Differences in Inhibitory Control and Resting Brain Metabolism between Older Chronic Users of Tetrahydrocannabinol (THC) or Cannabidiol (CBD)—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Measurements
2.3.1. Arm and Hand Function
2.3.2. Cognitive Function
2.3.3. Gait
2.3.4. Fall Risk
2.3.5. Static Posturography
2.3.6. Handgrip Strength Testing
2.4. MRI/PET Scans
2.5. Statistical Analysis
3. Results
3.1. Cognitive and Motor Tasks
3.2. Brain Image Analysis
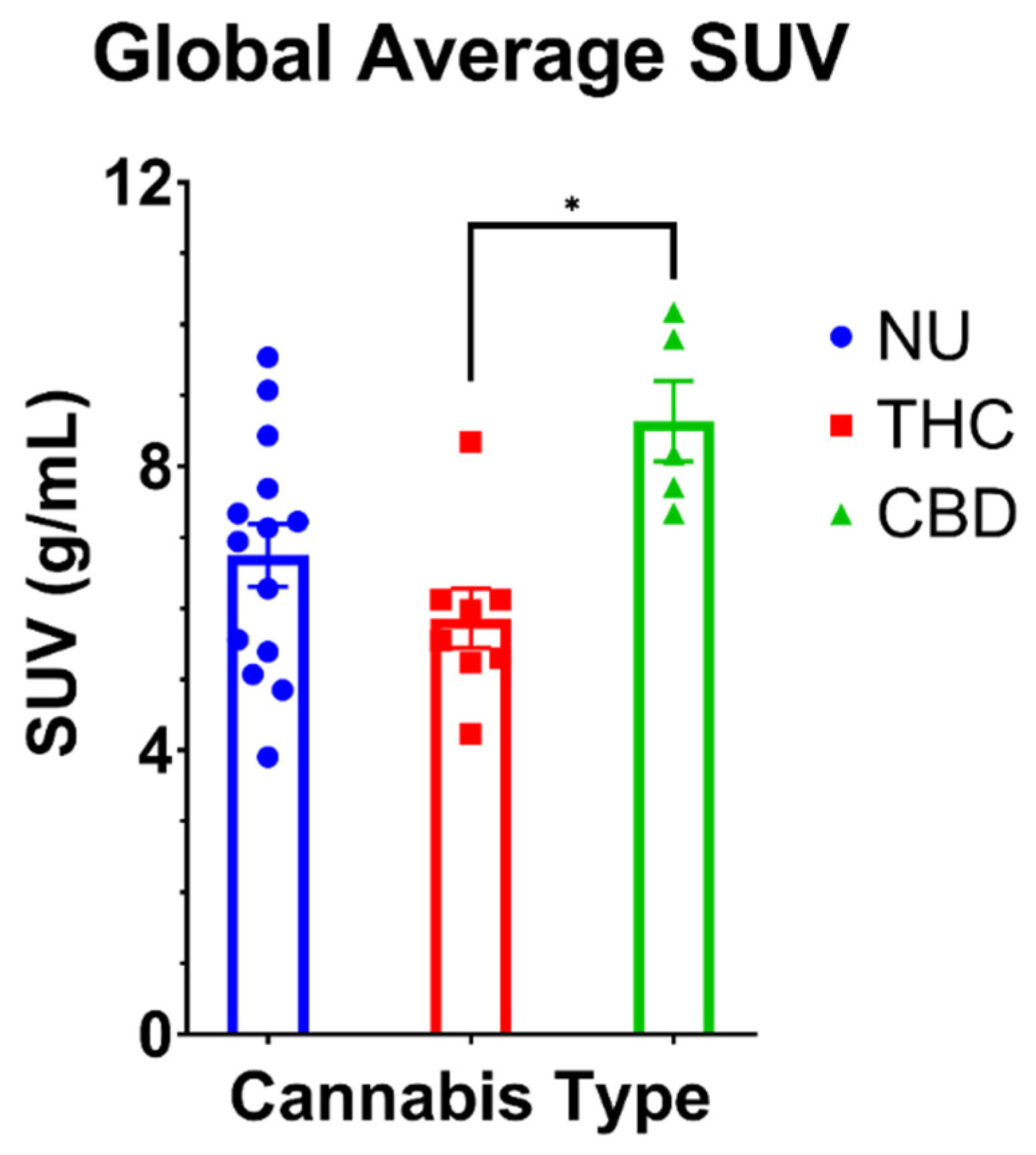
3.2.1. Global FDG SUV
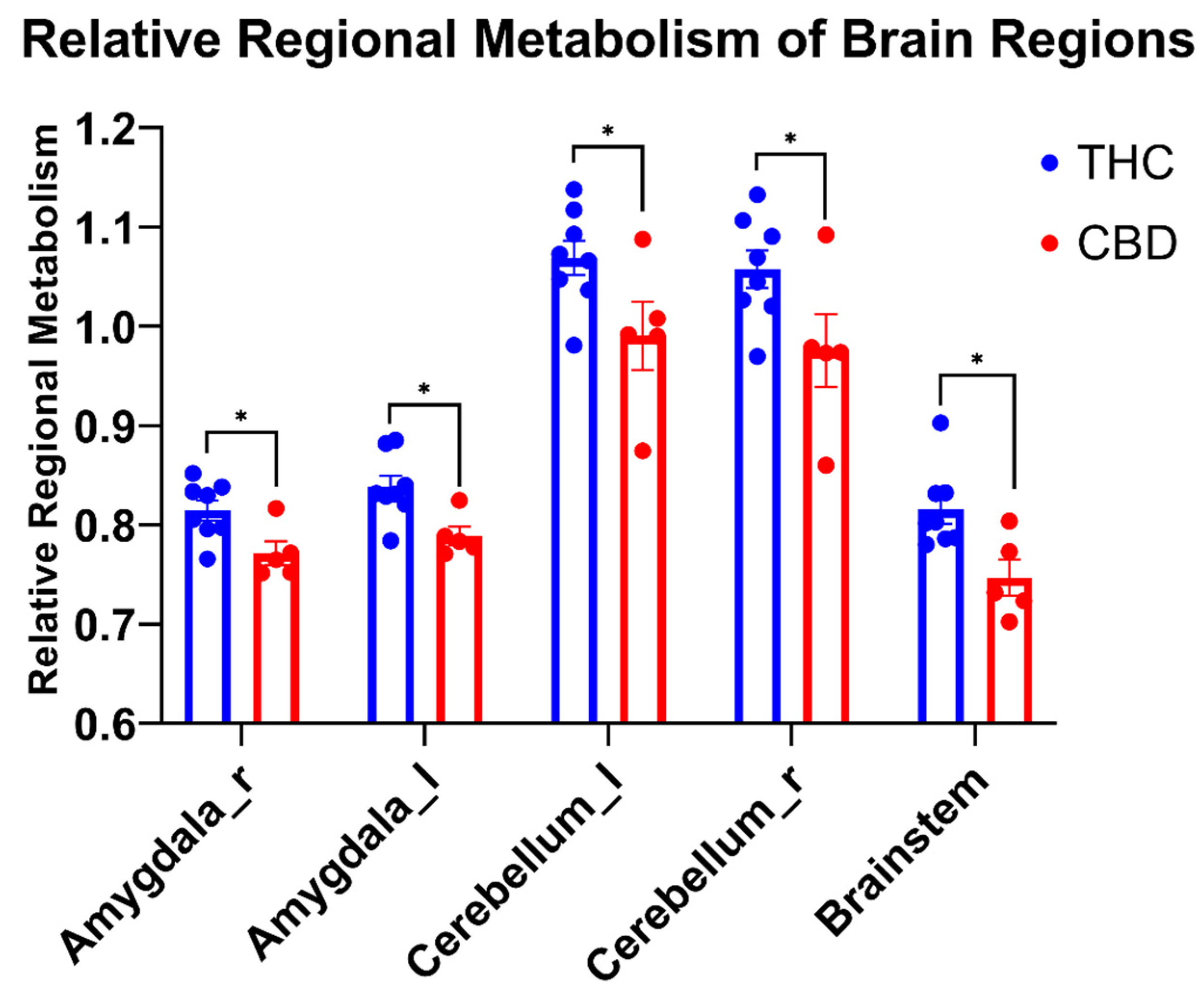
3.2.2. Relative Regional Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Moore, A.A. Prevention and screening of unhealthy substance use by older adults. Clin. Geriatr. Med. 2018, 34, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Palamar, J.J. Trends in cannabis use among older adults in the united states, 2015–2018. JAMA Intern. Med. 2020, 180, 609–611. [Google Scholar] [CrossRef]
- Kaskie, B.; Ayyagari, P.; Milavetz, G.; Shane, D.; Arora, K. The increasing use of cannabis among older americans: A public health crisis or viable policy alternative? Gerontologist 2017, 57, 1166–1172. [Google Scholar] [CrossRef]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Hindocha, C.; Green, S.F.; Wall, M.B.; Lees, R.; Petrilli, K.; Costello, H.; Ogunbiyi, M.O.; Bossong, M.G.; Freeman, T.P. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019, 195, 132–161. [Google Scholar] [CrossRef]
- Friedman, D.; Devinsky, O. Cannabinoids in the treatment of epilepsy. N. Engl. J. Med. 2015, 373, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S.; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Tsodikov, A.; Millman, J.; Bentley, H.; Gouaux, B.; Fishman, S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J. Pain 2008, 9, 506–521. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Ist Super. Sanita 2020, 56, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Scharf, E.L.; Hurt, R.T. Medical cannabis. Mayo Clin. Proc. 2018, 93, 1842–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusar-Poli, P.; Crippa, J.A.; Bhattacharyya, S.; Borgwardt, S.J.; Allen, P.; Martin-Santos, R.; Seal, M.; Surguladze, S.A.; O’Carrol, C.; Atakan, Z.; et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry 2009, 66, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a cannabidiol-rich cannabis extract in the mouse model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.M. A review of human studies assessing cannabidiol’s (cbd) therapeutic actions and potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef]
