Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Histology and Immunohistochemistry
2.4. Detection of Reactive Oxygen Species (ROS)
2.5. Immunofluorescence
2.6. Statistics
3. Results
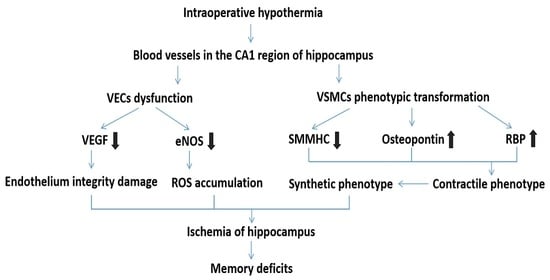
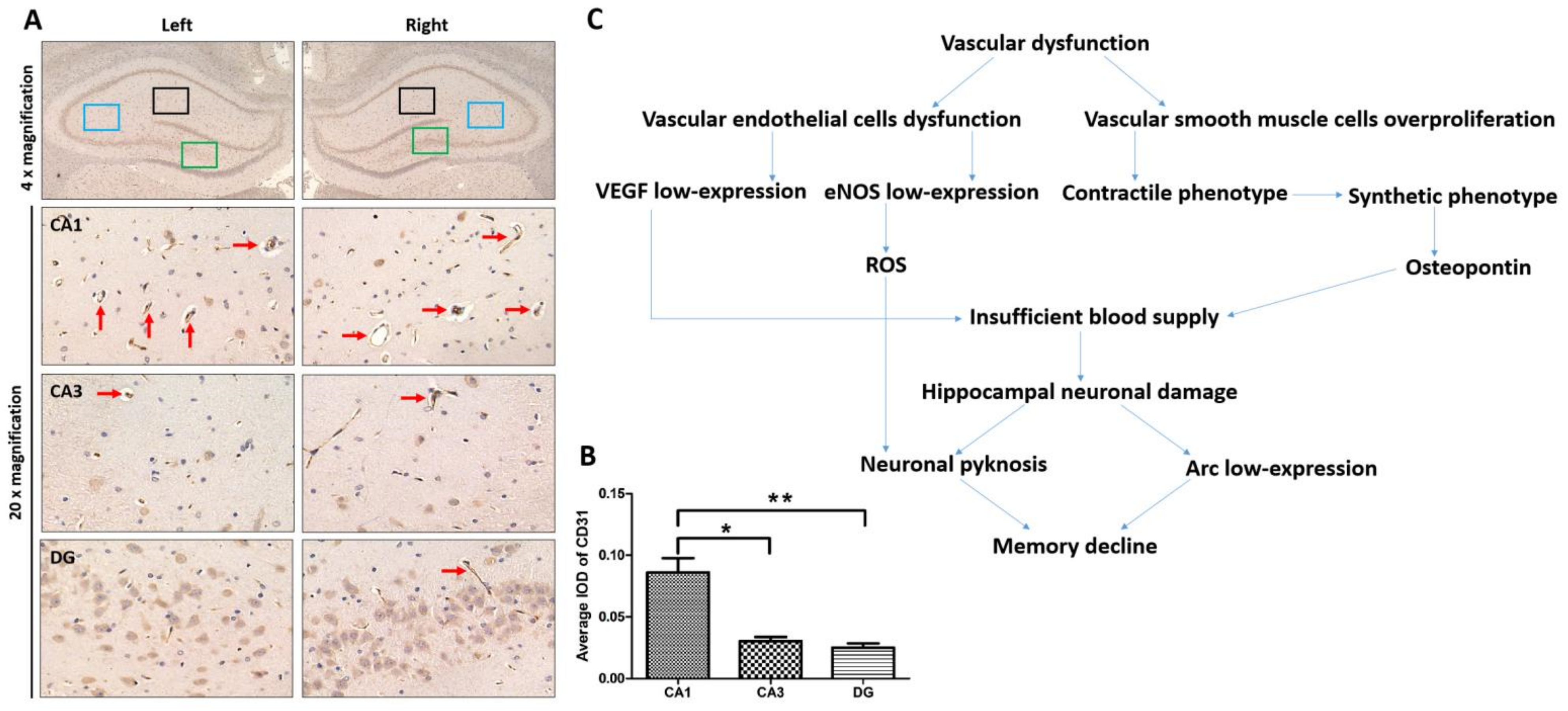
3.1. The Abundance of Blood Vessels in the CA1 Region of the Hippocampus and Its Relationship with Hippocampal Function
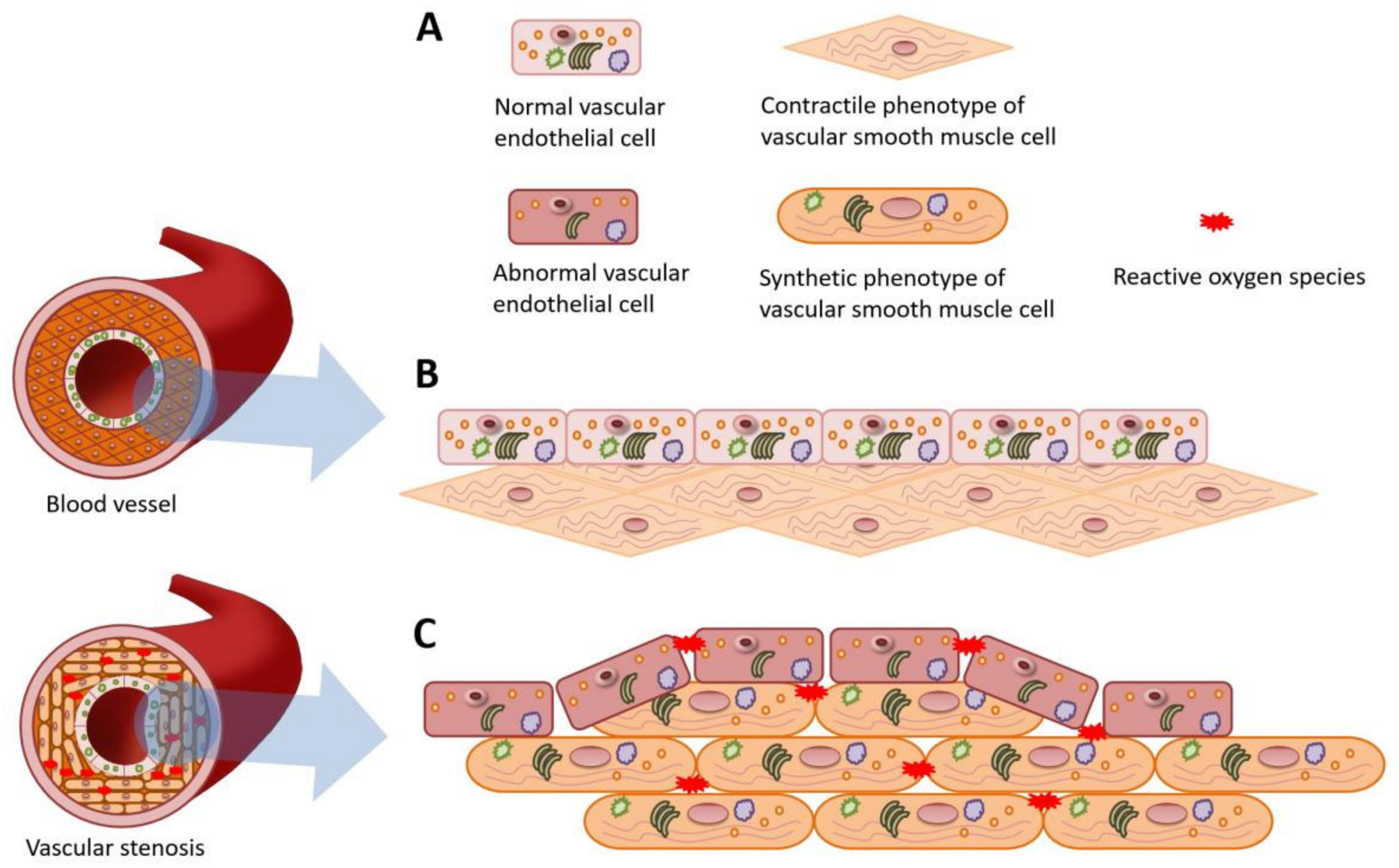
3.2. Intraoperative Hypothermia Caused Dysfunction of VECs and Phenotypic Transformation of VSMCs
3.3. The Overall Impact of Intraoperative Hypothermia on the Blood Vessel
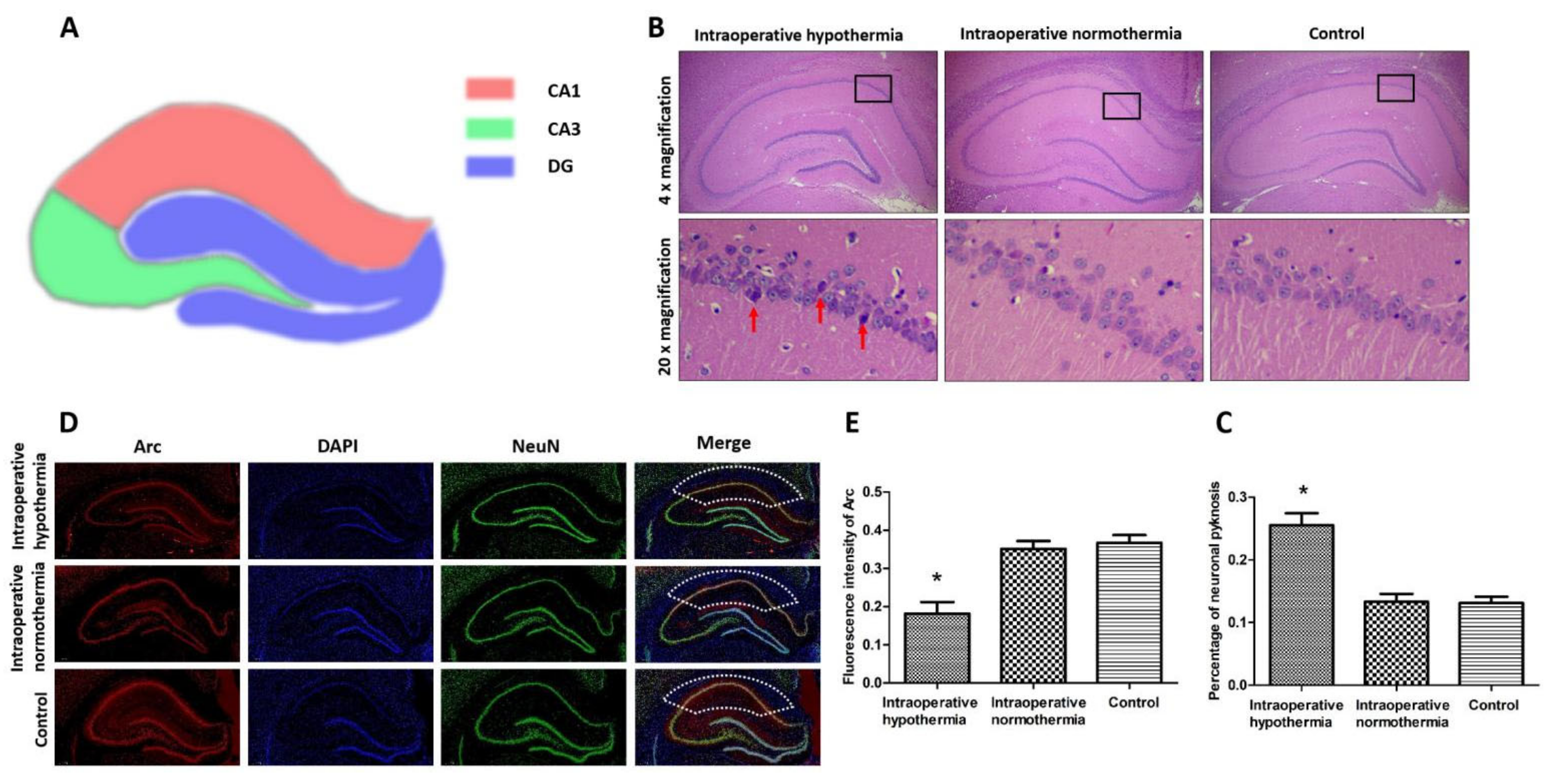
3.4. Intraoperative Hypothermia Damaged Hippocampal Neurons in the CA1 Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, K.C.; Tanner, E.J.; Frey, M.; Leitao, M.M.; Levine, D.A.; Gardner, G.J.; Sonoda, Y.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S. Intraoperative hypothermia during primary surgical cytoreduction for advanced ovarian cancer: Risk factors and associations with postoperative morbidity. Gynecol. Oncol. 2013, 131, 525–530. [Google Scholar] [CrossRef]
- Sari, S.; Aksoy, S.M.; But, A. The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int. J. Clin. Pract. 2021, 75, e14103. [Google Scholar] [CrossRef]
- Scott, A.V.; Stonemetz, J.L.; Wasey, J.O.; Johnson, D.J.; Rivers, R.J.; Koch, C.G.; Frank, S.M. Compliance with Surgical Care Improvement Project for Body Temperature Management (SCIP Inf-10) Is Associated with Improved Clinical Outcomes. Anesthesiology 2015, 123, 116–125. [Google Scholar] [CrossRef]
- Yi, J.; Lei, Y.; Xu, S.; Si, Y.; Li, S.; Xia, Z.; Shi, Y.; Gu, X.; Yu, J.; Xu, G.; et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS ONE 2017, 12, e0177221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Bramham, C.R. Arc/Arg3.1 function in long-term synaptic plasticity: Emerging mechanisms and unresolved issues. Eur. J. Neurosci. 2021, 54, 6696–6712. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, Z.; Zeng, Q.; Kuang, H.; Wang, L.P.; Tsien, J.Z.; Cao, X. Genetic Enhancement of Memory and Long-Term Potentiation but Not CA1 Long-Term Depression in NR2B Transgenic Rats. PLoS ONE 2009, 4, e7486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabresi, P.; Gubellini, P.; Centonze, D.; Picconi, B.; Bernardi, G.; Chergui, K.; Svenningsson, P.; Fienberg, A.A.; Greengard, P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000, 20, 8443–8451. [Google Scholar] [CrossRef] [PubMed]
