Pentraxin-3 in the Spinal Dorsal Horn Upregulates Nectin-1 Expression in Neuropathic Pain after Spinal Nerve Damage in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Surgical Procedure
2.3. Reagents and Administration
2.4. Behavioral Tests
2.5. Immunoblot
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
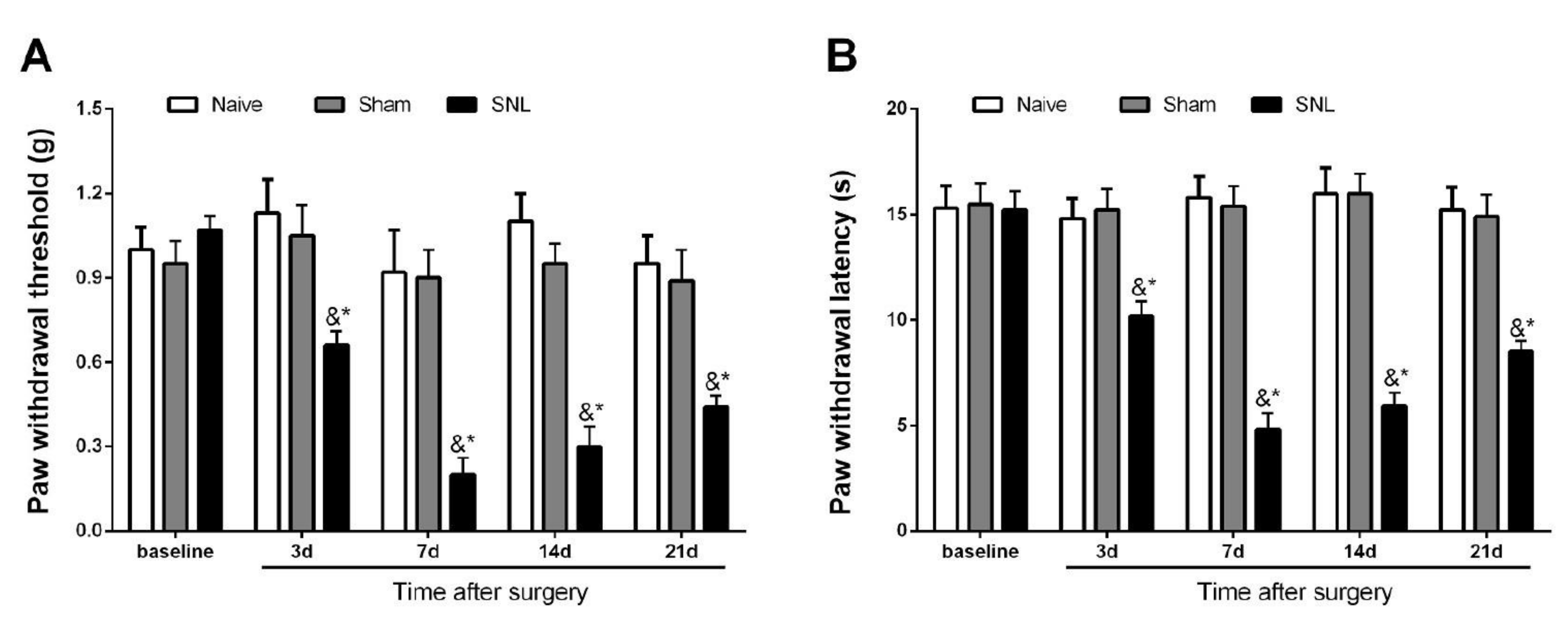
3.1. Initiation and Persistence of Mechanical Allodynia and Thermal Hyperalgesia Following Spinal Nerve Trauma in Male Mice
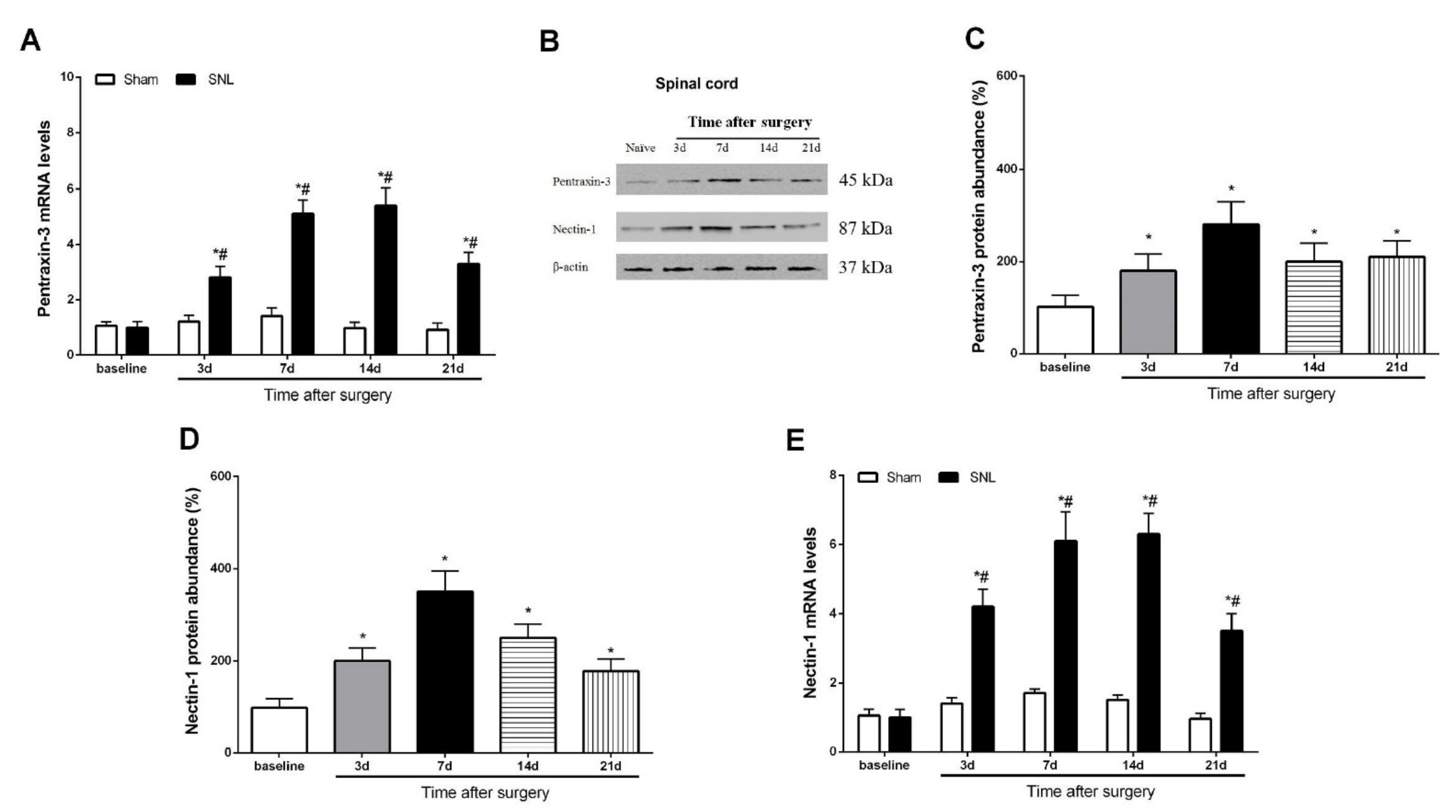
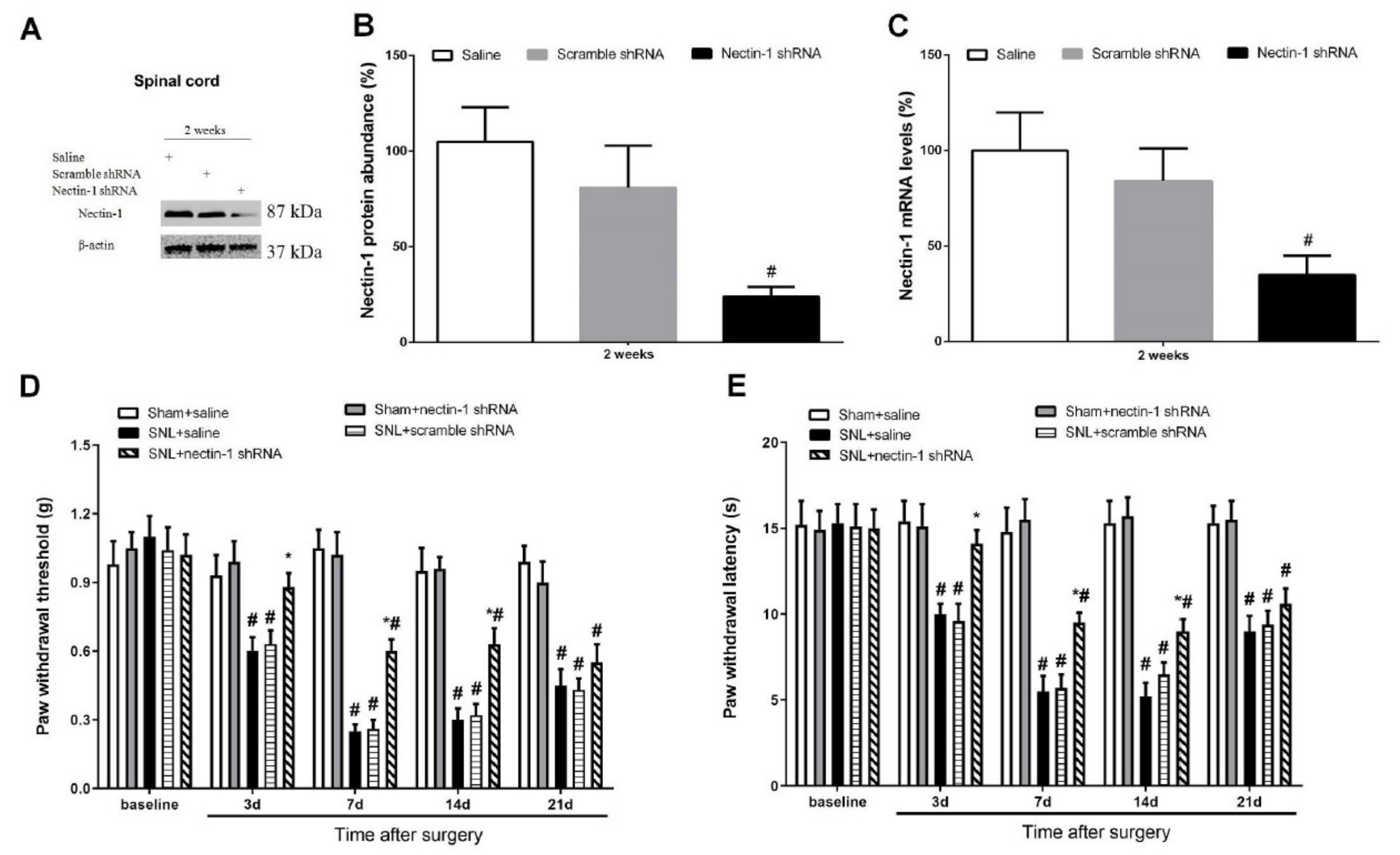
3.2. Pentraxin-3 and Nectin-1 Are Upregulated in the Spinal Cord Dorsal Horn upon Spinal Nerve Injury in Male Mice
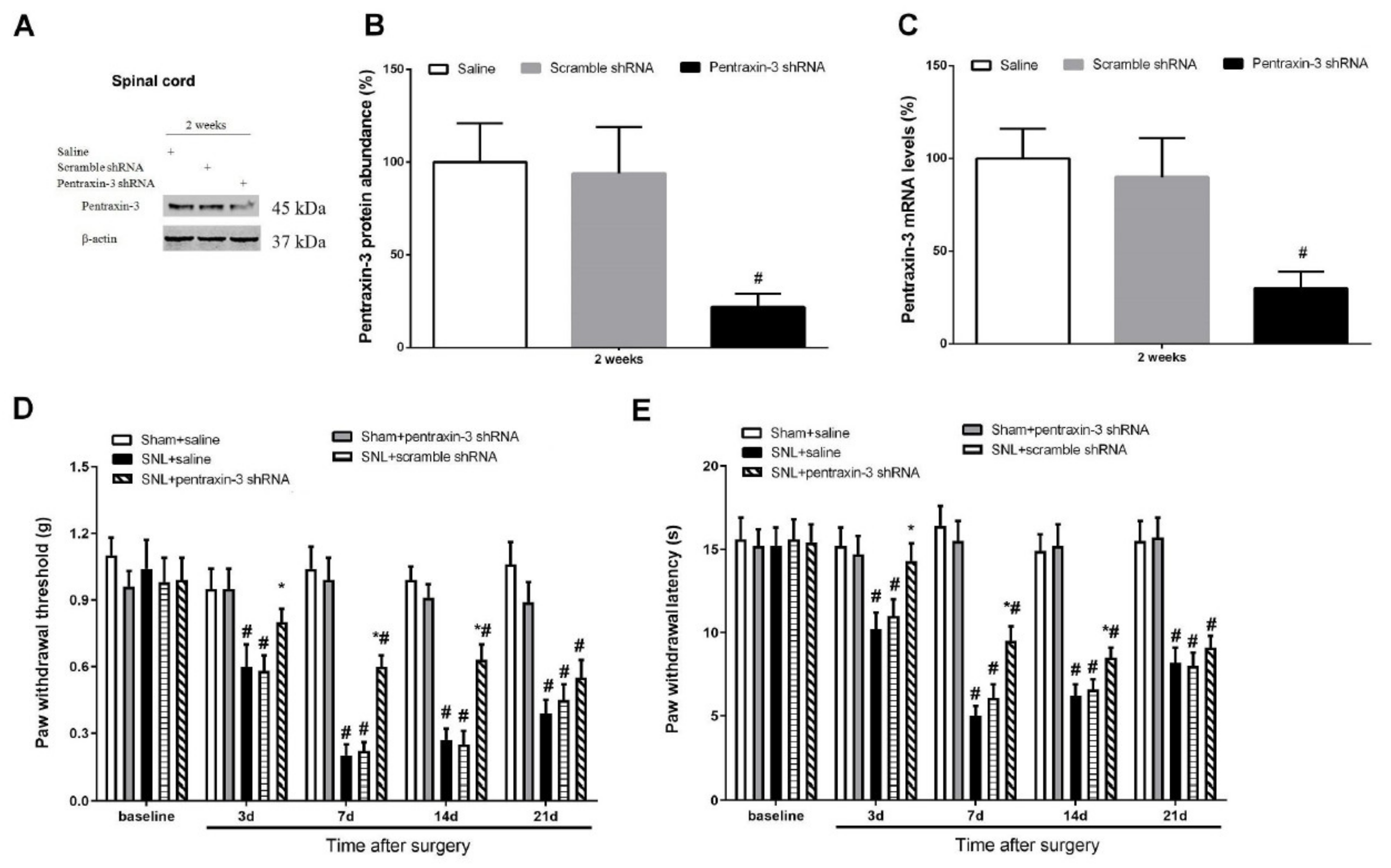
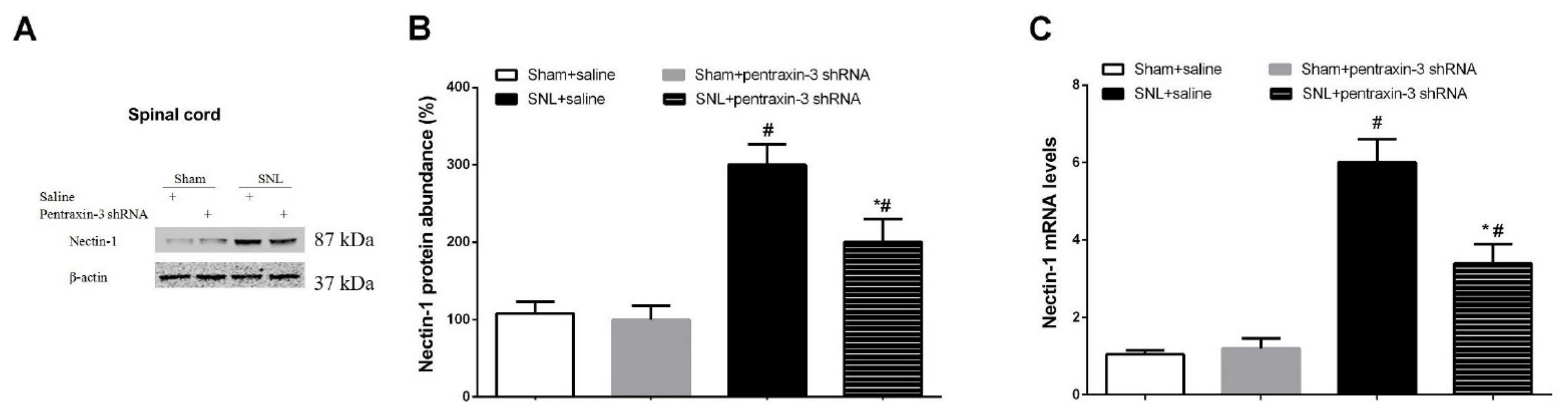
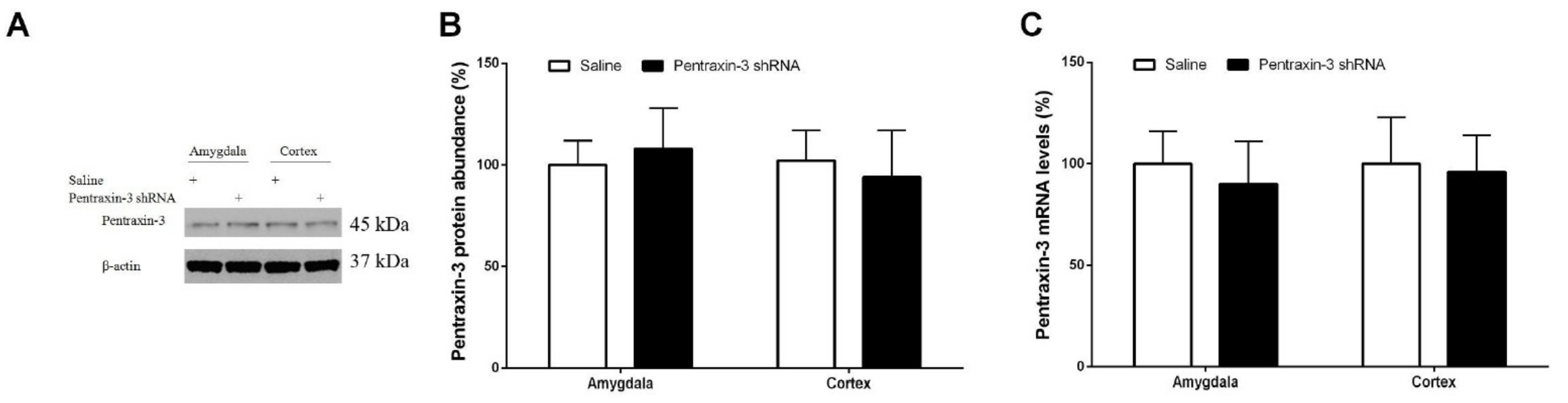