- Gorey, C.; Kuhns, L.; Smaragdi, E.; Kroon, E.; Cousijn, J. Age-related differences in the impact of cannabis use on the brain and cognition: A systematic review. Eur Arch. Psychiatry Clin. Neurosci. 2019, 269, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Workman, C.D.; Fietsam, A.C.; Sosnoff, J.; Rudroff, T. Increased likelihood of falling in older cannabis users vs. Non-users. Brain Sci. 2021, 11, 134. [Google Scholar] [CrossRef]
- Workman, C.D.; Sosnoff, J.J.; Rudroff, T. Disparity between perceptual fall risk and physiological fall risk in older cannabis users: A pilot study. Int. J. Environ. Res. Public Health 2021, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.S.; Rogowska, J.; Kanayama, G.; Jon, D.I.; Gruber, S.; Simpson, N.; Cherayil, M.; Pope, H.G.; Yurgelun-Todd, D.A. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: An fMRI study. Drug Alcohol Depend. 2004, 76, 261–271. [Google Scholar] [CrossRef]
- Ahmed, A.I.; van den Elsen, G.A.; Colbers, A.; Kramers, C.; Burger, D.M.; van der Marck, M.A.; Olde Rikkert, M.G. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berlin) 2015, 232, 2587–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.I.; van den Elsen, G.A.; Colbers, A.; van der Marck, M.A.; Burger, D.M.; Feuth, T.B.; Rikkert, M.G.; Kramers, C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: A randomized controlled trial. Eur. Neuropsychopharmacol. 2014, 24, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.J.; Weiss, S.R.; Condon, T.P. Drugs of abuse and the aging brain. Neuropsychopharmacology 2008, 33, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yücel, M.; Solowij, N. Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Crane, N.A.; Schuster, R.M.; Fusar-Poli, P.; Gonzalez, R. Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev. 2013, 23, 117–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; Gonzalez, R.; Bloomfield, M.A.; Curran, H.V.; Baler, R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef]
- Sagar, K.A.; Gruber, S.A. Marijuana matters: Reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. Int. Rev. Psychiatry 2018, 30, 251–267. [Google Scholar] [CrossRef]
- Scahill, R.I.; Frost, C.; Jenkins, R.; Whitwell, J.L.; Rossor, M.N.; Fox, N.C. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003, 60, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Eckert, M.A.; Keren, N.I.; Roberts, D.R.; Calhoun, V.D.; Harris, K.C. Age-related changes in processing speed: Unique contributions of cerebellar and prefrontal cortex. Front. Hum. Neurosci. 2010, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batalla, A.; Bos, J.; Postma, A.; Bossong, M.G. The impact of cannabidiol on human brain function: A systematic review. Front. Pharmacol. 2020, 11, 618184. [Google Scholar] [CrossRef] [PubMed]
