Serum Lactate Dehydrogenase to Phosphate Ratio as an Independent Predictor for Adverse Outcome of Microsurgical Clipping for Ruptured Intracranial Aneurysm: A Propensity-Score Matching Analysis
Abstract
:1. Introduction
2. Methods
3. Patient Characteristics
4. Preoperative Management
5. Statistical Analysis
6. Results
6.1. The Primary Clinical Characteristics of rIA Patients
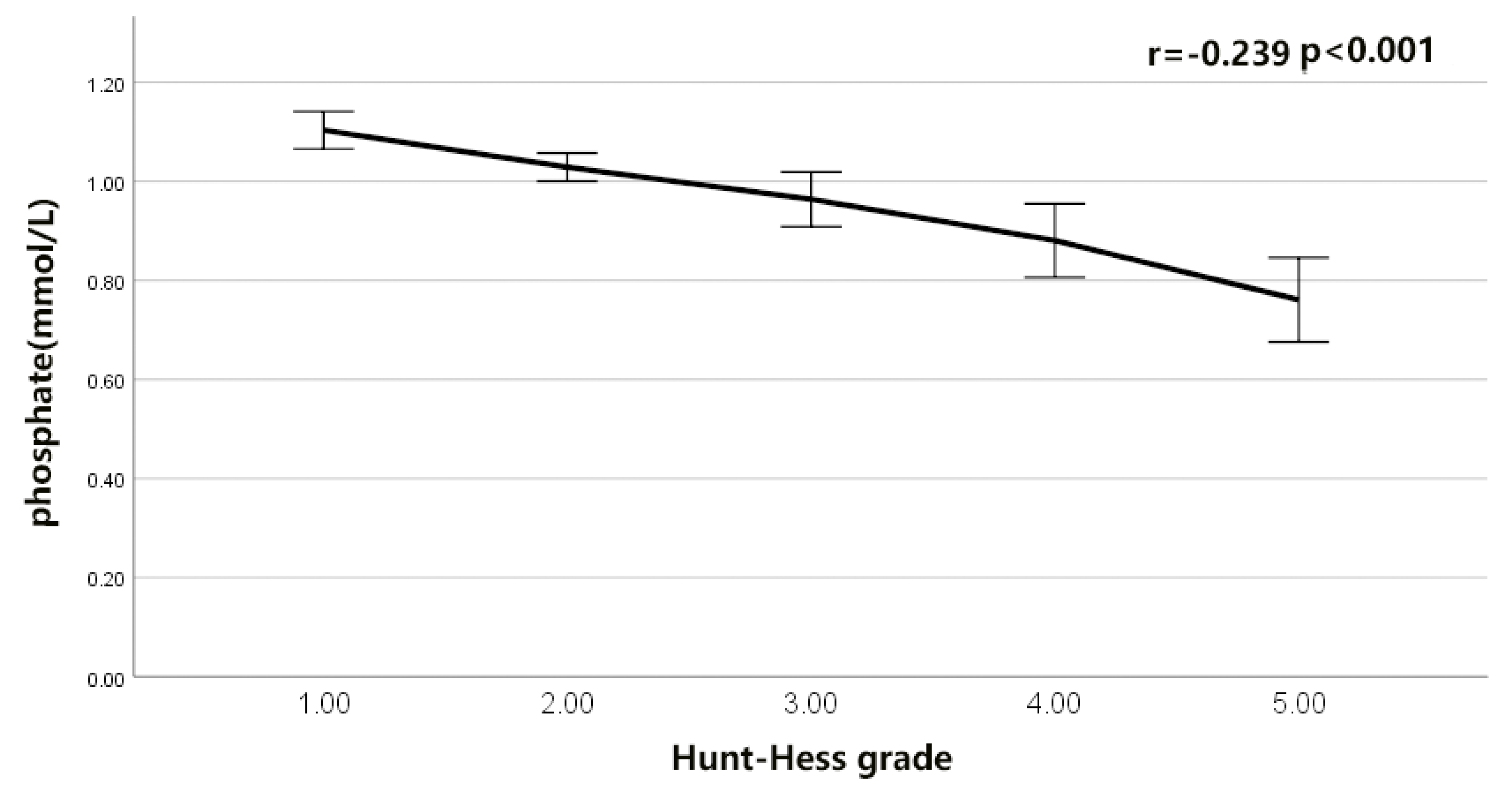
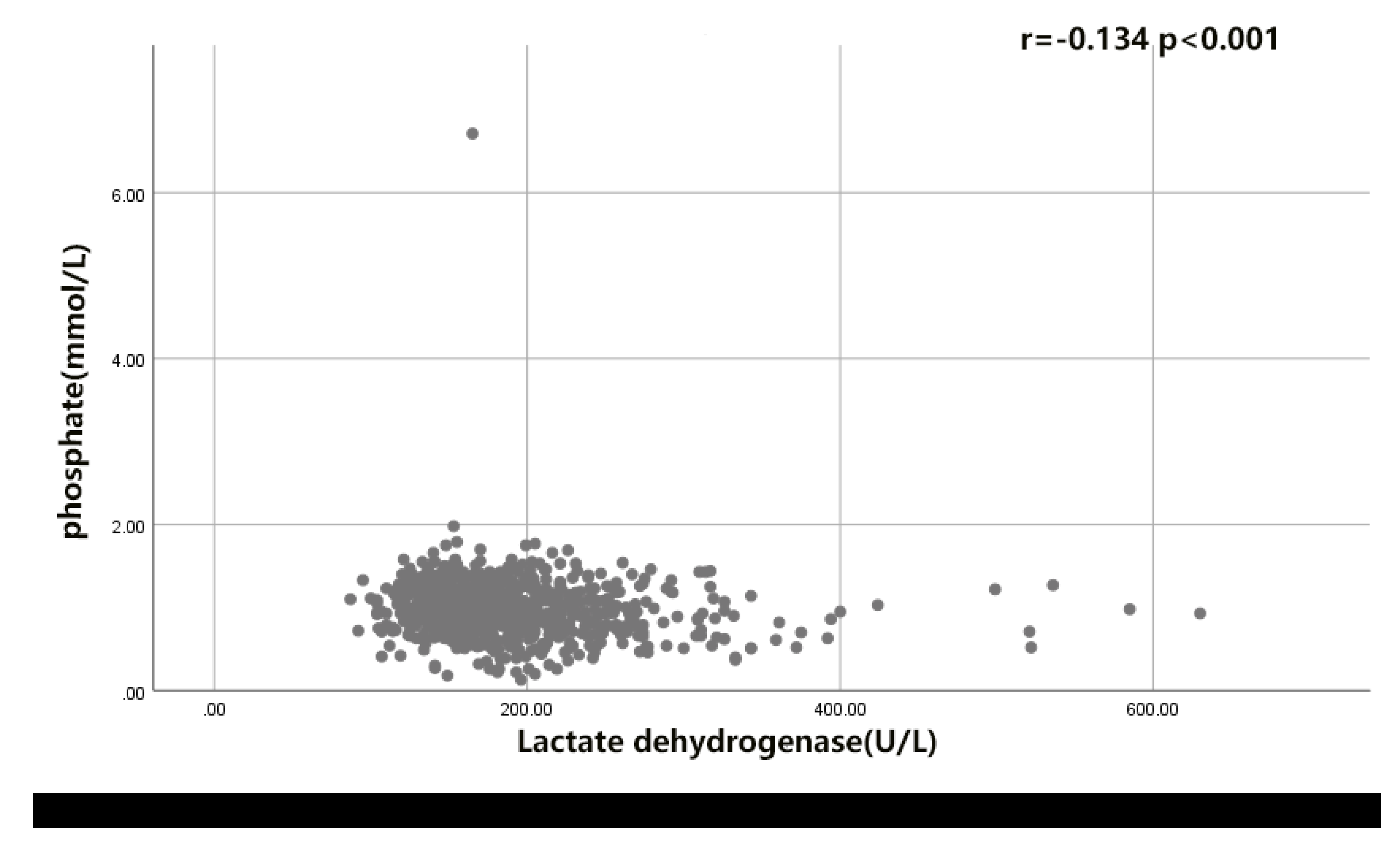
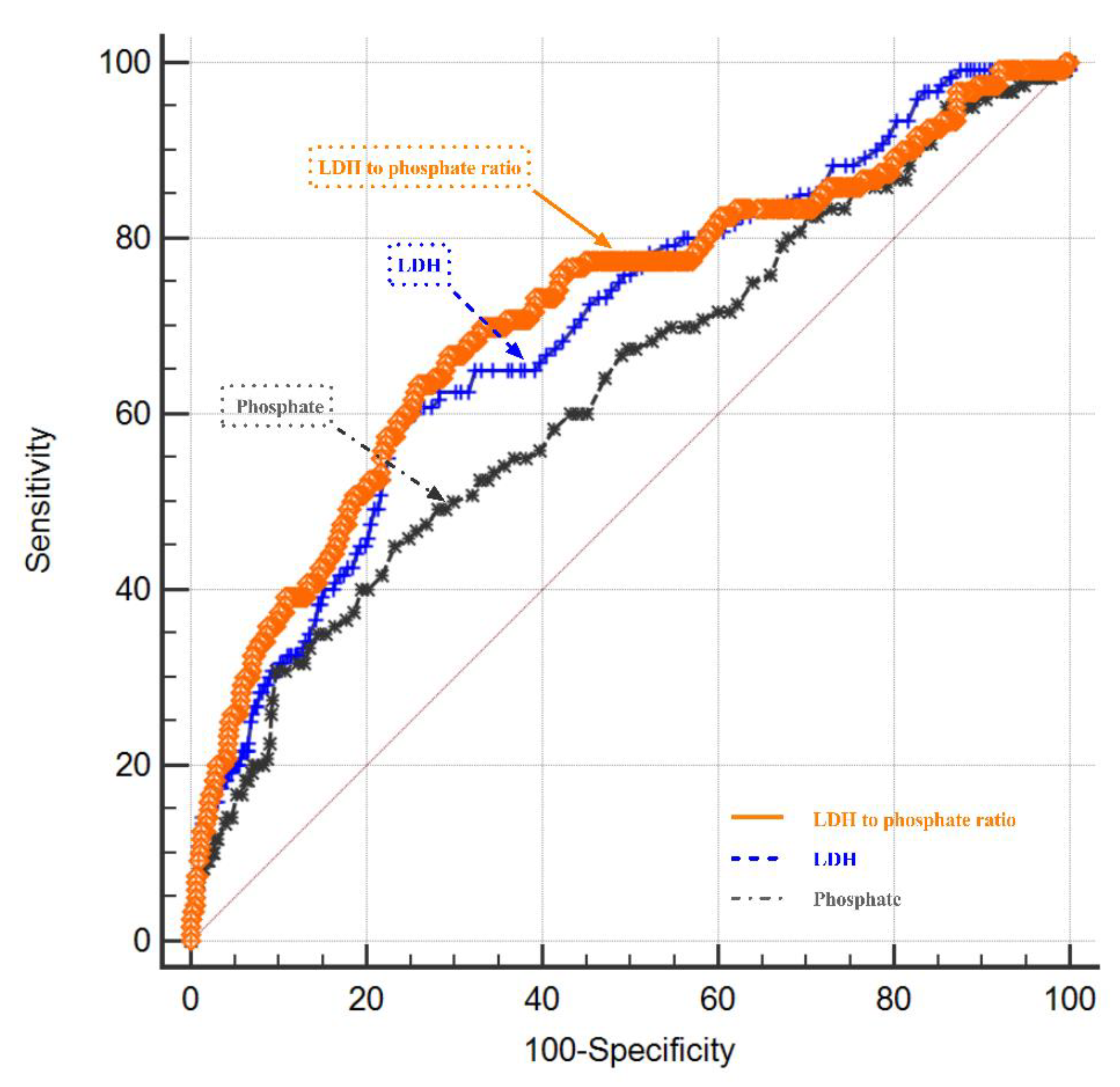
6.2. The Correlation of LDH- Phosphate Ratio with the Functional Outcome of rIA Patients
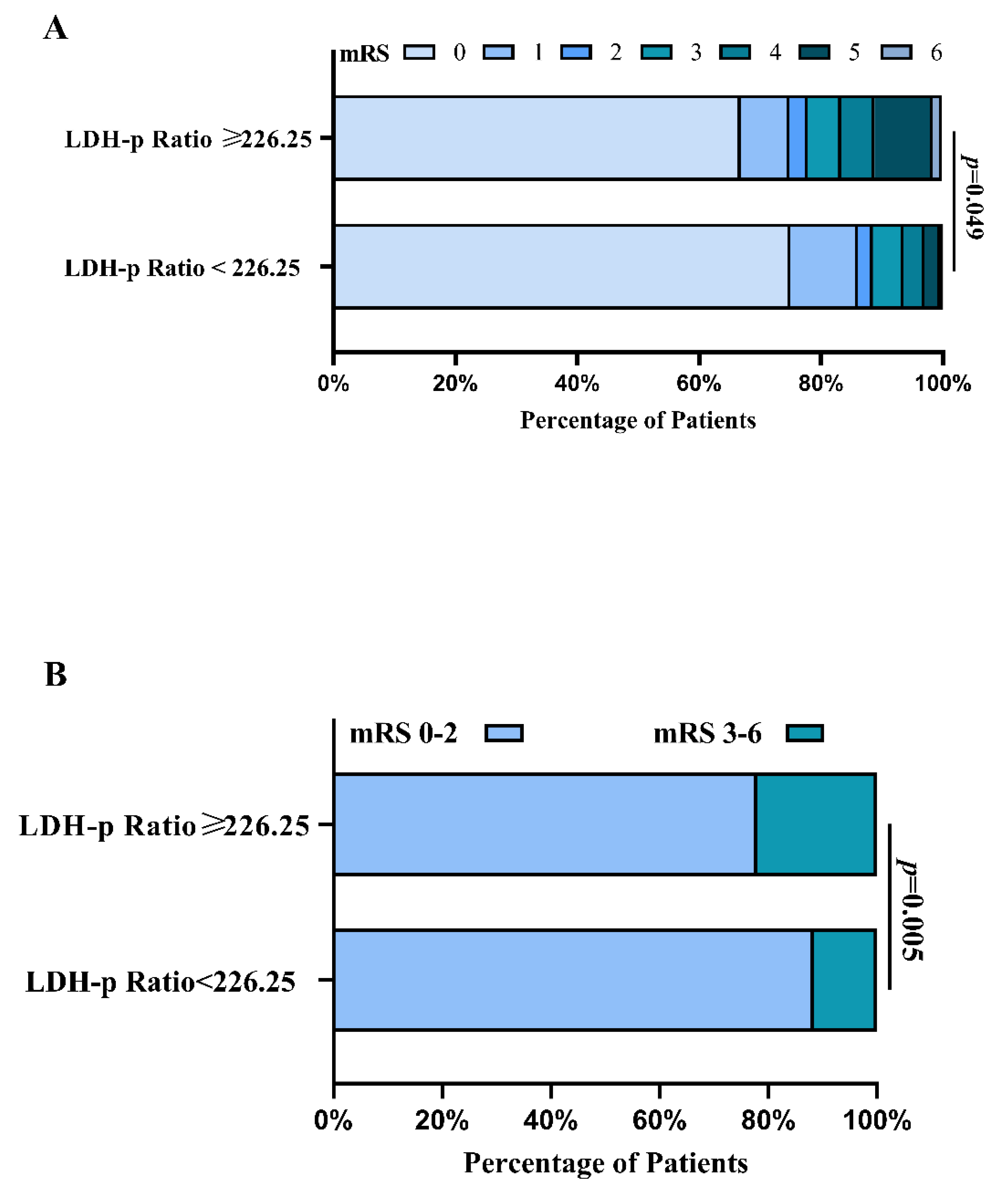
6.3. The Outcomes of rIA Patients in PSM Analysis
7. Discussion
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cahill, J.; Calvert, J.W.; Zhang, J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006, 26, 1341–1353. [Google Scholar] [CrossRef] [Green Version]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, F.R.; Cataneo, D.C.; Cataneo, A.J. C-reactive protein and vasospasm after aneurysmal subarachnoid hemorrhage. Acta Cir. Bras. 2014, 29, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Lan, F.; Zhang, Y. Associations between C-reactive protein and white blood cell count, occurrence of delayed cerebral ischemia and poor outcome following aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Acta Neurol. Belg. 2021, 121, 1311–1324. [Google Scholar] [CrossRef]
- Lucke-Wold, B.; Hosaka, K.; Dodd, W.; Motwani, K.; Laurent, D.; Martinez, M.; Hoh, B. Interleukin-6: Important Mediator of Vasospasm Following Subarachnoid Hemorrhage. Curr. Neurovasc. Res. 2021, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, X.T.; Wang, J.Q.; Wang, C.Y.; Zheng, W.L.; Pan, Z.M.; Xu, Z.B.; Li, X.Y.; Zhang, Y.B. Admission Neutrophil-Lymphocyte Ratio Predicts Rebleeding Following Aneurismal Subarachnoid Hemorrhage. World Neurosurg. 2020, 138, e317–e322. [Google Scholar] [CrossRef]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Shishido, H.; Ogawa, D.; Okauchi, M.; Shindo, A.; Kawanishi, M.; Tamiya, T.; Kuroda, Y. Blood Glucose Variability: A Strong Independent Predictor of Neurological Outcomes in Aneurysmal Subarachnoid Hemorrhage. J. Intensiv. Care Med. 2018, 33, 189–195. [Google Scholar] [CrossRef]