3.3. Pentraxin-3 Knockdown Reduces Neuropathic Pain Behaviors and Spinal Nectin-1 Expression after Peripheral Nerve Damage in Male Mice
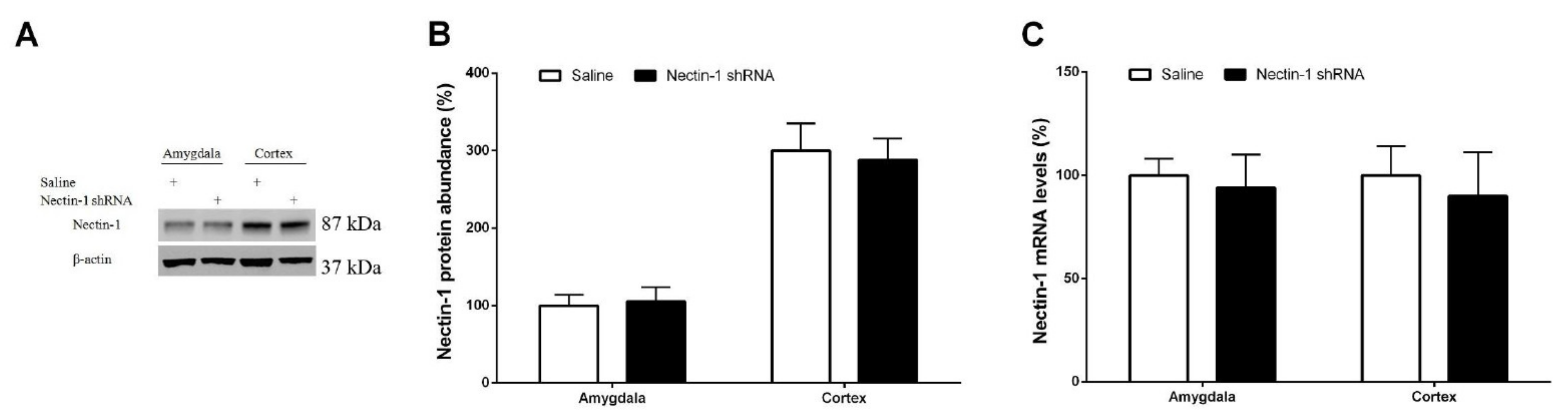
3.4. Spinal Nectin-1 Deficiency Impairs the Development of Neuropathic Pain after Peripheral Nerve Damage in Male Mice
3.5. Inhibition of Recombinant Pentraxin-3 Evokes Acute Pain Behaviors by Spinal Nectin-1 Knockdown in Male Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johannes, C.B.; Le, T.K.; Zhou, X.; Johnston, J.A.; Dworkin, R.H. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J. Pain 2010, 11, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- St John Smith, E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef]
- Luo, C.; Kuner, T.; Kuner, R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014, 37, 343–355. [Google Scholar] [CrossRef]