- Nikolaienko, O.; Patil, S.; Eriksen, M.S.; Bramham, C.R. Arc protein: A flexible hub for synaptic plasticity and cognition. Semin. Cell Dev. Biol. 2018, 77, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Verhage, M.; Maia, A.S.; Plomp, J.J.; Brussaard, A.B.; Heeroma, J.H.; Vermeer, H.; Toonen, R.F.; Hammer, R.E.; van Den, T.K.; Berg, T.V.D.; et al. Synaptic Assembly of the Brain in the Absence of Neurotransmitter Secretion. Science 2000, 287, 864–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.; Teng, J.; Guo, L.; Wang, Y.; Zhang, L.; Gao, J.; Liu, L. Mild hyperhomocysteinemia induces blood–brain barrier dysfunction but not neuroinflammation in the cerebral cortex and hippocampus of wild-type mice. Can. J. Physiol. Pharmacol. 2021, 99, 847–856. [Google Scholar] [CrossRef]
- Han, X.-J.; Shi, Z.-S.; Xia, L.-X.; Zhu, L.-H.; Zeng, L.; Nie, J.-H.; Xu, Z.-C.; Ruan, Y.-W. Changes in synaptic plasticity and expression of glutamate receptor subunits in the CA1 and CA3 areas of the hippocampus after transient global ischemia. Neuroscience 2016, 327, 64–78. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yin, X.; Zhang, J.; Li, A.; Gong, H.; Luo, Q.; Zhang, H.; Gao, Z.; Jiang, H. High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Natl. Sci. Rev. 2019, 6, 1223–1238. [Google Scholar] [CrossRef]
- Pluta, R.; Bogucka-Kocka, A.; Ułamek-Kozioł, M.; Bogucki, J.; Januszewski, S.; Kocki, J.; Czuczwar, S.J. Ischemic tau protein gene induction as an additional key factor driving development of Alzheimer’s phenotype changes in CA1 area of hippocampus in an ischemic model of Alzheimer’s disease. Pharmacol. Rep. 2018, 70, 881–884. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; Di Pellegrino, G. Context-dependent extinction of threat memories: Influences of healthy aging. Sci. Rep. 2018, 8, 12592. [Google Scholar] [CrossRef]
- Zhang, H.; Roman, R.J.; Fan, F. Hippocampus is more susceptible to hypoxic injury: Has the Rosetta Stone of regional variation in neurovascular coupling been deciphered? Geroscience 2022, 44, 127–130. [Google Scholar] [CrossRef]
