Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants
Abstract
1. Introduction
2. Materials and Methods
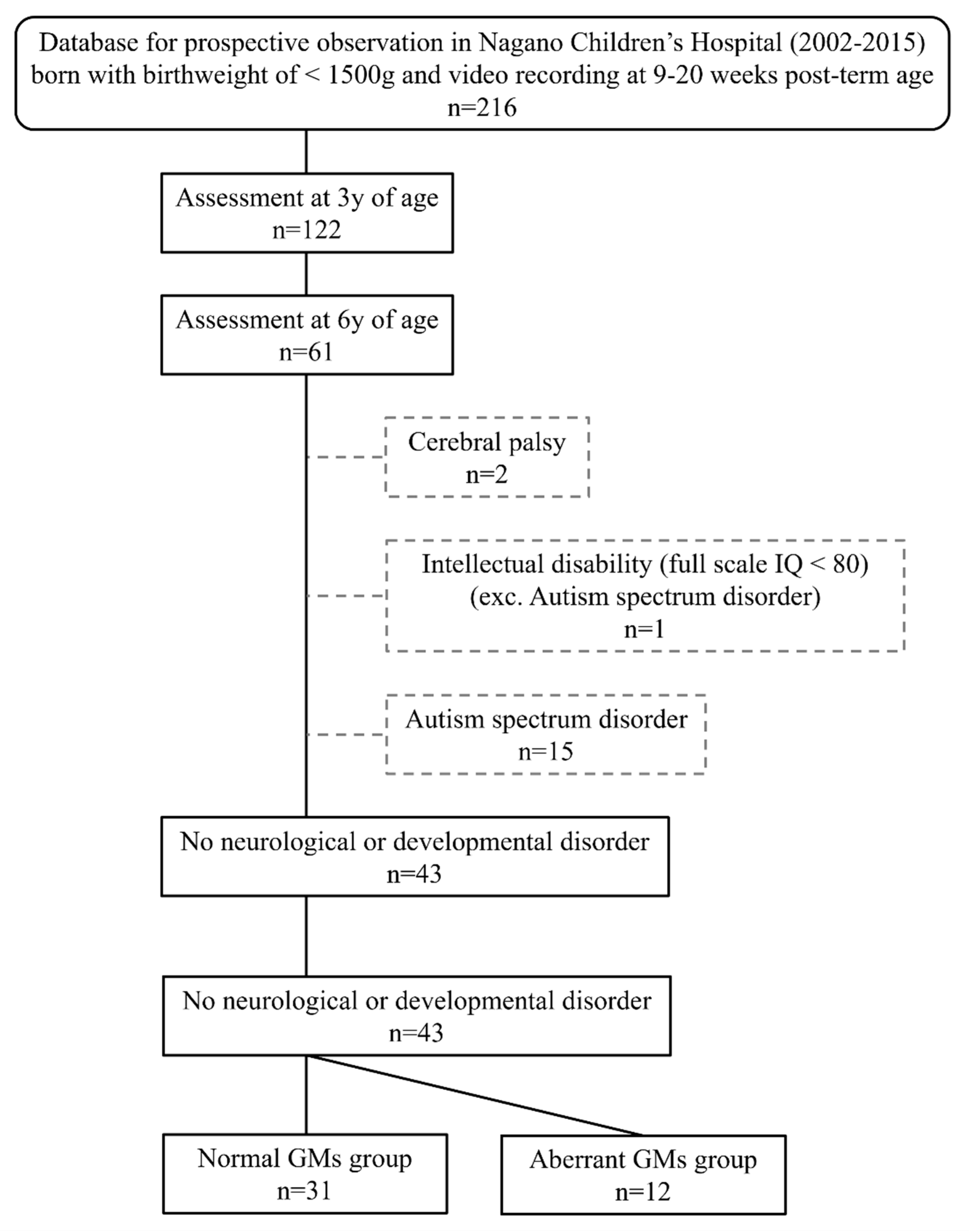
2.1. Participants
2.2. Assessment of GMs
2.3. Developmental Assessment at 3 and 6 Years of Age
2.4. Data Analysis
3. Results
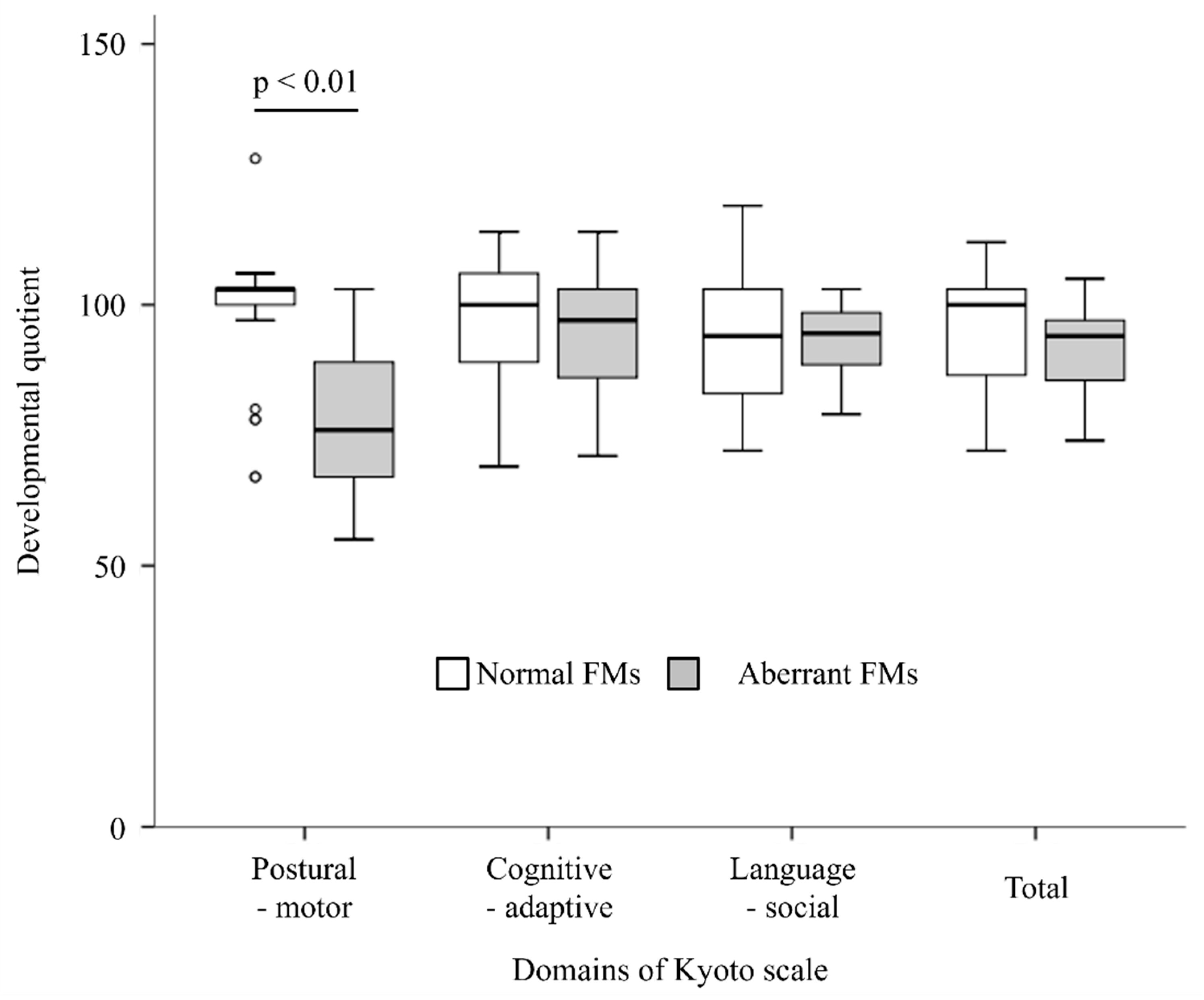
3.1. DQ at 3 Years of Age
3.2. Items Related to Motor Function in the DQ Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isayama, T.; Lee, S.K.; Mori, R.; Kusuda, S.; Fujimura, M.; Ye, X.Y.; Shah, P.S. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics 2012, 130, e957–e965. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, S.; Fujimura, M.; Uchiyama, A.; Totsu, S.; Matsunami, K. Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr. Res. 2012, 72, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Severud, R.; Kerbeshian, J.; Klug, M.G. Prenatal and perinatal risk factors for autism. J. Perinat. Med. 1999, 27, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in young adulthood for very-low-birth-weight infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.A.; De Luca, C.R.; Doyle, L.W.; Roberts, G.; Anderson, P.J. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013, 131, e1053–e1061. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Marlow, N. Preterm Birth and Childhood Psychiatric Disorders. Pediatr. Res. 2011, 69, 11–18. [Google Scholar] [CrossRef]
- Larsson, H.J.; Eaton, W.W.; Madsen, K.M.; Vestergaard, M.; Olesen, A.V.; Agerbo, E.; Schendel, D.; Thorsen, P.; Mortensen, P.B. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. Am. J. Epidemiol. 2005, 161, 916–925, discussion 916–925. [Google Scholar] [CrossRef]
- Schendel, D.; Bhasin, T.K. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 2008, 121, 1155–1164. [Google Scholar] [CrossRef]
- Williams, K.; Helmer, M.; Duncan, G.W.; Peat, J.K.; Mellis, C.M. Perinatal and maternal risk factors for autism spectrum disorders in New South Wales, Australia. Child Care Health Dev. 2008, 34, 249–256. [Google Scholar] [CrossRef]
- Edwards, J.; Berube, M.; Erlandson, K.; Haug, S.; Johnstone, H.; Meagher, M.; Sarkodee-Adoo, S.; Zwicker, J.G. Developmental Coordination Disorder in School-Aged Children Born Very Preterm and/or at Very Low Birth Weight: A Systematic Review. J. Dev. Behav. Pediatr. 2011, 32, 678–687. [Google Scholar] [CrossRef]