- Liu, P.; Song, C.; Wang, C.; Li, Y.; Su, L.; Li, J.; Zhao, Q.; Wang, Z.; Shen, M.; Wang, G.; et al. Spinal SNAP-25 regulates membrane trafficking of GluA1- containing AMPA receptors in spinal injury-induced neuropathic pain in rats. Neurosci. Lett. 2020, 715, 134616. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Xie, X.; Zhao, H.; Gao, Y.; Li, Y.; Xu, X.; Zhang, X.; Ke, C.; Liu, J. Promotion of bone cancer pain development by decorin is accompanied by modification of excitatory synaptic molecules in the spinal cord. Mol. Pain 2019, 15, 1744806919864253. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Wang, Z.; Song, C.; Yu, Y.; Wang, G.; Li, J.; Wang, C.; Zhang, L. Spinal caspase-6 regulates AMPA receptor trafficking and dendritic spine plasticity through netrin-1 in postoperative pain after orthopedic surgery for tibial fracture in mice. Pain 2020, 162, 124–134. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Zhao, Q.; Li, Y.; Song, C.; Wang, C.; Yu, Y.; Wang, G. Spinal Protein Kinase Mζ Regulates α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor Trafficking and Dendritic Spine Plasticity via Kalirin-7 in the Pathogenesis of Remifentanil-induced Postincisional Hyperalgesia in Rats. Anesthesiology 2018, 129, 173–186. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, Y.; Song, C.; Liu, P.; Wang, C.; Li, Y.; Cui, W.; Xie, K.; Zhang, L.; Wang, G. Spinal hevin mediates membrane trafficking of GluA1-containing AMPA receptors in remifentanil-induced postoperative hyperalgesia in mice. Neurosci. Lett. 2020, 722, 134855. [Google Scholar] [CrossRef]
