Abstract
Objective: The aim of this study was to evaluate ten exercise interventions (YOGA: yoga training, RT: resistance training, AQU: aquatic training, TAI: Taiji Qigong training, TRD: treadmill training, VR: virtual reality training, DANCE: musical dance training, WKT: walking training, CYC: cycling training, BDJ: Baduanjin Qigong training) on motor function in Parkinson’s disease (PD) patients. Design: Through searching PubMed, Embase, Cochrane Library, Web of Science, and CNKI, only randomized controlled trials (RCTs) were collected to study the effects of the ten exercise interventions on motor function in patients with Parkinson’s disease. The included studies were evaluated for methodological quality by the Cochrane bias risk assessment tool. Results: The RCTs were collected between the earliest available date and April 2022. Sixty RCTs were included and the total sample size used in the study was 2859. The results of the network meta-analysis showed that DANCE can significantly improve patients’ Berg Balance Scale (BBS) (SUCRA = 78.4%); DANCE can significantly decline patients’ Unified Parkinson’s Disease Rating Scale score (UPDRS) (SUCRA = 72.3%) and YOGA can significantly decline patients’ Timed-Up-and-Go score (TUGT) (SUCRA = 78.0%). Conclusion: Based on the network meta-analysis and SUCRA ranking, we can state that dance, yoga, virtual reality training and resistance training offers better advantages than other exercise interventions for patients’ motor function.
1. Introduction
Parkinson’s disease has become the second most prevalent neurodegenerative disease worldwide [1], affecting the quality of life and physical and mental health of more than six million people [2]. Parkinson’s disease can cause a number of motor dysfunctions that can seriously affect the lives of patients and place a significant burden on their families [3].
There is no complete cure for Parkinson’s disease, only a way to alleviate its symptoms to some extent [4]. Medication is currently the primary option for Parkinson’s disease relief, but the side effects and development of drug resistance or the cost of medication have limited the widespread use of medication in clinical practice and has become a long-term option for patients [5]. Is there a treatment option that is less costly and has almost no side effects? Physical exercise has made good progress in the treatment of other degenerative diseases due to its great ease of handling and the absence of side effects [6,7,8]. As a result of research and studies, it has been noted in relevant Parkinson’s disease rehabilitation studies that physical exercise can be of considerable help in improving motor function and slowing down the progression of Parkinson’s disease in people with it [9]. Previous studies have consistently shown that physical activity has considerable benefits for maintaining brain health, improving motor performance and enhancing quality of life in people with Parkinson’s disease [10].
However, for physical activity, different exercise programs have different characteristics and may have different effects on people with Parkinson’s disease, and a previous meta-analysis has only compared the effects of a particular exercise type relative to a control group for people with Parkinson’s disease [11,12,13,14]. There is still a lack of evidence-based recommendations as to which exercise programme is most suitable for improving motor function in people with Parkinson’s disease. It is therefore particularly important to find an exercise modality within a complex exercise programme that is suitable for improving the symptoms associated with motor function in patients with Parkinson’s disease, especially when physicians are considering the use of exercise prescriptions to treat patients with Parkinson’s disease.
Network meta-analysis is a recent evidence-based technique that uses direct or indirect comparisons to compare the effects of multiple interventions on a disease and to estimate the rank order of each treatment [15]. Therefore, in this study we used network meta-analysis to compare different exercise programmes (aquatic training, cycling, walking exercises, treadmill exercises, yoga exercises, taijiquan qigong, baduanjin qigong, musical dance exercises, virtual reality exercises and resistance exercises) in order to assess the effect of these exercise programmes on the motor function of Parkinson’s patients and to provide patients and clinicians with a better understanding of the effects of these programmes. The aim is to evaluate the effects of these exercise programmes on motor function in Parkinson’s patients and to provide evidence-based recommendations for patients and clinicians.
2. Materials and Methods
2.1. Search Strategy
The researchers in this paper searched five electronic databases (Pubmed, EMBASE, the Cochrane Central Register of Controlled Trials, Web of Science and CNKI) from their creation to April 2022. The search strategy was constructed around the PICOS tool: (P) Population: people with Parkinson’s disease; (I) Intervention: exercise; (C) Comparator: control group with only usual care and appropriate rehabilitation measures (including usual balance training); (O) Outcomes: motor tests for people with Parkinson’s disease. (S) Study type: RCTs. The detailed search strategy is shown in Table 1 (Pubmed is used as an example)

Table 1.
Search strategy on PubMed.
2.2. Inclusion Criteria
(1) An experimental group with different exercise modalities as an intervention for Parkinson’s disease; (2) a control group with routine care and rehabilitation of patients only; (3) a clinical randomised controlled trial; and (4) outcome indicators including at least one of the following: Unified Parkinson’s Disease Rating Scale (UPDRS) score [UPDRS or Movement Disorder Society-Unified Parkinson’s disease rating scale scores (MDS-UPDRS)], Berg Balance Scale (BBS) score, Timed-Up-and-Go (TUG) score.
2.3. Exclusion Criteria
(1) Studies with incomplete or unreported data; and (2) Studies from non-randomized controlled trials [including quasi-randomized controlled trials, animal studies, protocols, conference abstracts, case reports or correspondence].
2.4. Study Selection
The literature was screened and excluded using the literature management software Zotero. Two researchers first screened the titles of the literature for duplication, non-randomised controlled trial studies, review papers, conference papers, protocols and correspondence. The abstracts of the literature were then read by two researchers to identify literature for inclusion and to exclude literature. Finally, the remaining literature was read in full by both researchers and further identified for inclusion. During this process, both researchers independently screened the literature and finally compared the remaining literature; if it was the same then it was ultimately included, and if it was different then it was discussed and resolved by a third researcher.
2.5. Data Extraction
A seven-item, standardised and pre-selected data extraction form was used to record data for inclusion in the study under the following headings: (1) author, (2) year of publication, (3) country, (4) study period, (5) sample size, (6) mean age, and (7) details of the exercise intervention.