- Kindred, J.H.; Honce, J.M.; Kwak, J.J.; Rudroff, T. Multiple sclerosis, cannabis use, and clinical disability: A preliminary [(18)f]-fluorodeoxyglucose positron emission tomography study. Cannabis Cannabinoid Res. 2018, 3, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, C.D.; Kindred, J.H.; Boles Ponto, L.L.; Kamholz, J.; Rudroff, T. The effects of chronic delta-9-tetrahydrocannabinol (thc) and cannabidiol (cbd) use on cerebral glucose metabolism in multiple sclerosis: A pilot study. Appl. Physiol. Nutr. Metab. 2020, 45, 450–452. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Kanosue, K.; Montero-Odasso, M.; Ishii, K. Association between hypometabolism in the supplementary motor area and fear of falling in older adults. Front. Aging Neurosci. 2017, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Devanand, D.P.; Mikhno, A.; Pelton, G.H.; Cuasay, K.; Pradhaban, G.; Dileep Kumar, J.S.; Upton, N.; Lai, R.; Gunn, R.N.; Libri, V.; et al. Pittsburgh compound b (11c-pib) and fluorodeoxyglucose (18 f-FDG) PET in patients with alzheimer disease, mild cognitive impairment, and healthy controls. J. Geriatr. Psychiatry Neurol. 2010, 23, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Blinkenberg, M.; Rune, K.; Jensen, C.V.; Ravnborg, M.; Kyllingsbaek, S.; Holm, S.; Paulson, O.B.; Sørensen, P.S. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in ms. Neurology 2000, 54, 558–564. [Google Scholar] [CrossRef]
- Robinson, M.M.; Lowe, V.J.; Nair, K.S. Increased brain glucose uptake after 12 weeks of aerobic high-intensity interval training in young and older adults. J. Clin. Endocrinol. Metab. 2018, 103, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Constant, E.L.; de Volder, A.G.; Ivanoiu, A.; Bol, A.; Labar, D.; Seghers, A.; Cosnard, G.; Melin, J.; Daumerie, C. Cerebral blood flow and glucose metabolism in hypothyroidism: A positron emission tomography study. J. Clin. Endocrinol. Metab. 2001, 86, 3864–3870. [Google Scholar] [CrossRef]
- Huisman, M.C.; van Golen, L.W.; Hoetjes, N.J.; Greuter, H.N.; Schober, P.; Ijzerman, R.G.; Diamant, M.; Lammertsma, A.A. Cerebral blood flow and glucose metabolism in healthy volunteers measured using a high-resolution PET scanner. EJNMMI Res. 2012, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/ct: Eanm procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Coleman, R.E.; Guiberteau, M.J.; Brown, M.L.; Royal, H.D.; Siegel, B.A.; Townsend, D.W.; Berland, L.L.; Parker, J.A.; Hubner, K.; et al. Procedure guideline for tumor imaging with 18f-FDG PET/ct 1.0. J. Nucl. Med. 2006, 47, 885–895. [Google Scholar] [PubMed]