- Holsti, L.; Grunau, R.V.; Whitfield, M.F. Developmental coordination disorder in extremely low birth weight children at nine years. J. Dev. Behav. Pediatr. 2002, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lingam, R.; Hunt, L.; Golding, J.; Jongmans, M.; Emond, A. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: A UK population-based study. Pediatrics 2009, 123, e693–e700. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Yoon, S.W.; Mackay, M.; Petrie-Thomas, J.; Rogers, M.; Synnes, A.R. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch. Dis. Child. 2013, 98, 118–122. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.; Rigoli, D.; Piek, J. Developmental coordination disorder and internalizing problems in children: The environmental stress hypothesis elaborated. Dev. Rev. 2013, 33, 224–238. [Google Scholar] [CrossRef]
- Lingam, R.; Jongmans, M.J.; Ellis, M.; Hunt, L.P.; Golding, J.; Emond, A. Mental health difficulties in children with developmental coordination disorder. Pediatrics 2012, 129, e882–e891. [Google Scholar] [CrossRef]
- Missiuna, C.; Cairney, J.; Pollock, N.; Campbell, W.; Russell, D.J.; Macdonald, K.; Schmidt, L.; Heath, N.; Veldhuizen, S.; Cousins, M. Psychological distress in children with developmental coordination disorder and attention-deficit hyperactivity disorder. Res. Dev. Disabil. 2014, 35, 1198–1207. [Google Scholar] [CrossRef]
- Morgan, C.; Romeo, D.M.; Chorna, O.; Novak, I.; Galea, C.; Del Secco, S.; Guzzetta, A. The Pooled Diagnostic Accuracy of Neuroimaging, General Movements, and Neurological Examination for Diagnosing Cerebral Palsy Early in High-Risk Infants: A Case Control Study. J. Clin. Med. 2019, 8, 1879. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Caesar, R.; Colditz, P.B.; Cioni, G.; Boyd, R.N. Clinical tools used in young infants born very preterm to predict motor and cognitive delay (not cerebral palsy): A systematic review. Dev. Med. Child Neurol. 2021, 63, 387–395. [Google Scholar] [CrossRef]
- Prechtl, H.F. State of the art of a new functional assessment of the young nervous system. An early predictor of cerebral palsy. Early Hum. Dev. 1997, 50, 1–11. [Google Scholar] [CrossRef]