2.6. Risk of Bias of Individual Studies
Two researchers independently assessed the risk of bias (ROB), in accordance with the Cochrane Handbook version 5.1.0 tool for assessing ROB in RCTs. The following seven domains were considered: (1) randomized sequence generation, (2) treatment allocation concealment, blinding of (3) participants and (4) personnel, (5) incomplete outcome data, (6) selective reporting, and (7) other sources of bias. Trials were categorized into three levels of ROB by the number of components for which high ROB potentially existed: high risk (five or more), moderate risk (three or four), and low risk (two or less) [16].
2.7. Data Analysis
In studies where exercise is the intervention, all variables are continuous variables and are expressed as means with standard deviation (SD) [17]. Continuous variables in the study will be reported as mean difference (MD = absolute difference between the means of two groups, defined as the difference in means between the treatment and control groups and calculated using the same scale) or standardised mean difference (SMD = mean difference in outcome between groups/standard deviation of outcome between subjects, used to combine data when trials have different scales) with 95% confidence intervals (CI) and analysis. As there are certainly potential differences across studies, we chose a random effects model for analysis rather than a fixed effects model [18].
We used Stata software (version 15.1) and performed NMA aggregation and analysis using Markov chain Monte Carlo simulation chains in a Bayesian-based framework according to the PRISMA NMA instruction manual [19,20]. We used the nodal method to quantify and demonstrate the agreement between indirect and direct comparisons, calculated through the instructions in the Stata software, and if the p-value > 0.05, the consistency was verified [21].
Stata software was used to present and describe network diagrams of different movement interventions. In the generated network diagrams, each node represents a different motor intervention and a different control condition, and the lines connecting the nodes represent direct head-to-head comparisons between interventions. The size of each node and the width of the connecting lines are proportional to the number of studies [22].
The intervention hierarchy was summarized and reported as a P score. The P score is considered as a frequentist analogue to surface under the cumulative ranking curve (SUCRA) values and measures the extent of certainty that a treatment is better than another treatment, averaged over all competing treatments. The P score ranges from 0 to 1, where 1 indicates the best treatment with no uncertainty and 0 indicates the worst treatment with no uncertainty. While the P score or SUCRA can be usefully re-expressed as the percentage of effectiveness or acceptability of the exercise interventions, such scores should be interpreted cautiously unless there are actual clinically meaningful differences between interventions [23]. To check for the presence of bias due to small-scale studies, which may lead to publication bias in NMA, a network funnel plot was generated and visually inspected using the criterion of symmetry [24].
3. Results
3.1. Study and Identification and Selection
A total of 6431 documents were retrieved from the electronic database, and an additional nine documents were manually searched. After eliminating duplicates, the remaining 5483 documents were read for titles and abstracts, and 5176 documents were again excluded. The remaining 307 documents were read in full and 247 documents were again excluded (for reasons including non-randomised controlled trials, incomplete data, conference papers and failure to meet the interventions included in this review), leaving a final remaining 60 documents to be included in this study (Figure 1).
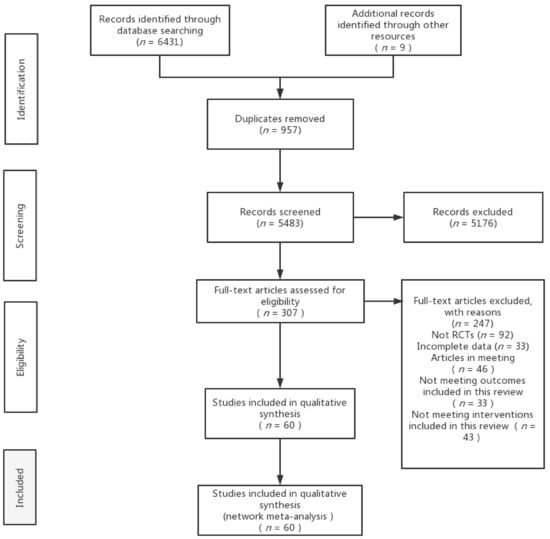
Figure 1.
Flow diagram of literature selection.
3.2. Quality Assessment of the Included Studies
Nineteen studies were defined as low risk, 11 as high risk and 30 as medium risk. Only four of these studies achieved the simultaneous blinding of subjects and measurers, but as the intervention in these studies was exercise, it was difficult to achieve simultaneous blinding of subjects and measurers as both the patients themselves and their relatives had to sign an informed consent form before the experiment was conducted. Specific details will be presented in Supplementary Table S1.
3.3. Characteristics of the Included Studies
In total, we included studies from 60 randomised controlled trials, which included 2859 patients diagnosed with Parkinson’s disease. Interventions in the control group included Baduanjin Qigong training (three studies) [25,26,27], walking training (three studies) [28,29,30], treadmill training (three studies) [31,32,33], aquatic training (nine studies) [34,35,36,37,38,39,40,41,42], Taiji Qigong training (eight studies) [43,44,45,46,47,48,49,50], musical dance training (10 studies) [51,52,53,54,55,56,57,58,59,60], yoga training (six studies) [61,62,63,64,65,66], cycling training (five studies) [67,68,69,70,71], resistance training (six studies) [64,72,73,74,75,76], and virtual reality training (seven studies) [57,71,77,78,79,80,81]. Thirty-one studies reported BBS as an outcome indicator, 41 studies reported UPDRS as an outcome indicator and 38 studies reported TUGT as an outcome indicator. These studies are from East Asia (17 studies), the Americas (21 studies), Europe (17 studies), Oceania (three studies) and Central Asia (two studies). The characteristics of the included studies are shown in Table 2.

Table 2.
Characteristics of the studies included in the meta-analysis.
3.4. Network Meta-Analysis
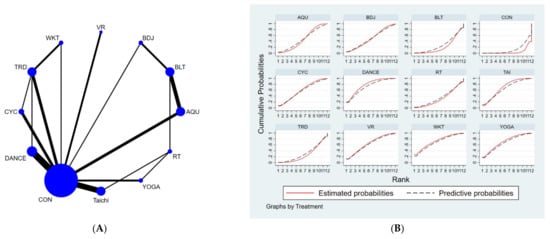
Figure 2.