- Cutter, G.R.; Baier, M.L.; Rudick, R.A.; Cookfair, D.L.; Fischer, J.S.; Petkau, J.; Syndulko, K.; Weinshenker, B.G.; Antel, J.P.; Confavreux, C.; et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999, 122 Pt 5, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Liewald, D.; Nissan, J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The deary-liewald reaction time task. Behav. Res. Methods 2011, 43, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Lajoie, Y.; Gallagher, S.P. Predicting falls within the elderly community: Comparison of postural sway, reaction time, the berg balance scale and the activities-specific balance confidence (abc) scale for comparing fallers and non-fallers. Arch. Gerontol. Geriatr. 2004, 38, 11–26. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Filiatrault, J.; Gauvin, L.; Fournier, M.; Parisien, M.; Robitaille, Y.; Laforest, S.; Corriveau, H.; Richard, L. Evidence of the psychometric qualities of a simplified version of the activities-specific balance confidence scale for community-dwelling seniors. Arch. Phys. Med. Rehabil. 2007, 88, 664–672. [Google Scholar] [CrossRef]
- Hsu, J. Multiple Comparisons: Theory and Methods; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Pocuca, N.; Walter, T.J.; Minassian, A.; Young, J.W.; Geyer, M.A.; Perry, W. The effects of cannabis use on cognitive function in healthy aging: A systematic scoping review. Arch. Clin. Neuropsychol. 2020, 36, 673–685. [Google Scholar] [CrossRef]
- Burggren, A.C.; Siddarth, P.; Mahmood, Z.; London, E.D.; Harrison, T.M.; Merrill, D.A.; Small, G.W.; Bookheimer, S.Y. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis Cannabinoid Res. 2018, 3, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Thayer, R.E.; YorkWilliams, S.L.; Hutchison, K.E.; Bryan, A.D. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res. Neuroimaging 2019, 285, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sznitman, S.R.; Vulfsons, S.; Meiri, D.; Weinstein, G. Medical cannabis and cognitive performance in middle to old adults treated for chronic pain. Drug Alcohol Rev. 2021, 40, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Anderson, J.E.; Richler, J.; Wallner-Allen, K.; Beaumont, J.L.; Conway, K.P.; Gershon, R.; Weintraub, S. Nih toolbox cognition battery (cb): Validation of executive function measures in adults. J. Int. Neuropsychol. Soc. 2014, 20, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Toki, S.; Siegle, G.J.; Takamura, M.; Takaishi, Y.; Yoshimura, S.; Okada, G.; Matsumoto, T.; Nakao, T.; Muranaka, H.; et al. Increased amygdala reactivity following early life stress: A potential resilience enhancer role. BMC Psychiatry 2017, 17, 27. [Google Scholar] [CrossRef] [Green Version]
- Gottwald, B.; Mihajlovic, Z.; Wilde, B.; Mehdorn, H.M. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia 2003, 41, 1452–1460. [Google Scholar] [CrossRef]