- Bian, L.; Liu, L.; Wang, C.; Hussain, M.; Yuan, Y.; Liu, G.; Wang, W.; Zhao, X. Hyperglycemia within day 14 of aneurysmal subarachnoid hemorrhage predicts 1-year mortality. Clin. Neurol. Neurosurg. 2013, 115, 959–964. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Q.; Zhang, Y.; Qiu, W.; Gao, H. Elevated Glucose-Potassium Ratio Predicts Preoperative Rebleeding in Patients With Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 795376. [Google Scholar] [CrossRef]
- Zhang, D.; Zhuang, Z.; Wei, Y.; Liu, X.; Li, W.; Gao, Y.; Li, J.; Hang, C. Association of Admission Serum Glucose-Phosphate Ratio with Severity and Prognosis of Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 127, e1145–e1151. [Google Scholar] [CrossRef] [PubMed]
- Podlasek, S.J.; McPherson, R.A.; Threatte, G.A. Characterization of apparent lactate dehydrogenase isoenzyme 6: A lactate-independent dehydrogenase. Clin. Chem. 1984, 30, 266–270. [Google Scholar] [CrossRef]
- Darkwah Oppong, M.; Gembruch, O.; Herten, A.; Frantsev, R.; Chihi, M.; Dammann, P.; El Hindy, N.; Forsting, M.; Sure, U.; Jabbarli, R. Intraventricular Hemorrhage Caused by Subarachnoid Hemorrhage: Does the Severity Matter? World Neurosurg. 2018, 111, e693–e702. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, M.; Schott, U.; Nordstrom, C.H.; Romner, B.; Reinstrup, P. Increased lactate levels impair the coagulation system--a potential contributing factor to progressive hemorrhage after traumatic brain injury. J. Neurosurg. Anesthesiol. 2006, 18, 200–204. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, Y.B.; Wang, J.Q.; Zhang, X.T.; Pan, Z.M.; Chen, L.X. Association Between Serum Lactate Dehydrogenase Level and Hematoma Expansion in Patients with Primary Intracerebral Hemorrhage: A Propensity-Matched Analysis. World Neurosurg. 2022, 160, e579–e590. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, H.; Chen, G.; Shangguan, H.; Yu, L.; Lin, Z.; Lin, Y.; Yao, P.; Kang, D. Higher Serum Levels of Lactate Dehydrogenase Before Microsurgery Predict Poor Outcome of Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 720574. [Google Scholar] [CrossRef] [PubMed]
