Effects of Dynamic Sitting Exercise with Delayed Visual Feedback in the Early Post-Stroke Phase: A Pilot Double-Blinded Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Apparatus
2.4. Intervention
2.5. Outcomes
2.6. Statistical Analysis
3. Results
Effects of the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.; Tallis, R.C. Balance Disability After Stroke. Phys. Ther. 2006, 86, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasseel-Ponche, S.; Yelnik, A.P.; Bonan, I.V. Motor Strategies of Postural Control After Hemispheric Stroke. Neurophysiol. Clin. 2015, 45, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Geurts, A.C.; de Haart, M.; van Nes, I.J.; Duysens, J. A Review of Standing Balance Recovery from Stroke. Gait Posture 2005, 22, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.T.; Wang, C.M.; Chung, C.Y.; Chen, C.L. Effects of Visual Feedback Rhythmic Weight-Shift Training on Hemiplegic Stroke Patients. Clin. Rehabil. 2004, 18, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Taly, A.B.; Gupta, A.; Kumar, S.; Murali, T. Post-Stroke Balance Training: Role of Force Platform with Visual Feedback Technique. J. Neurol. Sci. 2009, 287, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.S. Balance Retraining After Stroke Using Force Platform Biofeedback. Phys. Ther. 1997, 77, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, L.; Giannoni, P.; Marinelli, L.; Casadio, M. Effects of Continuous Visual Feedback During Sitting Balance Training in Chronic Stroke Survivors. J. Neuroeng. Rehabil. 2017, 14, 107. [Google Scholar] [CrossRef]
- Wiskerke, E.; van Dijk, M.; Thuwis, R.; Vandekerckhove, C.; Myny, C.; Kool, J.; Dejaeger, E.; Beyens, H.; Verheyden, G. Maximum Weight-Shifts in Sitting in Non-Ambulatory People with Stroke Are Related to Trunk Control and Balance: A Cross-Sectional Study. Gait Posture 2021, 83, 121–126. [Google Scholar] [CrossRef]
- Morgan, P. The Relationship Between Sitting Balance and Mobility Outcome in Stroke. Aust. J. Physiother. 1994, 40, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Veerbeek, J.M.; Van Wegen, E.E.; Harmeling-Van der Wel, B.C.H.; Kwakkel, G.; EPOS Investigators. Is Accurate Prediction of Gait in Nonambulatory Stroke Patients Possible Within 72 Hours Poststroke? The EPOS Study. Neurorehabilit. Neural Repair 2011, 25, 268–274. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, J.W.; Kim, B.R.; Jung, H.J.; Choi, D.H.; Lee, J. Predicting Independence of Gait by Assessing Sitting Balance Through Sitting Posturography in Patients with Subacute Hemiplegic Stroke. Top. Stroke Rehabil. 2021, 28, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, G.; Nieuwboer, A.; De Wit, L.D.; Feys, H.; Schuback, B.; Baert, I.; Jenni, W.; Schupp, W.; Thijs, V.; De Weerdt, W. Trunk Performance After Stroke: An Eye Catching Predictor of Functional Outcome. J. Neurol. Neurosurg. Psychiatry 2007, 78, 694–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, S.; Yamashita, H.; Shigenobu, M.; Watanabe, A.; Hiramoto, K.; Takimoto, Y.; Arakawa, R.; Kurisu, K.; Yamawaki, S.; Kitaoka, T. Sitting Balance as an Early Predictor of Functional Improvement in Association with Depressive Symptoms in Stroke Patients. Psychiatry Clin. Neurosci. 2007, 61, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Van Nes, I.J.; Nienhuis, B.; Latour, H.; Geurts, A.C. Posturographic Assessment of Sitting Balance Recovery in the Subacute Phase of Stroke. Gait Posture 2008, 28, 507–512. [Google Scholar] [CrossRef]
- Fujino, Y.; Amimoto, K.; Fukata, K.; Ishihara, S.; Makita, S.; Takahashi, H. Does Training Sitting Balance on a Platform Tilted 10° to the Weak Side Improve Trunk Control in the Acute Phase After Stroke? a Randomized, Controlled Trial. Top. Stroke Rehabil. 2016, 23, 43–49. [Google Scholar] [CrossRef]
- Dai, S.; Piscicelli, C.; Clarac, E.; Baciu, M.; Hommel, M.; Pérennou, D. Balance, Lateropulsion, and Gait Disorders in Subacute Stroke. Neurology 2021, 96, e2147–e2159. [Google Scholar] [CrossRef]
- Taylor, D.; Ashburn, A.; Ward, C.D. Asymmetrical Trunk Posture, Unilateral Neglect and Motor Performance Following Stroke. Clin. Rehabil. 1994, 8, 48–52. [Google Scholar] [CrossRef]
- Tessem, S.; Hagstrøm, N.; Fallang, B. Weight Distribution in Standing and Sitting Positions, and Weight Transfer During Reaching Tasks, in Seated Stroke Subjects and Healthy Subjects. Physiother. Res. Int. 2007, 12, 82–94. [Google Scholar] [CrossRef]
- Verheyden, G.; van Duijnhoven, H.J.; Burnett, M.; Littlewood, J.; Kunkel, D.; Ashburn, A.M.; Stroke Association Rehabilitation Research Centre. Kinematic Analysis of Head, Trunk, and Pelvis Movement When People Early After Stroke Reach Sideways. Neurorehabilit. Neural Repair. 2011, 25, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Kerr, A.; Clark, A.; Pomeroy, V.M. Neuromechanical Differences Between Successful and Failed Sit-to-Stand Movements and Response to Rehabilitation Early After Stroke. Neurorehabilit. Neural Repair 2019, 33, 395–403. [Google Scholar] [CrossRef]