- Burns, H.D.; Van Laere, K.; Sanabria-Bohórquez, S.; Hamill, T.G.; Bormans, G.; Eng, W.S.; Gibson, R.; Ryan, C.; Connolly, B.; Patel, S.; et al. [18f]mk-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 9800–9805. [Google Scholar] [CrossRef] [Green Version]
- Demirakca, T.; Sartorius, A.; Ende, G.; Meyer, N.; Welzel, H.; Skopp, G.; Mann, K.; Hermann, D. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug Alcohol Depend. 2011, 114, 242–245. [Google Scholar] [CrossRef]
- Yücel, M.; Lorenzetti, V.; Suo, C.; Zalesky, A.; Fornito, A.; Takagi, M.J.; Lubman, D.I.; Solowij, N. Hippocampal harms, protection and recovery following regular cannabis use. Transl. Psychiatry 2016, 6, e710. [Google Scholar] [CrossRef] [Green Version]
- Zalesky, A.; Solowij, N.; Yücel, M.; Lubman, D.I.; Takagi, M.; Harding, I.H.; Lorenzetti, V.; Wang, R.; Searle, K.; Pantelis, C.; et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain 2012, 135, 2245–2255. [Google Scholar] [CrossRef] [Green Version]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis addiction and the brain: A review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef] [Green Version]
- Heitzeg, M.M.; Cope, L.M.; Martz, M.E.; Hardee, J.E.; Zucker, R.A. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev. Cogn. Neurosci. 2015, 16, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spechler, P.A.; Orr, C.A.; Chaarani, B.; Kan, K.J.; Mackey, S.; Morton, A.; Snowe, M.P.; Hudson, K.E.; Althoff, R.R.; Higgins, S.T.; et al. Cannabis use in early adolescence: Evidence of amygdala hypersensitivity to signals of threat. Dev. Cogn. Neurosci. 2015, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Somaini, L.; Manfredini, M.; Amore, M.; Zaimovic, A.; Raggi, M.A.; Leonardi, C.; Gerra, M.L.; Donnini, C.; Gerra, G. Psychobiological responses to unpleasant emotions in cannabis users. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 47–57. [Google Scholar] [CrossRef]
- Cuttler, C.; Spradlin, A.; Nusbaum, A.T.; Whitney, P.; Hinson, J.M.; McLaughlin, R.J. Blunted stress reactivity in chronic cannabis users. Psychopharmacology (Berlin) 2017, 234, 2299–2309. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Etkin, A.; Prater, K.E.; Schatzberg, A.F.; Menon, V.; Greicius, M.D. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry 2009, 66, 1361–1372. [Google Scholar] [CrossRef] [Green Version]