- Einspieler, C.; Prechtl, H.F.; Bos, A.F.; Ferrari, F.; Cioni, G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term, and Young Infants; Mac Keith Press: London, UK, 2004. [Google Scholar]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, J.L.; Cioni, G.; Einspieler, C.; Maathuis, C.G.; Pascale, R.; Bos, A.F. Early motor repertoire is related to level of self-mobility in children with cerebral palsy at school age. Dev. Med. Child Neurol. 2009, 51, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Prechtl, H.F.R. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Yang, H.; Bartl-Pokorny, K.D.; Chi, X.; Zang, F.-F.; Marschik, P.B.; Guzzetta, A.; Ferrari, F.; Bos, A.F.; Cioni, G. Are sporadic fidgety movements as clinically relevant as is their absence? Early Hum. Dev. 2015, 91, 247–252. [Google Scholar] [CrossRef]
- Prechtl, H.F.R.; Einspieler, C.; Cioni, G.; Bos, A.F.; Ferrari, F.; Sontheimer, D. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997, 349, 1361–1363. [Google Scholar] [CrossRef]
- Yang, H.; Einspieler, C.; Shi, W.; Marschik, P.B.; Wang, Y.; Cao, Y.; Li, H.; Liao, Y.-G.; Shao, X.-M. Cerebral palsy in children: Movements and postures during early infancy, dependent on preterm vs. full term birth. Early Hum. Dev. 2012, 88, 837–843. [Google Scholar] [CrossRef]
- Bruggink, J.L.M.; Einspieler, C.; Butcher, P.R.; Van Braeckel, K.N.J.A.; Prechtl, H.F.R.; Bos, A.F. The Quality of the Early Motor Repertoire in Preterm Infants Predicts Minor Neurologic Dysfunction at School Age. J. Pediatr. 2008, 153, 32–39.e1. [Google Scholar] [CrossRef]
- Einspieler, C.; Marschik, P.B.; Milioti, S.; Nakajima, Y.; Bos, A.F.; Prechtl, H.F.R. Are abnormal fidgety movements an early marker for complex minor neurological dysfunction at puberty? Early Hum. Dev. 2007, 83, 521–525. [Google Scholar] [CrossRef]
- Nakajima, Y.; Einspieler, C.; Marschik, P.B.; Bos, A.F.; Prechtl, H.F.R. Does a detailed assessment of poor repertoire general movements help to identify those infants who will develop normally? Early Hum. Dev. 2006, 82, 53–59. [Google Scholar] [CrossRef]
- Hyde, C.; Fuelscher, I.; Williams, J. Neurophysiological Approaches to Understanding Motor Control in DCD: Current Trends and Future Directions. Curr. Dev. Disord. Rep. 2019, 6, 78–86. [Google Scholar] [CrossRef]
- Pearsall-Jones, J.G.; Piek, J.P.; Levy, F. Developmental Coordination Disorder and cerebral palsy: Categories or a continuum? Hum. Mov. Sci. 2010, 29, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Hyde, C.; Spittle, A. Developmental Coordination Disorder and Cerebral Palsy: Is There a Continuum? Curr. Dev. Disord. Rep. 2014, 1, 118–124. [Google Scholar] [CrossRef][Green Version]