- Larsen, V.H.; Waldau, T.; Gravesen, H.; Siggaard-Andersen, O. Erythrocyte 2,3-diphosphoglycerate depletion associated with hypophosphatemia detected by routine arterial blood gas analysis. Scand. J. Clin. Lab. Investig. Suppl. 1996, 224, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Schwartz, A.; Boniel, A.; Koyfman, L.; Boyko, M.; Kutz, R.; Klein, M. Clinical outcome of critically ill patients with thrombocytopenia and hypophosphatemia in the early stage of sepsis. Anaesthesiol. Intensive Ther. 2016, 48, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, E.; Yeh, D.D.; Quraishi, S.A.; Johnson, E.A.; Kaafarani, H.; Lee, J.; King, D.R.; DeMoya, M.; Fagenholz, P.; Butler, K.; et al. Hypophosphatemia in Enterally Fed Patients in the Surgical Intensive Care Unit. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2017, 32, 252–257. [Google Scholar] [CrossRef]
- Junttila, E.; Koskenkari, J.; Ala-Kokko, T. Hypophosphatemia after nontraumatic intracranial hemorrhage. Acta Anaesthesiol. Scand. 2017, 61, 641–649. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, H.; Zheng, K.; Li, Z.; Xiong, Y.; Tan, X.; Zhong, M. Preoperative and postoperative predictors of long-term outcome after endovascular treatment of poor-grade aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 126, 1764–1771. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.S.; Zhang, Z.H.; Zhou, X.M.; Gao, Y.Y.; Liu, G.J.; Wang, H.; Wu, L.Y.; Li, W.; Hang, C.H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef]
- Batt, A.M.; Ferrari, L. Manifestations of chemically induced liver damage. Clin. Chem. 1995, 41, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Dorneburg, C.; Fischer, M.; Barth, T.F.E.; Mueller-Klieser, W.; Hero, B.; Gecht, J.; Carter, D.R.; De Preter, K.; Mayer, B.; Christner, L.; et al. LDHA in neuroblastoma is associated with poor outcome and its depletion decreases neuroblastoma growth independent of aerobic glycolysis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5772–5783. [Google Scholar] [CrossRef] [Green Version]