- Cauda, F.; Cavanna, A.E.; D’Agata, F.; Sacco, K.; Duca, S.; Geminiani, G.C. Functional connectivity and coactivation of the nucleus accumbens: A combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci. 2011, 23, 2864–2877. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.C.; Becker, N.; Apps, R.; Jones, M.W. Back to front: Cerebellar connections and interactions with the prefrontal cortex. Front. Syst. Neurosci. 2014, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Farley, S.J.; Radley, J.J.; Freeman, J.H. Amygdala modulation of cerebellar learning. J. Neurosci. 2016, 36, 2190–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 2012, 61, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Krook-Magnuson, E. Cognitive collaborations: Bidirectional functional connectivity between the cerebellum and the hippocampus. Front. Syst. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglói, K.; Doeller, C.F.; Paradis, A.L.; Benchenane, K.; Berthoz, A.; Burgess, N.; Rondi-Reig, L. Interaction between hippocampus and cerebellum crus i in sequence-based but not place-based navigation. Cereb. Cortex 2015, 25, 4146–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinney, D.L.; Cassidy, M.P.; Collier, L.M.; Martin, B.R.; Wiley, J.L.; Selley, D.E.; Sim-Selley, L.J. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2008, 324, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Gillespie, H.; Mullani, N.; Tancredi, L.; Grant, C.; Valentine, A.; Hollister, L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996, 67, 29–38. [Google Scholar] [CrossRef]
- Sneider, J.T.; Pope, H.G., Jr.; Silveri, M.M.; Simpson, N.S.; Gruber, S.A.; Yurgelun-Todd, D.A. Altered regional blood volume in chronic cannabis smokers. Exp. Clin. Psychopharmacol. 2006, 14, 422–428. [Google Scholar] [CrossRef]
- Sneider, J.T.; Pope, H.G., Jr.; Silveri, M.M.; Simpson, N.S.; Gruber, S.A.; Yurgelun-Todd, D.A. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur. Neuropsychopharmacol. 2008, 18, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Bentourkia, M.; Bol, A.; Ivanoiu, A.; Labar, D.; Sibomana, M.; Coppens, A.; Michel, C.; Cosnard, G.; De Volder, A.G. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: Effect of aging. J. Neurol. Sci. 2000, 181, 19–28. [Google Scholar] [CrossRef]
- Chang, L.; Yakupov, R.; Cloak, C.; Ernst, T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 2006, 129, 1096–1112. [Google Scholar] [CrossRef] [Green Version]
- Block, R.I.; O’Leary, D.S.; Hichwa, R.D.; Augustinack, J.C.; Boles Ponto, L.L.; Ghoneim, M.M.; Arndt, S.; Hurtig, R.R.; Watkins, G.L.; Hall, J.A.; et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol. Biochem. Behav. 2002, 72, 237–250. [Google Scholar] [CrossRef]
- Behan, B.; Connolly, C.G.; Datwani, S.; Doucet, M.; Ivanovic, J.; Morioka, R.; Stone, A.; Watts, R.; Smyth, B.; Garavan, H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 2014, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bolla, K.I.; Eldreth, D.A.; Matochik, J.A.; Cadet, J.L. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 2005, 26, 480–492. [Google Scholar] [CrossRef]