(A). NMA figure for UPDRS. (B). SUCRA plot for UPDRS.
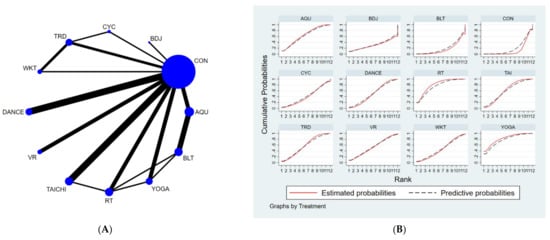
Figure 3.
(A) NMA figure for TUGT, (B) SUCRA plot for TUGT.
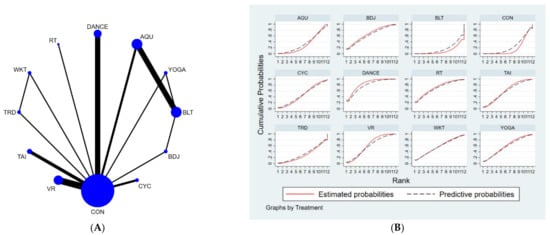
Figure 4.
(A) NMA figure for BBS, (B) SUCRA plot for BBS.
3.4.1. Unified Parkinson’s Disease Rating Scale-Motor (UPDRS-Motor)
All p-values for indirect and direct comparisons between all studies were tested for consistency and inconsistency, and all p-values were greater than 0.05, indicating that the effect of consistency between studies was acceptable. Details will be shown in Supplementary Table S2.
The results of the network meta-analysis showed that relative to the control group’s routine measures, musical dance exercises [MD = −4.9, 95% CI = (−7.57, −2.23)], walking exercises [MD = −4.79, 95% CI = (−9.05, −0.53)], yoga exercises [MD =−4.51, 95% CI = (−8.02, −1.00)], Taijiquan practice [MD = −4.26, 95% CI = (−6.63, −1.88)], virtual reality practice [MD = −4.12, 95% CI = (−7.34, −0.91)], cycling practice [MD = −3.70, 95% CI = (−6.65, −0.75)], aquatic exercise [MD = −2.93, CL = (−5.38, −0.48)], water exercise [MD = −2.93, 95% CI = (− 5.38, −0.48)] were superior to the control group in reducing UPDRS scores, the details of which are shown in Table 3. The probability ranking of the different exercise interventions in terms of reducing UPDRS scores was ranked first in the SUCRA for dance exercises (SUCRA = 72.3% as shown in Figure 2B).

Table 3.
League table on UPDRS.
3.4.2. Timed-Up-and-Go Test (TUGT)
All p-values for indirect and direct comparisons between all studies were tested for consistency and inconsistency, and all p-values were greater than 0.05, indicating that the effect of consistency between studies was acceptable. Details are shown in Supplementary Table S3.
The results of the network meta-analysis showed that, relative to the control group (usual care) for routine measures, yoga exercises [MD = −2.4, 95% CI = (−4.14, −0.65)], resistance training [MD = −2.19, CL = (−3.41, −0.97)], water exercise exercises [MD = −1.67, 95% CI = (−3.3, −0.03)], tai chi exercise [MD = −1.56, 95% CI = (−2.59, −0.54)] and musical dance exercise [MD = −1.24. 95% CI = (−2.48, −0.01)] were superior to the control group (usual care) in reducing TUGT time; relative to the control group (balance exercise), yoga exercise [MD = −2.5, 95% CI = (−4.96, −0.04)], resistance exercises [MD = −2.3, 95% CI = (−4.18, −0.42)] and aquatic exercises [MD = −1.78, 95% CI = (−3.14, −0.42)] were better than the control group (balance exercises) in reducing TUGT time, the details of which are shown in Table 4. The probability ranking of the different exercise interventions in terms of time to TUGT reduction was ranked first by yoga practice in the SUCRA (SUCRA = 78.0% as shown in Figure 3B).

Table 4.
League table on TUGT.
3.4.3. Berge Balance Scale
All p-values for indirect and direct comparisons between all studies were tested for consistency and inconsistency, and all p-values were greater than 0.05, indicating that the effect of consistency between studies was acceptable. Details are shown in Supplementary Table S4.
The results of the network meta-analysis showed that musical dance exercises [MD = 7.07, CL = (1.47, 12.68)] and Badaunjin Qigong exercises [MD = 5.51, 95% CI = (0.46, 10.55) were superior to the control group (balance exercises) in increasing BBS scores relative to the control group (balance exercises) for the usual measures. Relative to the control group (usual care), musical dance exercises [MD = 5.81, 95% CI = (2.45, 9.17)] and virtual reality exercises [MD = 3.6, 95% CI = (0.96, 6.24)] were superior to the control group (usual care) in terms of increasing BBS scores, the details will be shown in Table 5. The probability ranking of the different exercise interventions in terms of increasing BBS scores was ranked first by dance exercises in the SUCRA (SUCRA = 78.4% as shown in Figure 4B).

Table 5.
League table on BBS.
3.5. Publication Bias Test
We constructed separate funnel plots for all outcome indicators to test for possible publication bias. Visual inspection of the funnel plots did not reveal any significant publication bias [82]. Details as shown in Figure 5.

Figure 5.
Funnel plot on publication bias. (A): UPDRS; (B): TUG; (C): BBS.
4. Discussion
In this study we compared the effectiveness of different exercise interventions to improve motor function in people with Parkinson’s disease. A total of 60 studies including 10 different exercise programmes were included, including 2589 patients diagnosed with Parkinson’s disease, which is a fairly large sample size. Our study showed that dance practice to music was the best exercise intervention in terms of increasing BBS test scores, dance practice to music was also the best exercise intervention in terms of decreasing UPDRS-Motor scores, but yoga training showed better results in terms of decreasing the duration of TUGT. Overall, however, we believe that dance practice to music is perhaps the most appropriate intervention for improving motor function in Parkinson’s disease.