- Dean, C.M.; Channon, E.F.; Hall, J.M. Sitting Training Early After Stroke Improves Sitting Ability and Quality and Carries Over to Standing up but Not to Walking: A Randomised Controlled Trial. Aust. J. Physiother. 2007, 53, 97–102. [Google Scholar] [CrossRef]
- Grant, P.M.; Dall, P.M.; Kerr, A. Daily and Hourly Frequency of the Sit to Stand Movement in Older Adults: A Comparison of Day Hospital, Rehabilitation Ward and Community Living Groups. Aging Clin. Exp. Res. 2011, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.T.; Liaw, M.Y.; Wong, M.K.; Tang, F.T.; Lee, M.Y.; Lin, P.S. The Sit-to-Stand Movement in Stroke Patients and Its Correlation with Falling. Arch. Phys. Med. Rehabil. 1998, 79, 1043–1046. [Google Scholar] [CrossRef]
- Pollock, A.; Gray, C.; Culham, E.; Durward, B.R.; Langhorne, P. Interventions for Improving Sit-to-Stand Ability Following Stroke. Cochrane Database Syst. Rev. 2014, 2014, CD007232. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An Internal Model for Sensorimotor Integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef]
- Imamizu, H.; Miyauchi, S.; Tamada, T.; Sasaki, Y.; Takino, R.; Pütz, B.; Yoshioka, T.; Kawato, M. Human Cerebellar Activity Reflecting an Acquired Internal Model of a New Tool. Nature 2000, 403, 192–195. [Google Scholar] [CrossRef]
- Wolpert, D.M. Computational Approaches to Motor Control. Trends Cogn. Sci. 1997, 1, 209–216. [Google Scholar] [CrossRef]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Wolpert, D.M.; Miall, R.C.; Kawato, M. Internal Models in the Cerebellum. Trends Cogn. Sci. 1998, 2, 338–347. [Google Scholar] [CrossRef]
- Rougier, P. Optimising the Visual Feedback Technique for Improving Upright Stance Maintenance by Delaying Its Display: Behavioural Effects on Healthy Adults. Gait Posture 2004, 19, 154–163. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Näf, O.B.; Bauer, C.M.; Zange, C.; Rast, F.M. Validity and Variability of Center of Pressure Measures to Quantify Trunk Control in Stroke Patients During Quiet Sitting and Reaching Tasks. Gait Posture 2020, 76, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Benaim, C.; Pérennou, D.A.; Villy, J.; Rousseaux, M.; Pelissier, J.Y. Validation of a Standardized Assessment of Postural Control in Stroke Patients: The Postural Assessment Scale for Stroke Patients (PASS). Stroke 1999, 30, 1862–1868. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.F.; Hsueh, I.P.; Tang, P.F.; Sheu, C.F.; Hsieh, C.L. Analysis and Comparison of the Psychometric Properties of Three Balance Measures for Stroke Patients. Stroke 2002, 33, 1022–1027. [Google Scholar] [CrossRef]
- Whitney, S.L.; Wrisley, D.M.; Marchetti, G.F.; Gee, M.A.; Redfern, M.S.; Furman, J.M. Clinical Measurement of Sit-to-Stand Performance in People with Balance Disorders: Validity of Data for the Five-Times-Sit-to-Stand Test. Phys. Ther. 2005, 85, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, T.; Liu, M.; Sonoda, S.; Domen, K.; Chino, N. The Stroke Impairment Assessment Set: Its Internal Consistency and Predictive Validity. Arch. Phys. Med. Rehabil. 2000, 81, 863–868. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A New Tool to Measure Motor Impairment of the Trunk After Stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical Gait Assessment in the Neurologically Impaired. Reliability and Meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The Structure and Stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Fielding, S.; Fayers, P.; Ramsay, C.R. Analysing Randomised Controlled Trials With Missing Data: Choice of Approach Affects Conclusions. Contemp. Clin. Trials. 2012, 33, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods. 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukata, K.; Amimoto, K.; Inoue, M.; Sekine, D.; Inoue, M.; Fujino, Y.; Makita, S.; Takahashi, H. Effects of Diagonally Aligned Sitting Training with a Tilted Surface on Sitting Balance for Low Sitting Performance in the Early Phase After Stroke: A Randomised Controlled Trial. Disabil. Rehabil. 2021, 43, 1973–1981. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Yagi, M.; Yasunaga, H.; Matsui, H.; Morita, K.; Fushimi, K.; Fujimoto, M.; Koyama, T.; Fujitani, J. Impact of Rehabilitation on Outcomes in Patients with Ischemic Stroke: A Nationwide Retrospective Cohort Study in Japan. Stroke 2017, 48, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Amimoto, K.; Chiba, Y.; Sekine, D.; Fukata, K.; Fujino, Y.; Takahashi, H.; Makita, S. Effect of Exercise Involving Standing Weight Shifting to the Nonparetic Side on an Inclined Surface in the Early Phase After a Stroke: A Randomized Controlled Trial. Phys. Ther. 2021, 101, pzab114. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, B.; Mansfield, A. Visual Feedback of the Centre of Gravity to Optimize Standing Balance. Gait Posture 2015, 41, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.W.; Hu, M.H.; Tang, P.F.; Sheu, C.F.; Hsieh, C.L. A Comparison of Psychometric Properties of the Smart Balance Master System and the Postural Assessment Scale for Stroke in People Who Have Had Mild Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 374–380. [Google Scholar] [CrossRef]
- Genthon, N.; Rougier, P.; Gissot, A.S.; Froger, J.; Pélissier, J.; Pérennou, D. Contribution of Each Lower Limb to Upright Standing in Stroke Participants. Stroke 2008, 39, 1793–1799. [Google Scholar] [CrossRef]

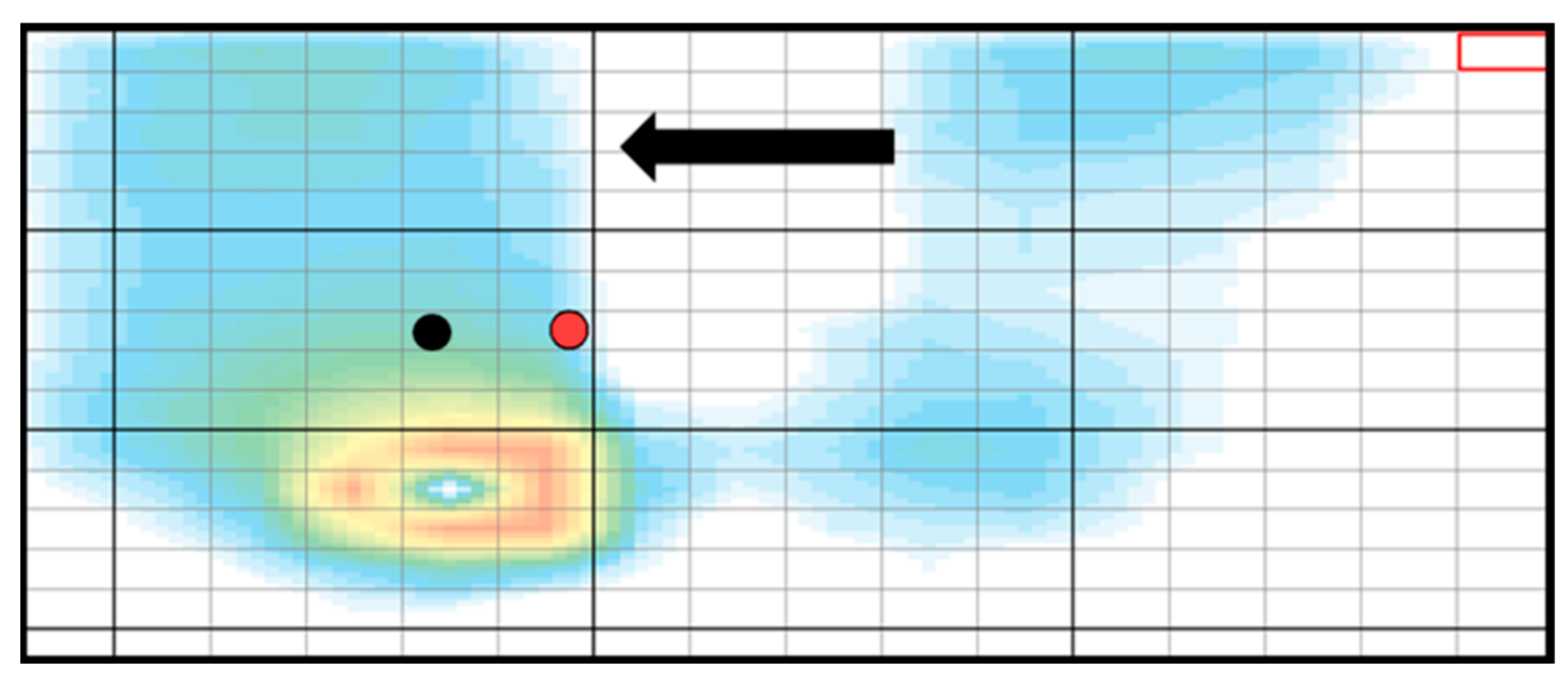
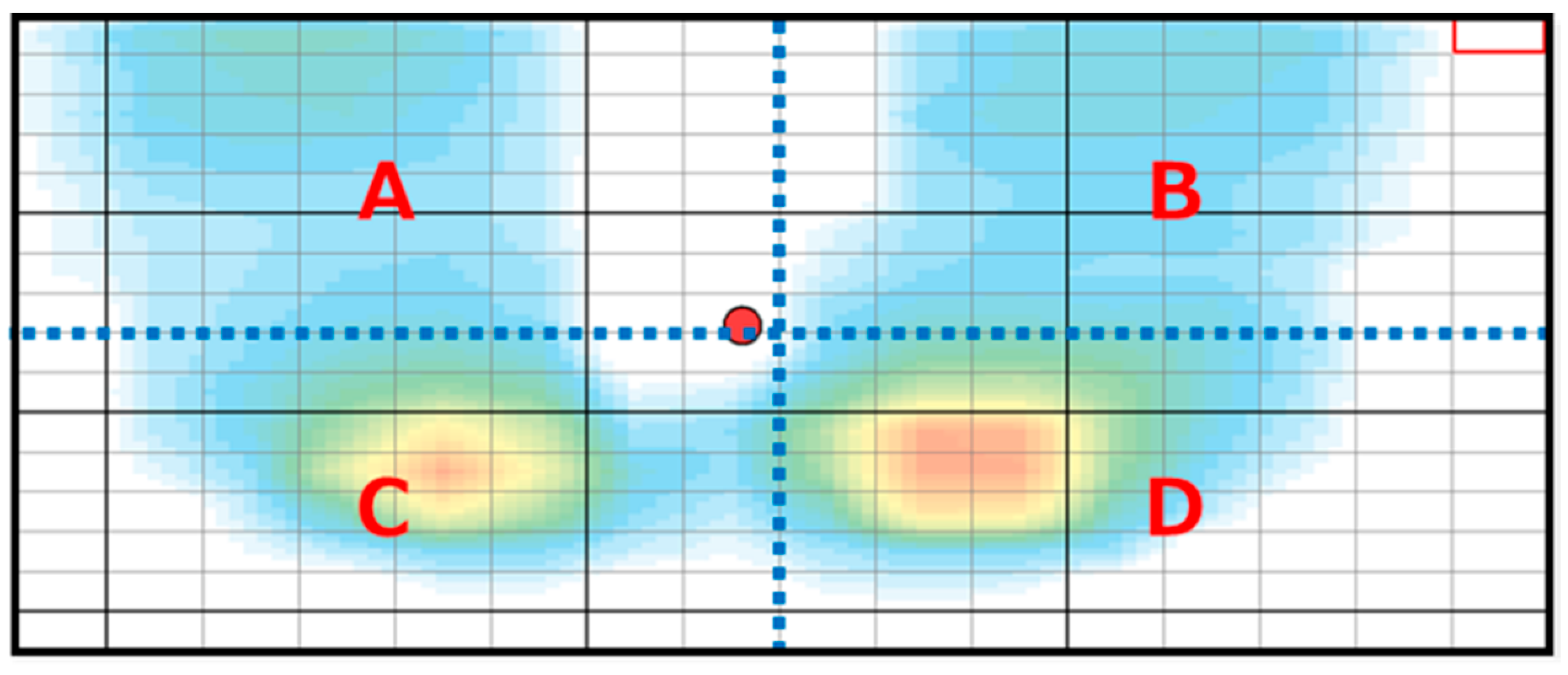

| Experimental Group | Control Group | p Value | |
|---|---|---|---|
| Sex (female/male) | 4/9 | 4/10 | 0.901 |
| Age (years), mean ± SD | 67.2 ± 14.3 | 66.1 ± 11.6 | 0.841 |
| Lesion side (right/left) | 6/7 | 9/5 | 0.343 |
| Etiology (infarction/hemorrhage) | 8/5 | 7/7 | 0.547 |
| Days from stroke onset (days), mean ± SD | 14.2 ± 4.0 | 17.5 ± 6.9 | 0.148 |
| Length of hospital stay (days), mean ± SD | 29.6 ± 7.4 | 31.9 ± 10.2 | 0.508 |
| SIAS-motor (0/1/2/3/4/5) | |||
| Upper limb | |||
| Knee to mouth | 5/1/1/4/2/0 | 3/3/3/2/3/0 | |
| Finger function | 6/1/0/4/2/0 | 5/3/2/1/3/0 | |
| Lower limb | |||
| Hip flexion | 4/3/0/4/2/0 | 1/4/3/3/3/0 | |
| Knee extension | 4/2/2/2/3/0 | 5/1/0/4/4/0 | |
| Foot tap | 8/0/0/2/3/0 | 5/1/3/4/1/0 | |
| Movement direction of lateral dynamic sitting exercise (paretic side/non-paretic side) | 8/5 | 7/7 | 0.547 |
| Experimental Group | Control Group | Group Effect | Time Effect | Interaction Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | F Value (df) | p Value | F Value (df) | p Value | F Value (df) | p Value |
| Main outcome | ||||||||||
| PASS (Primary outcome) | 21.8 (18.3, 25.2) | 28.4 (24.9, 31.9) | 21.7 (18.4, 25.0) | 25.3 (21.9, 28.8) | 0.467 (1, 25) | 0.501 | 63.299 (1, 21) | <0.001 * | 5.425 (1, 21) | 0.030 |
| Secondary outcome | ||||||||||
| Static sitting task | ||||||||||
| Mean velocity of COP (mm/s) | 1.7 (1.3, 2.2) | 1.3 (0.8, 1.8) | 2.1 (1.6, 2.5) | 1.5 (1.0, 2.0) | 0.756 (1, 26) | 0.392 | 9.039 (1, 24) | 0.006 | 0.202 (1, 24) | 0.657 |