- Vaidya, J.G.; Block, R.I.; O’Leary, D.S.; Ponto, L.B.; Ghoneim, M.M.; Bechara, A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology 2012, 37, 618–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; CM, O.C.; Seal, M.; Allen, P.; et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef]
- Sadaka, A.H.; Ozuna, A.G.; Ortiz, R.J.; Kulkarni, P.; Johnson, C.T.; Bradshaw, H.B.; Cushing, B.S.; Li, A.L.; Hohmann, A.G.; Ferris, C.F. Cannabidiol has a unique effect on global brain activity: A pharmacological, functional MRI study in awake mice. J. Transl. Med. 2021, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Marti, M.; Mostallino, R.; Castelli, M.P. Sex and gender differences in the effects of novel psychoactive substances. Brain Sci. 2020, 10, 606. [Google Scholar] [CrossRef]
- Yoo, H.B.; DiMuzio, J.; Filbey, F.M. Interaction of cannabis use and aging: From molecule to mind. J. Dual Diagn. 2020, 16, 140–176. [Google Scholar] [CrossRef]
- Berry, A.S.; Jagust, W.J.; Hsu, M. Age-related variability in decision-making: Insights from neurochemistry. Cogn. Affect. Behav. Neurosci. 2019, 19, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Karrer, T.M.; Josef, A.K.; Mata, R.; Morris, E.D.; Samanez-Larkin, G.R. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiol. Aging 2017, 57, 36–46. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Alexoff, D.; Logan, J.; Jayne, M.; Wong, C.; Tomasi, D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl. Acad. Sci. USA 2014, 111, E3149–E3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascini, F.; Aiello, C.; Di Tanna, G. Increasing delta-9-tetrahydrocannabinol (δ-9-thc) content in herbal cannabis over time: Systematic review and meta-analysis. Curr. Drug Abus. Rev. 2012, 5, 32–40. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the united states. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| THC Users | CBD Users | Non-Users | |
|---|---|---|---|
| n (f) | 8 (4) | 5 (3) | 16 (9) |
| Age (yrs) | 59.3 ± 5.7 | 54.6 ± 2.1 | 58.2 ± 16.9 |
| Height (cm) | 171.1 ± 12.1 | 171.7 ± 7.5 | 157.6 ± 41.9 |
| Weight (kg) | 89.3 ± 20.5 | 97.6 ± 24.1 | 84.2 ± 31.6 |
| Duration of use (yrs) | 20.2 ± 8.7 | 1.4 ± 1.3 | n/a |
| Uses per week (days) | 5.6 ± 2.6 | 5.4 ± 1.5 | n/a |
| Uses per day (times) | 1.9 ± 1.1 | 1 ± 0 | n/a |
| THC:CBD per dose (mg; range) | 150:7.5–600:30 | 14:280–70:1400 | n/a |
| Study Variables; Mean ± SD | p-Value (Cohen’s d) | |||||
|---|---|---|---|---|---|---|
| THC Users | CBD Users | NU | THC Users vs. CBD Users | THC Users vs. NU | CBD Users vs. NU | |
| n | 8 | 5 | 16 | |||