- Daniele, S.; Giacomelli, C.; Zappelli, E.; Granchi, C.; Trincavelli, M.L.; Minutolo, F.; Martini, C. Lactate dehydrogenase-A inhibition induces human glioblastoma multiforme stem cell differentiation and death. Sci. Rep. 2015, 5, 15556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oba, C.; Kashiwagi, M.; Tanabe, T.; Nomura, S.; Ogino, M.; Matsuda, T.; Murata, S.; Nakamura, M.; Shirasu, A.; Inoue, K.; et al. Prognostic factors in the early phase of acute encephalopathy. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2018, 60, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Inamura, N.; Miyashita, N.; Hasegawa, S.; Kato, A.; Fukuda, Y.; Saitoh, A.; Kondo, E.; Teranishi, H.; Wakabayashi, T.; Akaike, H.; et al. Management of refractory Mycoplasma pneumoniae pneumonia: Utility of measuring serum lactate dehydrogenase level. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2014, 20, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Zhou, D.X.; Zhao, L.M.; Li, G.R.; Deng, X.L. Equol is neuroprotective during focal cerebral ischemia and reperfusion that involves p-Src and gp91(phox). Curr. Neurovascular Res. 2014, 11, 367–377. [Google Scholar] [CrossRef]
- Hu, W.; Dang, X.B.; Wang, G.; Li, S.; Zhang, Y.L. Peroxiredoxin-3 attenuates traumatic neuronal injury through preservation of mitochondrial function. Neurochem. Int. 2018, 114, 120–126. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Li, H.; Yan, H.; Zhang, Z.; Zhou, C.; Chen, Q.; Ye, Z.; Hang, C. Biphasic activation of nuclear factor-kappaB and expression of p65 and c-Rel following traumatic neuronal injury. Int. J. Mol. Med. 2018, 41, 3203–3210. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.J.; Shukla, P.K.; Mohanty, S.; Reddy, Y.J. Predictive value of serum lactate dehydrogenase in head injury. J. Neurol. Neurosurg. Psychiatry 1978, 41, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Engelke, S.; Bridgers, S.; Saldanha, R.L.; Trought, W.S. Cerebrospinal fluid lactate dehydrogenase in neonatal intracranial hemorrhage. Am. J. Med. Sci. 1986, 291, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.A.; Sheehan, K.M.; Tipirneni, R.; Roark, C.D.; Pandey, A.S.; Thompson, B.G.; Rajajee, V. The Association Between Spontaneous Hyperventilation, Delayed Cerebral Ischemia, and Poor Neurological Outcome in Patients with Subarachnoid Hemorrhage. Neurocrit Care 2015, 23, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, S.; Wang, H.; Chen, G.; Li, C.; Lin, Y.; Yao, P.; Kang, D. Admission Lower Serum Phosphate Ion Levels Predict Acute Hydrocephalus of Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2022, 12, 759963. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, M.; Stone, E.; Braude, S. Persistent, progressive hypophosphataemia after voluntary hyperventilation. Clin. Sci. 2000, 98, 619–625. [Google Scholar] [CrossRef]
- Body, J.J.; Cryer, P.E.; Offord, K.P.; Heath, H., 3rd. Epinephrine is a Hypophosphatemic Hormone in Man. J. Clin. Investig. 1983, 71, 572–578. [Google Scholar] [CrossRef] [Green Version]



| Variable | Value |
|---|---|
| No. of patients | 856 |
| Mean age in years (range) | 54.5 (10–86) |
| Female | 519 (60.6) |
| Smoking | 178 (20.8) |
| Drink | 85 (9.9) |
| Medical history | |
| Hypertension | 379 (44.3) |
| Diabetes | 50 (5.8) |
| Coronary heart disease | 11 (1.3) |
| Cerebral stroke | 15 (1.8) |
| aneurysm location | |
| anterior circulation | 694 (81.1) |
| posterior circulation | 9 (1.1) |
| multiple aneurysm | 153 (17.9) |
| Hunt-Hess grade | |
| I | 145 (16.9) |
| II | 313 (36.6) |
| III | 264 (30.8) |
| IV | 88 (10.3) |
| V | 46 (5.4) |
| Fisher | |
| 1 | 250 (29.2) |
| 2 | 244 (28.5) |
| 3 | 171 (20.0) |
| 4 | 191 (22.3) |
| Hydrocephalus | 168 (19.6) |
| Laboratory values | |
| White blood cell, ×109/L, mean ± SD | 10.009 ± 4.347 |
| Hemoglobin (g/L) | 128.627 ± 18.680 |
| Glucose (mmol/L), mean ± SD | 6.580 ± 3.155 |