The most common symptom of Parkinson’s disease is a significant reduction in muscle control compared to the pre-existing condition, manifesting as frozen gait, reduced balance and a number of other problems. The BBS test is a comprehensive functional test that reflects the ability of Parkinson’s patients to actively shift their centre of gravity by examining their dynamic and static balance in a sitting or standing position [83], and its results are more accurate and acceptable. For Parkinson’s patients, dancing to music is a difficult and challenging exercise that requires a certain amount of proprioceptive control of the body in a state of balance before the muscle groups responsible for performing the motor function are activated [55]. During exercise, the non-periodic activities associated with dance such as starts, stops, rotations, side steps and displacements in different directions all have a beneficial effect on the training of the patient’s body responsiveness and body posture prediction, which in turn allows the basal neural network to show a larger shared network community associated with the motor cortex, resulting in an increased priority level of connectivity between the basal ganglia and the premotor areas [9,84,85], thus improving the Parkinson’s patient. This in turn improves the balance of Parkinson’s patients in different states and thus improves their scores on the BBS test. Our results demonstrate that dance training to music has a statistically significant beneficial effect on the balance of Parkinson’s patients compared to other exercises, and that there is a statistically significant difference compared to the control group, which is also consistent with previous studies [14,86,87].
In addition, Parkinson’s impairs motor function not only in terms of balance [88], but also in the mouth muscles associated with speech, facial muscles associated with facial expressions and limb muscles associated with alternating movements [89]. The UPDRS-III scale is the most commonly used international measure of motor function in Parkinson’s disease and provides a comprehensive measure of improvement or deterioration in motor function in Parkinson’s patients [90]. In our study, it was shown that all exercises, regardless of type, had a role in reducing UPDRS scale scores relative to the no-exercise control group, but dance training to music was the most useful exercise intervention among the different exercises included in this study, which is consistent with previous research findings [87,91,92]. As a physical activity, dance exercise not only enhances the physical and cognitive aspects of exercise, but also enhances the therapeutic effect on motor function in Parkinson’s disease with the help of rhythmic music [93]. The improvement in motor function may be the result of the increased cognitive attention of the patient while performing the exercises [94]. Imaging evidence suggests that the improvement in motor function with dance practice appears to be related to the emergence of higher-order neurological functions (for the patient, dance practice is a new form of physical activity), with altered neuroplasticity recorded by functional magnetic resonance, reflecting increased brain connectivity, particularly between the basal ganglia and cortical motor centres [95].
With age, older people experience varying degrees of decline in muscle strength and flexibility, which is common even in healthy older people [96]. The TUGT test is often used to test lower limb muscle strength in older people due to its ease of use and sensitivity [97], and our study shows that yoga and resistance training have unparalleled advantages in reducing TUGT testing times in people with Parkinson’s disease, which is in line with previous studies. The results are the same as in the original study [98]. At the same time, however, we made the further hypothesis that yoga combined with resistance training may be more beneficial than yoga or resistance training alone, with resistance training being beneficial in slowing muscle strength loss and promoting skeletal muscle hypertrophy [99] and yoga training being beneficial in maintaining joint flexibility and ligament elasticity [100], and that combining the two at the right dose (exercise duration and intensity) may have better effects. However, further studies are needed to prove our hypothesis.
Overall, our study has some clinical implications. First, dance exercises to music and yoga exercises have a significant effect in improving motor function in Parkinson’s disease. Furthermore, doctors can promote exercise as a good non-pharmacological treatment in the management of Parkinson’s disease.
5. Strengths and Limitations
First, our study included 60 studies and 2859 patients, which is a very large sample size, and we also built on the original review on the treatment of motor function in people with Parkinson’s disease by including two more novel interventions, aquatic exercises and virtual reality exercises, to compare with other interventions, which provides newer and more comprehensive evidence-based recommendations.
Secondly, our study shares some limitations with the studies on which it is based. Although we made every effort to control for study heterogeneity when including these original studies, heterogeneity between studies was unavoidable (e.g., the proportion of studies by region and between male and female participants).
Finally, in our study, readers should interpret the results with caution because of the small number of studies and the limited head-to-head direct comparative evidence for some interventions. It also highlights the need for further expansion of the relevant studies.
6. Conclusions
In our study, we recommended yoga for those who wanted to improve TUG and dance for those who wanted to improve balance. Overall, however, dance exercises to the rhythm of music, yoga, virtual reality training and resistance training are the most recommended exercise prescription for Parkinson’s patients who want to improve their overall motor function.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/12/6/698/s1, Table S1: Risk of bias for each included studies, Table S2: Consistency test for UPDRS, Table S3: Consistency test for TUGT, Table S4: Consistency test for BBS.
Author Contributions
Z.H. interpreted the data, wrote the initial manuscript, and was involved in the data analysis; X.Z. was responsible for the collection of all relevant papers; P.C. was responsible for the supervision of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of the study are available from the first author, upon reasonable request.
Acknowledgments
We thank all the reviewers for their assistance and support.
Conflicts of Interest
The authors declare that they have no conflicts of interest. Hao Zikang is currently an M.D student under the supervision of Chen Ping. His research is centered on rehabilitation medicine. Zhang Xiaodan is also an M.D student in the Department of Physical Education at Ocean University of China, a classmate of Hao Zikang and a student of Chen Ping. Chen Ping is a professor at the Department of Physical Exercise, Ocean University of China. The current research interests of Professor Chen’s group include: (1) athletic training; (2) rehabilitation of sports injury; (3) physical education.