| Cognitive tasks | ||||||
| RT Simple (ms) | 355.5 ± 73.1 | 315 ± 39. 2 | 328.3 ± 43.4 | 0.56 | 0.73 | 0.99 |
| RT Choice (ms) | 642 ± 172.0 | 507.2 ± 46.4 | 607.7 ± 79.5 | 0.12 | 0.99 | 0.25 |
| FT-C (ms) | 999.5 ± 142.8 | 852.4 ± 111.3 | 946.75 ± 108.0 | 0.12 | 0.94 | 0.40 |
| FT-I (ms) | 1166.9 ± 171.9 | 906.8 ± 129.3 | 1015.6 ± 112.8 | 0.01 (1.65) | 0.04 (1.13) | 0.37 |
| FT-E (ms) | 167.4 ± 99.1 | 54.4 ± 48.8 | 70.5 ± 63.6 | 0.04 (1.34) | 0.02 (1.26) | >0.99 |
| Motor Tasks | ||||||
| 30MWT time (s) | 25.4 ± 7.2 | 26.5 ± 4.1 | 25.5 ± 5.2 | 0.99 | 0.99 | 0.99 |
| 30MWT steps | 45.3 ± 8.2 | 46.8 ± 7.1 | 47.1 ± 7.6 | 0.99 | 0.99 | 0.99 |
| 30MWT velocity (m/s) | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.99 | 0.99 | 0.99 |
| AP Pathlength (cm) | 2.1 ± 0.3 | 2.5 ± 1.4 | 2.4 ± 0.9 | 0.99 | 0.99 | 0.99 |
| ML Pathlength (cm) | 0.8 ± 0.2 | 1.1 ± 0.5 | 0.9 ± 0.4 | 0.46 | 0.99 | 0.99 |
| COP area (cm2) | 1.1 ± 0.4 | 2.5 ± 0.9 | 8.9 ± 29.1 | 0.99 | 0.99 | 0.99 |
| Pegboard dom (s) | 25.2 ± 3.7 | 22.7 ± 2.7 | 22.2 ± 3.3 | 0.60 | 0.15 | 0.99 |
| Pegboard nondom (s) | 33.2 ± 10.5 | 36.6 ± 19.9 | 30.0 ± 12.1 | 0.99 | 0.80 | 0.51 |
| Grip Strength dom (kg) | 33.1 ± 9.2 | 38.1 ± 11.4 | 31.8 ± 13.2 | 0.99 | 0.99 | 0.95 |
| Grip Strength nondom (kg) | 33.2 ± 10.5 | 36.6 ± 19.9 | 30.0 ± 12.1 | 0.99 | 0.99 | 0.99 |
| Fall Risk (%) | 33.2 ± 45.4 | 9.8 ± 20.0 | 5.6 ± 11.9 | 0.40 | 0.07 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudroff, T.; Workman, C.D.; Gander, P.E.; Deters, J.R.; Ponto, L.L.B. Differences in Inhibitory Control and Resting Brain Metabolism between Older Chronic Users of Tetrahydrocannabinol (THC) or Cannabidiol (CBD)—A Pilot Study. Brain Sci. 2022, 12, 819. https://doi.org/10.3390/brainsci12070819
Rudroff T, Workman CD, Gander PE, Deters JR, Ponto LLB. Differences in Inhibitory Control and Resting Brain Metabolism between Older Chronic Users of Tetrahydrocannabinol (THC) or Cannabidiol (CBD)—A Pilot Study. Brain Sciences. 2022; 12(7):819. https://doi.org/10.3390/brainsci12070819
Chicago/Turabian StyleRudroff, Thorsten, Craig D. Workman, Phillip E. Gander, Justin R. Deters, and Laura L. Boles Ponto. 2022. "Differences in Inhibitory Control and Resting Brain Metabolism between Older Chronic Users of Tetrahydrocannabinol (THC) or Cannabidiol (CBD)—A Pilot Study" Brain Sciences 12, no. 7: 819. https://doi.org/10.3390/brainsci12070819
APA StyleRudroff, T., Workman, C. D., Gander, P. E., Deters, J. R., & Ponto, L. L. B. (2022). Differences in Inhibitory Control and Resting Brain Metabolism between Older Chronic Users of Tetrahydrocannabinol (THC) or Cannabidiol (CBD)—A Pilot Study. Brain Sciences, 12(7), 819. https://doi.org/10.3390/brainsci12070819