| Lactate dehydrogenase (U/L), mean ± SD | 186.914 ± 58.595 |
| serum phosphate (mmol/L), mean ± SD | 0.992 ± 0.348 |
| LDH-phosphate Ratio, mean ± SD | 217.509 ± 135.722 |
| Complication | |
| Postoperative intracranial hematoma | 71 (8.3) |
| Postoperative intracranial infection | 66 (7.7) |
| Postoperative Pneumonia | 220 (25.7) |
| Sepsis | 12 (1.4) |
| Delay ischemic neurological deficit | 115 (13.4) |
| General Information (n = 856) | Good Outcome | Poor Outcome | p−Value |
|---|---|---|---|
| (n = 736) | (n = 120) | ||
| Mean age in years (range) | 54.1 (10–86) | 57.4 (22–85) | 0.003 |
| Female | 439 (59.6) | 80 (66.7) | 0.144 |
| Smoking | 148 (20.1) | 30 (25.0) | 0.221 |
| Drink | 75 (10.2) | 10 (8.3) | 0.528 |
| Medical history | |||
| Hypertension | 317 (43.1) | 62 (51.7) | 0.079 |
| Diabetes | 39 (5.3) | 11 (9.2) | 0.094 |
| Coronary heart disease | 9 (1.2) | 2 (1.7) | 0.689 |
| Cerebral stroke | 13 (1.8) | 2 (1.7) | 0.939 |
| Aneurysm location | 0.177 | ||
| Anterior circulation | 604 (82.1) | 90 (75.0) | |
| Posterior circulation | 7 (1.0) | 2 (1.7) | |
| Multiple aneurysms | 125 (17.0) | 28 (23.3) | |
| Hunt−Hess grade | <0.001 | ||
| I | 138 (18.8) | 7 (5.8) | |
| II | 293 (39.8) | 20 (16.7) | |
| III | 233 (31.7) | 31 (25.8) | |
| IV | 54 (7.3) | 34 (28.3) | |
| V | 18 (2.4) | 28 (23.3) | |
| Fisher grade | <0.001 | ||
| 1 | 240 (32.6) | 10 (8.3) | |
| 2 | 229 (31.1) | 15 (12.5) | |
| 3 | 139 (18.9) | 32 (26.7) | |
| 4 | 128 (17.4) | 63 (52.5) | |
| Hydrocephalus | 115 (15.6) | 53 (44.2) | <0.001 |
| Lab values | |||
| White blood cell, ×109/L, mean ± SD | 9.634 ± 4.033 | 12.307 ± 5.394 | <0.001 |
| Hemoglobin (g/L) | 128.523 ± 18.252 | 129.267 ± 21.190 | 0.686 |
| Glucose (mmol/L), mean ± SD | 6.360 ± 3.122 | 7.928 ± 3.024 | 0.008 |
| Lactate dehydrogenase (U/L), mean ± SD | 180.378 ± 50.695 | 227 ± 83.125 | <0.001 |
| Serum phosphate (mmol/L), mean ± SD | 1.012 ± 0.347 | 0.863 ± 0.319 | <0.001 |
| LDH−phosphate Ratio, mean ± SD | 200.175 ± 107.290 | 323.826 ± 219.075 | <0.001 |
| Complication | |||