- Estefan, D.P.; Sánchez-Fibla, M.; Duff, A.; Principe, A.; Rocamora, R.; Zhang, H.; Axmacher, N.; Verschure, P.F.M.J. Coordinated representational reinstatement in the human hippocampus and lateral temporal cortex during episodic memory retrieval. Nat. Commun. 2019, 10, 2255. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, H.; Brys, M.; Glodzik, L.; Switalski, R.; Tsui, W.-H.; Haas, F.; Mcgorty, K.; Chen, Q.; de Leon, M.J. Hippocampal blood flow in normal aging measured with arterial spin labeling at 3T. Magn. Reson. Med. 2011, 65, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.-M.; Hornberger, M. Spatial navigation deficits—Overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef]
- Hort, J.; Vališ, M.; Kuča, K.; Angelucci, F. Vascular Cognitive Impairment: Information from Animal Models on the Pathogenic Mechanisms of Cognitive Deficits. Int. J. Mol. Sci. 2019, 20, 2405. [Google Scholar] [CrossRef] [Green Version]
- Peleli, M.; Zollbrecht, C.; Montenegro, M.F.; Hezel, M.; Zhong, J.; Persson, E.G.; Holmdahl, R.; Weitzberg, E.; Lundberg, J.O.; Carlström, M. Enhanced XOR activity in eNOS-deficient mice: Effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic. Biol. Med. 2016, 99, 472–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaman, S.; Leppänen, V.-M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanjare, M.; Kuo, F.; Gerecht, S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013, 97, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosun, Z.; McFetridge, P.S. Variation in Cardiac Pulse Frequencies Modulates vSMC Phenotype Switching During Vascular Remodeling. Cardiovasc. Eng. Technol. 2015, 6, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Bennick, R.A.; Nagengast, A.A.; DiAngelo, J.R. The SR proteins SF2 and RBP1 regulate triglyceride storage in the fat body of Drosophila. Biochem. Biophys. Res. Commun. 2019, 516, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.G.; Hawse, J.R.; Subramaniam, M.; Spelsberg, T.C. Retinoblastoma binding protein-1 (RBP1) is a Runx2 coactivator and promotes osteoblastic differentiation. BMC Musculoskelet. Disord. 2010, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Senatorov, V.V.; Friedman, A.R.; Milikovsky, D.Z.; Ofer, J.; Saar-Ashkenazy, R.; Charbash, A.; Jahan, N.; Chin, G.; Mihaly, E.; Lin, J.M.; et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 2019, 11, eaaw8283. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Che, S.; Elarova, I.; Chen, Y.; Jeanneteau, F.; Kranz, T.M.; Chao, M.V.; Counts, S.E.; et al. Selective decline of neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons and hippocampus proper: Correlation with cognitive performance and neuropathology in mild cognitive impairment and Alzheimer’s disease. Hippocampus 2019, 29, 422–439. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, M.-C.; Tsai, J.-S.; He, P.-L.; Luo, W.-T.; Chiu, I.-M.; Herschman, H.R.; Li, H.-J. Exosomal 2′,3′-CNP from mesenchymal stem cells promotes hippocampus CA1 neurogenesis/neuritogenesis and contributes to rescue of cognition/learning deficiencies of damaged brain. Stem Cells Transl. Med. 2020, 9, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, B.; Ghahari, L.; Safari, M.; Shahroozian, E.; Naeimi, S. Combination therapy of the granulocyte colony stimulating factor and intravenous lipid emulsion protect the hippocampus after global ischemia in rat: Focusing on CA1 region. Metab. Brain Dis. 2020, 35, 991–997. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, P.; Li, W.; Gao, M.; He, X.; Zheng, J.; Li, X.; Wang, X.; Wang, N.; Zhang, J.; et al. Pre- and Posttreatment With Edaravone Protects CA1 Hippocampus and Enhances Neurogenesis in the Subgranular Zone of Dentate Gyrus After Transient Global Cerebral Ischemia in Rats. ASN Neuro 2014, 6, 1759091414558417. [Google Scholar] [CrossRef]
- Wall, M.; Collins, D.R.; Chery, S.L.; Allen, Z.D.; Pastuzyn, E.D.; George, A.J.; Nikolova, V.D.; Moy, S.S.; Philpot, B.D.; Shepherd, J.D.; et al. The Temporal Dynamics of Arc Expression Regulate Cognitive Flexibility. Neuron 2018, 98, 1124–1132.e7. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.D.; Bear, M.F. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 2011, 14, 279–284. [Google Scholar] [CrossRef]
- Plath, N.; Ohana, O.; Dammermann, B.; Errington, M.L.; Schmitz, D.; Gross, C.; Mao, X.; Engelsberg, A.; Mahlke, C.; Welzl, H.; et al. Arc/Arg3.1 Is Essential for the Consolidation of Synaptic Plasticity and Memories. Neuron 2006, 52, 437–444. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; He, B.; Behrle, B.L.; Fejleh, M.P.C.; Cui, L.; Paule, M.G.; Greenfield, L.J. Ischemia-Induced Increase in Microvascular Phosphodiesterase 4D Expression in Rat Hippocampus Associated with Blood Brain Barrier Permeability: Effect of Age. ACS Chem. Neurosci. 2012, 3, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, T.; Huang, Y. The Effects of Intraoperative Hypothermia on Postoperative Cognitive Function in the Rat Hippocampus and Its Possible Mechanisms. Brain Sci. 2022, 12, 96. [Google Scholar] [CrossRef]