| Mean percentage BW on the paretic side (%) | 50.4 (46.4, 54.4) | 47.7 (43.5, 51.8) | 46.9 (43.1, 50.7) | 50.0 (45.8, 54.3) | 0.071 (1, 23) | 0.793 | 0.011 (1, 22) | 0.917 | 2.536 (1, 22) | 0.125 |
| Lateral sitting task to the paretic side | ||||||||||
| Mean velocity of COP (mm/s) | 2.8 (1.8, 3.7) | 2.8 (1.8, 3.7) | 3.6 (2.6, 4.5) | 3.4 (2.4, 4.3) | 1.296 (1, 25) | 0.266 | 0.235 (1, 21) | 0.633 | 0.251 (1, 21) | 0.622 |
| Mean percentage BW on the paretic side (%) | 69.7 (64.0, 75.3) | 84.3 (78.5, 90.2) | 77.5 (72.1, 83.0) | 82.3 (76.4, 88.3) | 0.719 (1, 26) | 0.404 | 22.540 (1, 24) | <0.001 * | 5.832 (1, 24) | 0.024 |
| Lateral sitting task to the non-paretic side | ||||||||||
| Mean velocity of COP (mm/s) | 2.4 (1.9, 2.9) | 2.4 (1.9, 2.9) | 2.6 (2.2, 3.1) | 2.2 (1.7, 2.8) | 0.014 (1, 25) | 0.907 | 1.104 (1, 23) | 0.304 | 0.817 (1, 23) | 0.375 |
| Mean percentage BW on the non-paretic side (%) | 74.0 (68.8, 79.2) | 83.0 (77.7, 88.4) | 76.8 (71.8, 81.8) | 77.7 (72.3, 83.2) | 0.157 (1, 24) | 0.696 | 7.629 (1, 22) | 0.011 | 5.025 (1, 22) | 0.035 |
| Forward sitting task | ||||||||||
| Mean velocity of COP (mm/s) | 2.6 (1.7, 3.5) | 2.6 (1.7, 3.5) | 3.3 (2.4, 4.1) | 3.5 (2.6, 4.4) | 1.910 (1, 22) | 0.181 | 0.179 (1, 19) | 0.677 | 0.270 (1, 19) | 0.609 |
| Mean percentage BW on the anterior direction (%) | 38.3 (34.4, 42.2) | 54.8 (50.8, 58.8) | 36.6 (33.0, 40.2) | 59.3 (55.4, 63.3) | 1.199 (1, 19) | 0.287 | 70.087 (1, 22) | <0.001 * | 1.757 (1, 22) | 0.199 |
| Five-times sit-to-stand task | 1.8 (0.7, 3.0) | 4.1 (3.0, 5.3) | 2.1 (1.1, 3.2) | 2.9 (1.7, 4.0) | 0.464 (1, 25) | 0.502 | 21.566 (1, 22) | <0.001 * | 5.728 (1, 22) | 0.026 |
| SIAS | 49.2 (42.9, 55.4) | 52.0 (45.7, 58.3) | 48.9 (42.8, 54.9) | 52 (45.5, 57.7) | 0.007 (1, 25) | 0.934 | 27.722 (1, 21) | <0.001 * | 0.011 (1, 21) | 0.917 |
| TIS | 12.1 (9.6, 14.6) | 17.2 (14.6, 19.7) | 13.0 (10.6, 15.4) | 16.0 (13.5, 18.6) | 0.005 (1, 25) | 0.945 | 40.875 (1, 22) | <0.001 * | 2.676 (1, 22) | 0.116 |
| FAC | 1.8 (1.4, 2.3) | 2.4 (2.0, 2.9) | 1.8 (1.4, 2.2) | 2.2 (1.8, 2.7) | 0.175 (1, 26) | 0.680 | 23.910 (1, 22) | <0.001 * | 0.312 (1, 22) | 0.582 |
| FIM-motor | 39.2 (32.2, 46.2) | 45.9 (38.8, 52.9) | 37.6 (30.9, 44.4) | 46.7 (39.8, 53.6) | 0.006 (1, 25) | 0.938 | 50.5 (1, 21) | <0.001 * | 1.223 (1, 21) | 0.281 |
| Outcomes | Power (1−β) | Effect Size | Mean Differences |
|---|---|---|---|