| Postoperative intracranial hematoma | 61 (8.3) | 10 (8.3) | 0.987 |
| Postoperative intracranial infection | 53 (7.2) | 13 (10.8) | 0.167 |
| Postoperative Pneumonia | 142 (19.3) | 78 (65.0) | <0.001 |
| Sepsis | 10 (1.4) | 2 (1.7) | 0.790 |
| Delay ischemic neurological deficit | 72 (9.8) | 43 (35.8) | <0.001 |
| Variable | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.016 | 0.996–1.037 | 0.118 |
| Hunt-Hess grade | 1.731 | 1.333–2.246 | <0.001 |
| Fisher | 1.428 | 1.108–1.842 | 0.006 |
| Hydrocephalus | 1.090 | 0.648–1.833 | 0.747 |
| White blood cell | 0.979 | 0.926–1.035 | 0.446 |
| Glucose | 1.025 | 0.964–1.089 | 0.432 |
| LDH- phosphate Ratio ≥ 226.25 | 1.967 | 1.185–3.266 | 0.009 |
| Pneumonia | 4.017 | 2.472–6.530 | <0.001 |
| Delay ischemic neurological deficit | 3.773 | 2.171–6.559 | <0.001 |
| General Information | LDH−Phosphate Ratio on Admission | LDH−Phosphate Ratio on Admission | p-Value |
|---|---|---|---|
| ≥226.25 | <226.25 | ||
| Pre–PS match | |||
| No. of patients | 267(30.9) | 589 (68.1) | |
| Mean age in years (range) | 55.9 (25–85) | 53.9 (10–86) | 0.016 |
| Hunt–Hess grade | |||
| I–III | 174(65.2) | 548(93.0) | <0.001 |
| IV–V | 93(34.8) | 41 (7.0) | |
| Fisher | |||
| 1–3 | 161 (60.3) | 506 (85.9) | <0.001 |
| 4 | 106 (39.7) | 85 (14.4) | |
| Hydrocephalus | 85 (31.8) | 83 (14.1) | <0.001 |
| Lab values | |||
| White blood cell, ×109/L, mean ± SD | 12.208 ± 4.728 | 9.011 ± 3.764 | <0.001 |
| Glucose (mmol/L), mean ± SD | 7.506 ± 2.574 | 6.159 ± 3.302 | <0.001 |
| Lactate dehydrogenase (U/L), mean ± SD | 233.004 ± 72.611 | 166.020 ± 34.718 | <0.001 |
| LDH–phosphate Ratio, mean ± SD | 357.445 ± 166.110 | 154.075 ± 37.147 | <0.001 |
| Complication | |||
| Pneumonia | 113 (42.3) | 107 (18.2) | <0.001 |
| Delay ischemic neurological deficit | 43 (16.1) | 72 (12.2) | 0.123 |
| Post–PS match | |||
| No. of patients | 199 | 199 | |
| Mean age in years (range) | 55.4 (25–85) | 55.6 (23–86) | 0.806 |
| Hunt-Hess grade | |||
| I–III | 154 (77.4) | 166 (83.4) | 0.130 |
| IV–V | 45 (22.6) | 33 (16.6) | |
| Fisher | |||