- Soltesz, I.; Losonczy, A. CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci. 2018, 21, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, C.F.; Santos, R.M.; Barbosa, R.M.; Cadenas, E.; Radi, R.; Laranjinha, J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic. Biol. Med. 2014, 73, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Xiang, Z.; Deng, X.; Fan, T.; Fu, R.; Geng, W.; Guo, R.; He, N.; Li, C.; Li, L.; et al. Incidence of Inadvertent Intraoperative Hypothermia and Its Risk Factors in Patients Undergoing General Anesthesia in Beijing: A Prospective Regional Survey. PLoS ONE 2015, 10, e0136136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.; Zhan, L.; Lei, Y.; Xu, S.; Si, Y.; Li, S.; Xia, Z.; Shi, Y.; Gu, X.; Yu, J.; et al. Establishment and Validation of a Prediction Equation to Estimate Risk of Intraoperative Hypothermia in Patients Receiving General Anesthesia. Sci. Rep. 2017, 7, 13927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Lun, Y.; Wu, X.; Xia, Q.; Zhang, X.; Xin, S.; Zhang, J. Association between the Hypomethylation of Osteopontin and Integrin β3 Promoters and Vascular Smooth Muscle Cell Phenotype Switching in Great Saphenous Varicose Veins. Int. J. Mol. Sci. 2014, 15, 18747–18761. [Google Scholar] [CrossRef]
- Faraci, F.M.; Breese, K.R. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ. Res. 1993, 72, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Northington, F.J.; Carhuapoma, J.R.; Falck, J.R.; Harder, D.R.; Traystman, R.J.; Koehler, R.C. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-d-aspartate. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1616–H1624. [Google Scholar] [CrossRef] [Green Version]
- Buerk, D.G.; Ances, B.M.; Greenbergbc, J.H.; Detrebc, J.A. Temporal Dynamics of Brain Tissue Nitric Oxide during Functional Forepaw Stimulation in Rats. NeuroImage 2003, 18, 1–9. [Google Scholar] [CrossRef]