- Williams, J.; Lee, K.J.; Anderson, P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2010, 52, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Society for the Kyoto Scale of Psychological Development Test. Shinpan K Shiki Hattatsu Kensahou 2001 Nenban [The Kyoto Scale of Psychological Development Test 2001]; Nakanishiya Shuppan: Kyoto, Japan, 2008. (In Japanese) [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Spittle, A.J.; Spencer-Smith, M.M.; Cheong, J.L.; Eeles, A.L.; Lee, K.J.; Anderson, P.J.; Doyle, L.W. General movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics 2013, 132, e452–e458. [Google Scholar] [CrossRef]
- Peyton, C.; Yang, E.; Msall, M.E.; Adde, L.; Støen, R.; Fjørtoft, T.; Bos, A.F.; Einspieler, C.; Zhou, Y.; Schreiber, M.D.; et al. White Matter Injury and General Movements in High-Risk Preterm Infants. Am. J. Neuroradiol. 2017, 38, 162. [Google Scholar] [CrossRef]
- Gima, H.; Watanabe, H.; Kihara, H.; Nakano, H.; Nakamura, T.; Taga, G. Assessment of fidgety movements in low-birth-weight infants based on visual observation and computer-based analysis. Rigaku Ryoho Gaku 2017, 44, 115–123, (In Japanese, English Abstract). [Google Scholar] [CrossRef]
- Pearsall-Jones, J.G.; Piek, J.P.; Rigoli, D.; Martin, N.C.; Levy, F. An investigation into etiological pathways of DCD and ADHD using a monozygotic twin design. Twin Res. Hum. Genet. 2009, 12, 381–391. [Google Scholar] [CrossRef]
- de Kieviet, J.F.; Pouwels, P.J.; Lafeber, H.N.; Vermeulen, R.J.; van Elburg, R.M.; Oosterlaan, J. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 2014, 18, 126–133. [Google Scholar] [CrossRef]
| Normal FMs (n = 31) | Aberrant FMs (n = 12) | |
|---|---|---|
| Male/female (n) a | 12/27 | 2/10 |
| Gestational age (weeks and days), mean ± SD b | 28 w 6 d ± 3 w 0 d | 27 w 5 d ± 2 w 6 d |
| Birth weight (g), mean ± SD b | 1050.1 ± 278.1 | 826.8 ± 233.8 * |
| Apgar score at 5 min, median (range) c | 8 (3–10) | 6 (3–9) * |
| Duration of hospital stay (days), median (range) c | 74 (28–194) | 108 (28–151) |
| Age at recording of spontaneous movements (weeks and days), mean ± SD b | 54 w 1 d ± 2 w 3 d | 54 w 0 d ± 2w 0 d |
| Age at DQ assessment (years and months), median (range) c | 3 y 0 m (2 y 11 m–3 y 2m) | 3 y 0 m (2 y 11 m–3 y 1 m) |