| Primary outcome | |||
| PASS | 0.55 | 0.84 | 3.08 |
| Secondary outcome | |||
| Static sitting task | |||
| Mean velocity of COP (mm/s) | 0.07 | 0.18 | 0.15 |
| Mean percentage BW on the paretic side (%) | 0.05 | 0.04 | 0.41 |
| Dynamic sitting task to the paretic side | |||
| Mean velocity of COP (mm/s) | 0.06 | 0.09 | 0.11 |
| Mean percentage BW on the paretic side (%) | 0.46 | 0.74 | 9.95 |
| Dynamic sitting task to the non-paretic side | |||
| Mean velocity of COP (mm/s) | 0.13 | 0.33 | 0.36 |
| Mean percentage BW on the non-paretic side (%) | 0.98 | 1.58 | 8.07 |
| Dynamic sitting to the anterior direction | |||
| Mean velocity of COP (mm/s) | 0.28 | 0.56 | 0.21 |
| Mean percentage BW on the anterior direction (%) | 0.19 | 0.43 | 6.56 |
| Number of successes in the five-times sit-to-stand task | 0.51 | 0.80 | 1.62 |
| SIAS | 0.05 | 0.03 | 0.11 |
| TIS | 0.35 | 0.63 | 2.19 |
| FAC | 0.07 | 0.15 | 0.12 |
| FIM-motor | 0.13 | 0.34 | 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, M.; Amimoto, K.; Shida, K.; Sekine, D.; Hasegawa, D.; Fukata, K.; Fujino, Y.; Makita, S.; Takahashi, H. Effects of Dynamic Sitting Exercise with Delayed Visual Feedback in the Early Post-Stroke Phase: A Pilot Double-Blinded Randomized Controlled Trial. Brain Sci. 2022, 12, 670. https://doi.org/10.3390/brainsci12050670
Inoue M, Amimoto K, Shida K, Sekine D, Hasegawa D, Fukata K, Fujino Y, Makita S, Takahashi H. Effects of Dynamic Sitting Exercise with Delayed Visual Feedback in the Early Post-Stroke Phase: A Pilot Double-Blinded Randomized Controlled Trial. Brain Sciences. 2022; 12(5):670. https://doi.org/10.3390/brainsci12050670
Chicago/Turabian StyleInoue, Masahide, Kazu Amimoto, Kohei Shida, Daisuke Sekine, Daichi Hasegawa, Kazuhiro Fukata, Yuji Fujino, Shigeru Makita, and Hidetoshi Takahashi. 2022. "Effects of Dynamic Sitting Exercise with Delayed Visual Feedback in the Early Post-Stroke Phase: A Pilot Double-Blinded Randomized Controlled Trial" Brain Sciences 12, no. 5: 670. https://doi.org/10.3390/brainsci12050670
APA StyleInoue, M., Amimoto, K., Shida, K., Sekine, D., Hasegawa, D., Fukata, K., Fujino, Y., Makita, S., & Takahashi, H. (2022). Effects of Dynamic Sitting Exercise with Delayed Visual Feedback in the Early Post-Stroke Phase: A Pilot Double-Blinded Randomized Controlled Trial. Brain Sciences, 12(5), 670. https://doi.org/10.3390/brainsci12050670