- Ren, X.-S.; Tong, Y.; Ling, L.; Chen, D.; Sun, H.-J.; Zhou, H.; Qi, X.-H.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; et al. NLRP3 Gene Deletion Attenuates Angiotensin II-Induced Phenotypic Transformation of Vascular Smooth Muscle Cells and Vascular Remodeling. Cell. Physiol. Biochem. 2017, 44, 2269–2280. [Google Scholar] [CrossRef]
- Cauli, B. Revisiting the role of neurons in neurovascular coupling. Front. Neuroenergetics 2010, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Li, T.; Ni, L.; Liu, X.; Wang, Z.; Liu, C. High glucose induces the expression of osteopontin in blood vessels in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016, 480, 201–207. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Ni, L.; Wang, Z.; Wang, W.; Shi, T.; Liu, X.; Liu, C. Perivascular adipose tissue alleviates inflammatory factors and stenosis in diabetic blood vessels. Biochem. Biophys. Res. Commun. 2016, 480, 147–152. [Google Scholar] [CrossRef]
- Yang, M.; Fang, J.; Liu, Q.; Wang, Y.; Zhang, Z. Role of ROS-TRPM7-ERK1/2 axis in high concentration glucose-mediated proliferation and phenotype switching of rat aortic vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2017, 494, 526–533. [Google Scholar] [CrossRef]
- Perosa, V.; Priester, A.; Ziegler, G.; Cardenas-Blanco, A.; Dobisch, L.; Spallazzi, M.; Assmann, A.; Maass, A.; Speck, O.; Oltmer, J.; et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain 2020, 143, 622–634. [Google Scholar] [CrossRef]
- Morin, J.-P.; Cerón-Solano, G.; Velázquez-Campos, G.; Pacheco-López, G.; Bermúdez-Rattoni, F.; Díaz-Cintra, S. Spatial Memory Impairment is Associated with Intraneural Amyloid-β Immunoreactivity and Dysfunctional Arc Expression in the Hippocampal-CA3 Region of a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 51, 69–79. [Google Scholar] [CrossRef]
- Fuentes, J.G.; Serrano, R.I.; Sánchez, S.C.; Donate, M.J.L.; Infante, E.R.; Jiménez, M.D.M.A.; Rabal, M.D.P.M. Neuropeptides in the developing human hippocampus under hypoxic–ischemic conditions. J. Anat. 2021, 239, 856–868. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Fan, F.; Booz, G.W.; Roman, R.J. Aging diabetes, deconstructing the cerebrovascular wall. Aging 2021, 13, 9158–9159. [Google Scholar] [CrossRef]