References
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. The Economic Burden of Physical Inactivity: A Global Analysis of Major Non-Communicable Diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, W.M.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Haelbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a Prescription for Patients with Various Diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- Andrade, A.; Siqueira, T.C.; D’Oliveira, A.; Dominski, F.H. Effects of Exercise in the Treatment of Alzheimer’s Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Aging Phys. Act. 2021, 1, 1–17. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Li, C.; Tsang, R.C.C.; Chen, Y.; Ge, Y.; Gao, Q. Effects of Exercise in Patients with Amyotrophic Lateral Sclerosis A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 801–810. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-Term Effects of Exercise and Physical Therapy in People with Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical Exercise Improves Quality of Life, Depressive Symptoms, and Cognition across Chronic Brain Disorders: A Transdiagnostic Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Wang, Y.T.; Liu, X.; Song, W.; Du, X. The Effect of Qigong-Based Therapy on Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2020, 34, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.O.; Scianni, A.; Rodrigues-de-Paula, F. Progressive Resistance Exercise Improves Strength and Physical Performance in People with Mild to Moderate Parkinson’s Disease: A Systematic Review. J. Physiother. 2013, 59, 7–13. [Google Scholar] [CrossRef]
- Flach, A.; Jaegers, L.; Krieger, M.; Bixler, E.; Kelly, P.; Weiss, E.P.; Ahmad, S.O. Endurance Exercise Improves Function in Individuals with Parkinson’s Disease: A Meta-Analysis. Neurosci. Lett. 2017, 659, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.; Hewitt, J. Dance as an Intervention for People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2014, 47, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.; Chaimani, A.; Li, T. Network Meta-Analysis: An Introduction for Clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Jueni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ-Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Li, D.; Chen, P. Effects of Aquatic Exercise and Land-Based Exercise on Cardiorespiratory Fitness, Motor Function, Balance, and Functional Independence in Stroke Patients—A Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2021, 11, 1097. [Google Scholar] [CrossRef]
- Jackson, D.; Riley, R.; White, I.R. Multivariate Meta-Analysis: Potential and Promise. Stat. Med. 2011, 30, 2481–2498. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Vats, D.; Flegal, J.M.; Jones, G.L. Multivariate Output Analysis for Markov Chain Monte Carlo. Biometrika 2019, 106, 321–337. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Demeco, A.; Moggio, L.; Marinaro, C.; Pino, I.; Barletta, M.; Petraroli, A.; Pepe, D.; Lavano, F.; Ammendolia, A. Comparative Effectiveness of Breathing Exercises in Patients with Chronic Obstructive Pulmonary Disease. Complementary Ther. Clin. Pract. 2020, 41, 101260. [Google Scholar] [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events A Systematic Review and Meta-Analysis. JAMA-J. Am. Med. Assoc. 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhuang, Y.; Kang, Y. Effect of Health Qigong Baduanjin on Fall Prevention in Individuals with Parkinson’s Disease. J. Am. Geriatr. Soc. 2016, 64, e227–e228. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Q.; Dong, S.; Cai, Z. Effects of Baduanjin and Balancer Exercise on Motor and Non-motor Symptoms of Parkinson’s Disease. Chin. J. Rehabil. Theory Pract. 2021, 27, 111–116. [Google Scholar]
- Shu, X.; Yang, W.; Jie, W. Effects of Baduanjin combined with balance mat training on lower limb motor function and trunk balance strength in elderly Parkinson’s patients. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2021, 21, 56–57. [Google Scholar] [CrossRef]
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A.; et al. Effects of a Nordic Walking Program on Motor and Non-Motor Symptoms, Functional Performance and Body Composition in Patients with Parkinson’s Disease. NeuroRehabilitation 2015, 37, 245–254. [Google Scholar] [CrossRef]
- Bang, D.-H.; Shin, W.-S. Effects of an Intensive Nordic Walking Intervention on the Balance Function and Walking Ability of Individuals with Parkinson’s Disease: A Randomized Controlled Pilot Trial. Aging Clin. Exp. Res. 2017, 29, 993–999. [Google Scholar] [CrossRef]
- Bello, O.; Sanchez, J.A.; Lopez-Alonso, V.; Márquez, G.; Morenilla, L.; Castro, X.; Giraldez, M.; Santos-García, D.; Fernandez-del-Olmo, M. The Effects of Treadmill or Overground Walking Training Program on Gait in Parkinson’s Disease. Gait Posture 2013, 38, 590–595. [Google Scholar] [CrossRef]
- Carvalho, A.; Barbirato, D.; Araujo, N.; Martins, J.V.; Cavalcanti, J.L.S.; Santos, T.M.; Coutinho, E.S.; Laks, J.; Deslandes, A.C. Comparison of Strength Training, Aerobic Training, and Additional Physical Therapy as Supplementary Treatments for Parkinson’s Disease: Pilot Study. Clin. Interv. Aging 2015, 10, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Katzel, L.I.; Ivey, F.M.; Sorkin, J.D.; Favors, K.; Anderson, K.E.; Smith, B.A.; Reich, S.G.; Weiner, W.J.; Macko, R.F. Randomized Clinical Trial of 3 Types of Physical Exercise for Patients with Parkinson Disease. JAMA Neurol. 2013, 70, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sage, M.D.; Almeida, Q.J. Symptom and Gait Changes after Sensory Attention Focused Exercise vs Aerobic Training in Parkinson’s Disease. Mov. Disord. 2009, 24, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Cruz, S. A Bicentric Controlled Study on the Effects of Aquatic Ai Chi in Parkinson Disease. Complementary Ther. Med. 2018, 36, 147–153. [Google Scholar] [CrossRef]