| Age at IQ assessment (years and months), median (range) c | 5 y 7 m (5 y 6 m–6 y 2 m) | 5 y 7 m (5 y 6 m–5 y 10 m) |
| Classification | Definition |
|---|---|
| Normal FMs | FMs are small movements of moderate speed and variable acceleration of neck, trunk, and limbs in all directions that are continual in the awake infant during fussing and crying. |
| Absence of FMs | No FMs can be observed, although other movements can occur. |
| Abnormal FMs | Abnormal FMs look like normal FMs, but their amplitude, speed, and jerkiness are moderately or greatly exaggerated. Abnormal FMs are rare. |
| Test Items Related to Motor Function | Normal FMs (n = 31) | Aberrant FMs (n = 12) | |
|---|---|---|---|
| 1 | Jump up with both feet in two or three repetitions | 93.5 | 83.3 |
| 2 | Jump at least two to three steps forward on one leg | 3.2 | 0 |
| 3 | Climb up and down the stairs by grasping the handrail (both feet may be placed together in each step) | 100 | 100 |
| 4 | Climb the stairs with alternating legs | 93.5 | 41.7 * |
| 5 | Jump from a 15–20 cm platform | 90.3 | 50.0 * |
| 6 | Stack five building blocks | 100 | 100 |
| 7 | Stack blocks to imitate a gate | 9.7 | 0 |
| 8 | Insert square plates into a hole | 100 | 100 |
| 9 | Fit the board into the frame | 100 | 100 |
| 10 | Fold paper (origami) | 29.0 | 33.3 |
| 11 | Hold boxes in hand and compare the weights | 22.6 | 8.3 |
| 12 | Draw a circular or spiral pattern | 100 | 100 |
| 13 | Draw a horizontal line | 93.5 | 91.7 |
| 14 | Draw a vertical line | 96.8 | 91.7 |
| 15 | Draw a cross | 64.5 | 66.7 |
| 16 | Draw a circle | 38.7 | 33.3 |
| 17 | Draw the necessary parts of an unfinished portrait | 9.7 | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gima, H.; Nakamura, T. Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants. Brain Sci. 2022, 12, 686. https://doi.org/10.3390/brainsci12060686
Gima H, Nakamura T. Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants. Brain Sciences. 2022; 12(6):686. https://doi.org/10.3390/brainsci12060686
Chicago/Turabian StyleGima, Hirotaka, and Tomohiko Nakamura. 2022. "Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants" Brain Sciences 12, no. 6: 686. https://doi.org/10.3390/brainsci12060686
APA StyleGima, H., & Nakamura, T. (2022). Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants. Brain Sciences, 12(6), 686. https://doi.org/10.3390/brainsci12060686





