 12, no. 7: 879. https://doi.org/10.3390/brainsci12070879
APA StyleDinkel, J. G., Lahmer, G., Mennecke, A., Hock, S. W., Richter-Schmidinger, T., Fietkau, R., Distel, L., Putz, F., Dörfler, A., & Schmidt, M. A. (2022). Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis. Brain Sciences, 12(7), 879. https://doi.org/10.3390/brainsci12070879