| 1–3 | 141 (70.9) | 141 (70.9) | 1.000 |
| 4 | 58 (29.1) | 58 (29.1) | |
| Hydrocephalus | 53 (26.6) | 50 (25.1) | 0.731 |
| Lab values | |||
| White blood cell, ×109/L, mean ± SD | 11.048 ± 3.997 | 11.180 ± 4.462 | 0.756 |
| Glucose (mmol/L), mean ± SD | 7.233 ± 2.468 | 7.132 ± 4.929 | 0.797 |
| Lactate dehydrogenase (U/L), mean ± SD | 229.141 ± 71.352 | 185.633 ± 38.186 | <0.001 |
| LDH-phosphate Ratio, mean ± SD | 341.684 ± 153.528 | 189.008 ± 26.814 | <0.001 |
| Complication | |||
| Pneumonia | 61 (30.7) | 60 (30.2) | 0.913 |
| Delay ischemic neurological deficit | 26 (13.1) | 22 (11.1) | 0.538 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhang, Y.; Wang, H.; Xie, X.; Lin, Y.; Yao, P.; Lin, Z.; Kang, D. Serum Lactate Dehydrogenase to Phosphate Ratio as an Independent Predictor for Adverse Outcome of Microsurgical Clipping for Ruptured Intracranial Aneurysm: A Propensity-Score Matching Analysis. Brain Sci. 2022, 12, 737. https://doi.org/10.3390/brainsci12060737
Zheng S, Zhang Y, Wang H, Xie X, Lin Y, Yao P, Lin Z, Kang D. Serum Lactate Dehydrogenase to Phosphate Ratio as an Independent Predictor for Adverse Outcome of Microsurgical Clipping for Ruptured Intracranial Aneurysm: A Propensity-Score Matching Analysis. Brain Sciences. 2022; 12(6):737. https://doi.org/10.3390/brainsci12060737
Chicago/Turabian StyleZheng, Shufa, Yibin Zhang, Haojie Wang, Xueling Xie, Yuanxiang Lin, Peisen Yao, Zhangya Lin, and Dezhi Kang. 2022. "Serum Lactate Dehydrogenase to Phosphate Ratio as an Independent Predictor for Adverse Outcome of Microsurgical Clipping for Ruptured Intracranial Aneurysm: A Propensity-Score Matching Analysis" Brain Sciences 12, no. 6: 737. https://doi.org/10.3390/brainsci12060737
APA StyleZheng, S., Zhang, Y., Wang, H., Xie, X., Lin, Y., Yao, P., Lin, Z., & Kang, D. (2022). Serum Lactate Dehydrogenase to Phosphate Ratio as an Independent Predictor for Adverse Outcome of Microsurgical Clipping for Ruptured Intracranial Aneurysm: A Propensity-Score Matching Analysis. Brain Sciences, 12(6), 737. https://doi.org/10.3390/brainsci12060737