- Carroll, L.M.; Volpe, D.; Morris, M.E.; Saunders, J.; Clifford, A.M. Aquatic Exercise Therapy for People with Parkinson Disease: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 631–638. [Google Scholar] [CrossRef]
- Vivas, J.; Arias, P.; Cudeiro, J. Aquatic Therapy versus Conventional Land-Based Therapy for Parkinson’s Disease: An Open-Label Pilot Study. Arch. Phys. Med. Rehabil. 2011, 92, 1202–1210. [Google Scholar] [CrossRef]
- Volpe, D.; Giantin, M.G.; Maestri, R.; Frazzitta, G. Comparing the Effects of Hydrotherapy and Land-Based Therapy on Balance in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study. Clin. Rehabil. 2014, 28, 1210–1217. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Feng, S.; Hou, W.; Zhang, Y. Effect of water—Based exercise on motor function, balance function and walking ability in patients with Parkinson’s disease. Chin. J. Contemp. Neurol. Neurosurg. 2017, 17, 346–351. [Google Scholar]
- Kurt, E.E.; Büyükturan, B.; Büyükturan, Ö.; Erdem, H.R.; Tuncay, F. Effects of Ai Chi on Balance, Quality of Life, Functional Mobility, and Motor Impairment in Patients with Parkinson’s Disease. Disabil. Rehabil. 2018, 40, 791–797. [Google Scholar] [CrossRef]
- Palamara, G.; Gotti, F.; Maestri, R.; Bera, R.; Gargantini, R.; Bossio, F.; Zivi, I.; Volpe, D.; Ferrazzoli, D.; Frazzitta, G. Land Plus Aquatic Therapy Versus Land-Based Rehabilitation Alone for the Treatment of Balance Dysfunction in Parkinson Disease: A Randomized Controlled Study With 6-Month Follow-Up. Arch. Phys. Med. Rehabil. 2017, 98, 1077–1085. [Google Scholar] [CrossRef]
- Clerici, I.; Maestri, R.; Bonetti, F.; Ortelli, P.; Volpe, D.; Ferrazzoli, D.; Frazzitta, G. Land Plus Aquatic Therapy Versus Land-Based Rehabilitation Alone for the Treatment of Freezing of Gait in Parkinson Disease: A Randomized Controlled Trial. Phys. Ther. 2019, 99, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.; Giantin, M.G.; Manuela, P.; Filippetto, C.; Pelosin, E.; Abbruzzese, G.; Antonini, A. Water-Based vs. Non-Water-Based Physiotherapy for Rehabilitation of Postural Deformities in Parkinson’s Disease: A Randomized Controlled Pilot Study. Clin. Rehabil. 2017, 31, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J. Effects of Therapeutic Tai Chi on Functional Fitness and Activities of Daily Living in Patients with Parkinson Disease. J. Exerc. Rehabil. 2016, 12, 499–503. [Google Scholar] [CrossRef] [PubMed]
- You, H.; She, J. Observation on the effectiveness of group therapy of Tai Chi balance exercise in improving the balance function and depressive state of Parkinson’s disease patients. Guizhou Med. J. 2020, 44, 1071–1072. [Google Scholar]
- Li, F.; Harmer, P.; Fitzgerald, K.; Eckstrom, E.; Stock, R.; Galver, J.; Maddalozzo, G.; Batya, S.S. Tai Chi and Postural Stability in Patients with Parkinson’s Disease. N. Engl. J. Med. 2012, 366, 511–519. [Google Scholar] [CrossRef]
- Vergara-Diaz, G.; Osypiuk, K.; Hausdorff, J.M.; Bonato, P.; Gow, B.J.; Miranda, J.G.; Sudarsky, L.R.; Tarsy, D.; Fox, M.D.; Gardiner, P.; et al. Tai Chi for Reducing Dual-Task Gait Variability, a Potential Mediator of Fall Risk in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Glob. Adv. Health Med. 2018, 7, 2164956118775385. [Google Scholar] [CrossRef]
- Hackney, M.E.; Earhart, G.M. Tai Chi Improves Balance and Mobility in People with Parkinson Disease. Gait Posture 2008, 28, 456–460. [Google Scholar] [CrossRef]
- Elkins, M. Tai Chi Improves Balance and Prevents Falls in People with Parkinson’s Disease. J. Physiother. 2014, 61, 44. [Google Scholar] [CrossRef][Green Version]
- Amano, S.; Nocera, J.R.; Vallabhajosula, S.; Juncos, J.L.; Gregor, R.J.; Waddell, D.E.; Wolf, S.L.; Hass, C.J. The Effect of Tai Chi Exercise on Gait Initiation and Gait Performance in Persons with Parkinson’s Disease. Parkinsonism Relat. Disord. 2013, 19, 955–960. [Google Scholar] [CrossRef]
- Choi, H.-J.; Garber, C.E.; Jun, T.-W.; Jin, Y.-S.; Chung, S.-J.; Kang, H.-J. Therapeutic Effects of Tai Chi in Patients with Parkinson’s Disease. ISRN Neurol. 2013, 2013, 548240. [Google Scholar] [CrossRef]
- Volpe, D.; Signorini, M.; Marchetto, A.; Lynch, T.; Morris, M.E. A Comparison of Irish Set Dancing and Exercises for People with Parkinson’s Disease: A Phase II Feasibility Study. BMC Geriatr. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, P. Analysis of the effects of rhythmic auditory stimulation combined with motor training on motor function in patients with Parkinson’s disease. Mod. Diagn. Treat. 2020, 31, 1129–1131. [Google Scholar]
- Michels, K.; Dubaz, O.; Hornthal, E.; Bega, D. “Dance Therapy” as a Psychotherapeutic Movement Intervention in Parkinson’s Disease. Complementary Ther. Med. 2018, 40, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, J.; Morris, M.E.; Bhriain, O.N.; Volpe, D.; Lynch, T.; Clifford, A.M. Dancing for Parkinson Disease: A Randomized Trial of Irish Set Dancing Compared with Usual Care. Arch. Phys. Med. Rehabil. 2017, 98, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Hackney, M.E.; Earhart, G.M. Effects of Dance on Movement Control in Parkinson’s Disease: A Comparison of Argentine Tango and American Ballroom. J. Rehabil. Med. 2009, 41, 475–481. [Google Scholar] [CrossRef]