- Mughal, A.; Harraz, O.F.; Gonzales, A.L.; Hill-Eubanks, D.; Nelson, M.T. PIP2 Improves Cerebral Blood Flow in a Mouse Model of Alzheimer’s Disease. Function 2021, 2, zqab010. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerez, J.A.; Riek, R. Neurodegenerative diseases distinguished through protein-structure analysis. Nature 2020, 578, 223–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Hirose, D.; Hatanaka, H.; Takenoshita, N.; Kaneko, Y.; Ogawa, Y.; Sakurai, H.; Hanyu, H. Role of Neuroimaging as a Biomarker for Neurodegenerative Diseases. Front. Neurol. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [Green Version]
- Brosseron, F.; Krauthausen, M.; Kummer, M.; Heneka, M.T. Body Fluid Cytokine Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Comparative Overview. Mol. Neurobiol. 2014, 50, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, H.; Zhao, J.; Chen, C.; Leak, R.K.; Xu, Y.; Vosler, P.; Chen, J.; Gao, Y.; Zhang, F. Drug-Induced Hypothermia in Stroke Models: Does it Always Protect? CNS Neurol. Disord. -Drug Targets 2013, 12, 371–380. [Google Scholar] [CrossRef]
- Curfman, G.D. Hypothermia to Protect the Brain. N. Engl. J. Med. 2002, 346, 546. [Google Scholar] [CrossRef]
- Olsen, T.S.; Weber, U.J.; Kammersgaard, L.P. Therapeutic hypothermia for acute stroke. Lancet Neurol. 2003, 2, 410–416. [Google Scholar] [CrossRef]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267–278. [Google Scholar] [CrossRef]

| Effect | R-Squared | F | p |
|---|---|---|---|
| CD31 | 0.9478 | 36.30 | 0.0027 |
| VEGF | 0.8281 | 14.45 | 0.0051 |
| eNOS | 0.9524 | 59.98 | 0.0001 |
| ROS | 0.8021 | 12.16 | 0.0077 |
| SMMHC | 0.9100 | 30.33 | 0.0007 |
| Osteopontin | 0.9632 | 78.55 | <0.0001 |
| RBP | 0.9472 | 53.85 | 0.0001 |
| Neuronal pyknosis | 0.8866 | 23.45 | 0.0015 |
| Fluorescence intensity | 0.8617 | 18.69 | 0.0026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xu, G.; Yi, J.; Huang, Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sci. 2022, 12, 692. https://doi.org/10.3390/brainsci12060692
Li T, Xu G, Yi J, Huang Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sciences. 2022; 12(6):692. https://doi.org/10.3390/brainsci12060692
Chicago/Turabian StyleLi, Tianjia, Guangyan Xu, Jie Yi, and Yuguang Huang. 2022. "Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus" Brain Sciences 12, no. 6: 692. https://doi.org/10.3390/brainsci12060692
APA StyleLi, T., Xu, G., Yi, J., & Huang, Y. (2022). Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sciences, 12(6), 692. https://doi.org/10.3390/brainsci12060692