- Rawson, K.S.; McNeely, M.E.; Duncan, R.P.; Pickett, K.A.; Perlmutter, J.S.; Earhart, G.M. Exercise and Parkinson Disease: Comparing Tango, Treadmill and Stretching. J. Neurol. Phys. Ther. 2019, 43, 26–32. [Google Scholar] [CrossRef]
- Song, J.; Paul, S.; Caetano, M.J.; Smith, S.; Dibble, L.; Love, R.; Schoene, D.; Menant, J.; Sherrington, C.; Lord, S.; et al. Home-Based Step Training Using Videogame Technology in People with Parkinson’s Disease: A Single-Blinded Randomised Controlled Trial. Clin. Rehabil. 2017, 32, 0269215517721593. [Google Scholar] [CrossRef]
- Duncan, R.P.; Earhart, G.M. Randomized Controlled Trial of Community-Based Dancing to Modify Disease Progression in Parkinson Disease. Neurorehabil. Neural. Repair. 2012, 26, 132–143. [Google Scholar] [CrossRef]
- Solla, P.; Cugusi, L.; Bertoli, M.; Cereatti, A.; Della Croce, U.; Pani, D.; Fadda, L.; Cannas, A.; Marrosu, F.; Defazio, G.; et al. Sardinian Folk Dance for Individuals with Parkinson’s Disease: A Randomized Controlled Pilot Trial. J. Altern. Complementary 2019, 25, 305–316. [Google Scholar] [CrossRef]
- Rios Romenets, S.; Anang, J.; Fereshtehnejad, S.-M.; Pelletier, A.; Postuma, R. Tango for Treatment of Motor and Non-Motor Manifestations in Parkinson’s Disease: A Randomized Control Study. Complementary Ther. Med. 2015, 23, 175–184. [Google Scholar] [CrossRef]
- Sharma, N.; Robbins, K.; Wagner, K.; Colgrove, Y. A Randomized Controlled Pilot Study of the Therapeutic Effects of Yoga in People with Parkinson’s Disease. Int. J. Yoga 2015, 8, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Mooney, K.; Richards, L.; Balachandran, A.; Sun, M.; Harriell, K.; Potiaumpai, M.; Signorile, J.F. Comparative Impacts of Tai Chi, Balance Training, and a Specially-Designed Yoga Program on Balance in Older Fallers. Arch. Phys. Med. Rehabil. 2014, 95, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Khuzema, A.; Brammatha, A.; Arul Selvan, V. Effect of Home-Based Tai Chi, Yoga or Conventional Balance Exercise on Functional Balance and Mobility among Persons with Idiopathic Parkinson’s Disease: An Experimental Study. Hong Kong Physiother. J. 2020, 40, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People with Parkinson Disease. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Cheung, C.; Bhimani, R.; Wyman, J.F.; Konczak, J.; Zhang, L.; Mishra, U.; Terluk, M.; Kartha, R.V.; Tuite, P. Effects of Yoga on Oxidative Stress, Motor Function, and Non-Motor Symptoms in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Pilot Feasibility Stud. 2018, 4, 162. [Google Scholar] [CrossRef]
- Van Puymbroeck, M.; Walter, A.; Hawkins, B.; Sharp, J.; Woschkolup, K.; Urrea-Mendoza, E.; Revilla, F.; Adams, E.; Schmid, A. Functional Improvements in Parkinson’s Disease Following a Randomized Trial of Yoga. Evid. -Based Complementary Altern. Med. 2018, 2018, 8516351. [Google Scholar] [CrossRef]
- Van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of Home-Based and Remotely Supervised Aerobic Exercise in Parkinson’s Disease: A Double-Blind, Randomised Controlled Trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise Increases Caudate Dopamine Release and Ventral Striatal Activation in Parkinson’s Disease. Mov. Disord. 2019, 34, 1891–1900. [Google Scholar] [CrossRef]
- Ridgel, A.L.; Ault, D.L. High-Cadence Cycling Promotes Sustained Improvement in Bradykinesia, Rigidity, and Mobility in Individuals with Mild-Moderate Parkinson’s Disease. Parkinsons Dis. 2019, 2019, 4076862. [Google Scholar] [CrossRef]
- Arcolin, I.; Pisano, F.; Delconte, C.; Godi, M.; Schieppati, M.; Mezzani, A.; Picco, D.; Grasso, M.; Nardone, A. Intensive Cycle Ergometer Training Improves Gait Speed and Endurance in Patients with Parkinson’s Disease: A Comparison with Treadmill Training. Restor. Neurol. Neurosci. 2016, 34, 125–138. [Google Scholar] [CrossRef]
- Tollár, J.; Nagy, F.; Hortobágyi, T. Vastly Different Exercise Programs Similarly Improve Parkinsonian Symptoms: A Randomized Clinical Trial. Gerontology 2019, 65, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Mei, G.; Wang, Z.; Li, Y. Effect of resistance training on the improvement of lower extremity muscle strength and balance function in patients with Parkinson’s disease. Chin. J. Gerontol. 2019, 39, 127–130. [Google Scholar]
- Leal, L.C.; Abrahin, O.; Rodrigues, R.P.; da Silva, M.C.; Araújo, A.P.; de Sousa, E.C.; Pimentel, C.P.; Cortinhas-Alves, E.A. Low-Volume Resistance Training Improves the Functional Capacity of Older Individuals with Parkinson’s Disease. Geriatr. Gerontol. Int. 2019, 19, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Moraes Filho, A.; Chaves, S.N.; Martins, W.R.; Tolentino, G.P.; de Cássia Pereira Pinto Homem, R.; Landim de Farias, G.; Fischer, B.L.; Oliveira, J.A.; Pereira, S.K.A.; Vidal, S.E.; et al. Progressive Resistance Training Improves Bradykinesia, Motor Symptoms and Functional Performance in Patients with Parkinson’s Disease. Clin. Interv. Aging 2020, 15, 87–95. [Google Scholar] [CrossRef]
- De Lima, T.A.; Ferreira-Moraes, R.; da Alves, W.M.G.C.; Alves, T.G.G.; Pimentel, C.P.; Sousa, E.C.; Abrahin, O.; Cortinhas-Alves, E.A. Resistance Training Reduces Depressive Symptoms in Elderly People with Parkinson Disease: A Controlled Randomized Study. Scand. J. Med. Sci. Sports 2019, 29, 1957–1967. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Paschen, S.; Kruse, A.; Raethjen, J.; Weisser, B.; Deuschl, G. Resistance versus Balance Training to Improve Postural Control in Parkinson’s Disease: A Randomized Rater Blinded Controlled Study. PLoS ONE 2015, 10, e0140584. [Google Scholar] [CrossRef]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Lo Monaco, R.; Parisi, A.; Padua, L. Comparison of Virtual Reality Rehabilitation and Conventional Rehabilitation in Parkinson’s Disease: A Randomised Controlled Trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef]
- Xia, M.; Jiang, Y.; Zhen, D.; Wang, Z.; Zhan, Z.; Lin, Z. Effect of somatosensory games on cognition and gait of patients with Parkinson’s disease. Clin. Focus 2020, 35, 900–903. [Google Scholar]
- Lee, N.-Y.; Lee, D.-K.; Song, H.-S. Effect of Virtual Reality Dance Exercise on the Balance, Activities of Daily Living, and Depressive Disorder Status of Parkinson’s Disease Patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef]
- Yuan, R.-Y.; Chen, S.-C.; Peng, C.-W.; Lin, Y.-N.; Chang, Y.-T.; Lai, C.-H. Effects of Interactive Video-Game–Based Exercise on Balance in Older Adults with Mild-to-Moderate Parkinson’s Disease. J. Neuroeng. Rehabil. 2020, 17, 91. [Google Scholar] [CrossRef]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the Nintendo Wii Combination with Conventional Exercises in the Rehabilitation of Individuals with Parkinson’s Disease: A Randomized Clinical Trial. NeuroRehabilitation 2019, 45, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Schmid, C.H.; Lau, J.; Trikalinos, T.A. Meta-Analyst: Software for Meta-Analysis of Binary, Continuous and Diagnostic Data. BMC Med. Res. Methodol. 2009, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Pickenbrock, H.M.; Diel, A.; Zapf, A. A Comparison between the Static Balance Test and the Berg Balance Scale: Validity, Reliability, and Comparative Resource Use. Clin. Rehabil. 2016, 30, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.H.; Su, Y.-H. Sensorimotor Synchronization: A Review of Recent Research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Diedrichsen, J.; Flanagan, J.R. Principles of Sensorimotor Learning. Nat. Rev. Neurosci. 2011, 12, 739–751. [Google Scholar] [CrossRef]
- Hidalgo-Agudo, R.D.; Lucena-Anton, D.; Luque-Moreno, C.; Marcos Heredia-Rizo, A.; Moral-Munoz, J.A. Additional Physical Interventions to Conventional Physical Therapy in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2020, 9, 1038. [Google Scholar] [CrossRef]
- De Almeida, H.S.; Porto, F.; Porretti, M.; Lopes, G.; Fiorot, D.; dos Bunn, P.S.; da Silva, E.B. Effect of Dance on Postural Control in People with Parkinson’s Disease: A Meta-Analysis Review. J. Aging Phys. Act. 2021, 29, 130–141. [Google Scholar] [CrossRef]
- Dirnberger, G.; Jahanshahi, M. Executive Dysfunction in Parkinson’s Disease: A Review. J. Neuropsychol. 2013, 7, 193–224. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The Clinical Symptoms of Parkinson’s Disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to Identify Tremor Dominant and Postural Instability/Gait Difficulty Groups with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale: Comparison with the Unified Parkinson’s Disease Rating Scale. Mov. Disord. 2013, 28, 668–670. [Google Scholar] [CrossRef]
- Hasan, S.M.; Alshafie, S.; Hasabo, E.A.; Saleh, M.; Elnaiem, W.; Qasem, A.; Alzu’bi, Y.O.; Khaled, A.; Zaazouee, M.S.; Ragab, K.M.; et al. Efficacy of Dance for Parkinson’s Disease: A Pooled Analysis of 372 Patients. J. Neurol. 2022, 269, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Bueno, C.; Deeks, J.J.; Cavero-Redondo, I.; Jolly, K.; Torres-Costoso, A.I.; Price, M.; Fernandez-Rodriguez, R.; Martinez-Vizcaino, V. Effect of Exercise on Motor Symptoms in Patients with Parkinson’s Disease: A Network Meta-Analysis. J. Geriatr. Phys. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Paris-Garcia, F. Influence of Dance Programmes on Gait Parameters and Physical Parameters of the Lower Body in Older People: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 1547. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Krause, J.; Krause, M.; Reischak-Oliveira, A. Dancing for Healthy Aging: Functional and Metabolic Perspectives. Altern. Ther. Health Med. 2019, 25, 44–63. [Google Scholar] [PubMed]
- Teixeira-Machado, L.; Arida, R.M.; de Jesus Mari, J. Dance for Neuroplasticity: A Descriptive Systematic Review. Neurosci. Biobehav. Rev. 2019, 96, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, Muscle Mass, and Muscle Strength in Relation to Functional Decline in Older Persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef]
- Yoo, J.E.; Jang, W.; Shin, D.W.; Jeong, S.-M.; Jung, H.-W.; Youn, J.; Han, K.; Kim, B. Timed Up and Go Test and the Risk of Parkinson’s Disease: A Nation-Wide Retrospective Cohort Study. Mov. Disord. 2020, 35, 1263–1267. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Y.; Guo, H.; Tang, C.; Chen, D.; Zhu, M. Effects of Aerobic Exercise and Mind-Body Exercise in Parkinson’s Disease: A Mixed-Treatment Comparison Analysis. Front. Aging Neurosci. 2021, 13, 739115. [Google Scholar] [CrossRef]
- Henwood, T.; Tuckett, A.; Edelstein, O.; Bartlett, H. Exercise in Later Life: The Older Adults’ Perspective about Resistance Training. Ageing Soc. 2011, 31, 1330–1349. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).