- Bottazzi, B.; Garlanda, C.; Salvatori, G.; Jeannin, P.; Manfredi, A.; Mantovani, A. Pentraxins as a key component of innate immunity. Curr. Opin. Immunol. 2006, 18, 10–15. [Google Scholar] [CrossRef]
- Zanier, E.R.; Brandi, G.; Peri, G.; Longhi, L.; Zoerle, T.; Tettamanti, M.; Garlanda, C.; Sigurta, A.; Valaperta, S.; Mantovani, A.; et al. Cerebrospinal fluid pentraxin 3 early after subarachnoid hemorrhage is associated with vasospasm. Intensive Care Med. 2011, 37, 302–309. [Google Scholar] [CrossRef]
- Rodriguez-Grande, B.; Swana, M.; Nguyen, L.; Englezou, P.; Maysami, S.; Allan, S.M.; Rothwell, N.J.; Garlanda, C.; Denes, A.; Pinteaux, E. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J. Cereb. Blood Flow Metab. 2014, 34, 480–488. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, H.; Zheng, J.F.; Guo, Z.D.; Huang, Z.J.; Wu, Y.; Zhong, J.J.; Sun, X.C.; Cheng, C.J. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen. Res. 2020, 15, 2318–2326. [Google Scholar]
- Fossati, G.; Pozzi, D.; Canzi, A.; Mirabella, F.; Valentino, S.; Morini, R.; Ghirardini, E.; Filipello, F.; Moretti, M.; Gotti, C.; et al. Pentraxin 3 regulates synaptic function by inducing AMPA receptor clustering via ECM remodeling and β1-integrin. EMBO J. 2019, 38, e99529. [Google Scholar] [CrossRef]
- Brügger-Andersen, T.; Pönitz, V.; Kontny, F.; Staines, H.; Grundt, H.; Sagara, M.; Nilsen, D.W. The long pentraxin 3 (PTX3): A novel prognostic inflammatory marker for mortality in acute chest pain. Thromb. Haemost. 2009, 102, 555–563. [Google Scholar] [CrossRef]
- Salcini, C.; Atasever-Arslan, B.; Sunter, G.; Gur, H.; Isik, F.B.; Saylan, C.C.; Yalcin, A.D. High Plasma Pentraxin 3 Levels in Diabetic Polyneuropathy Patients with Nociceptive Pain. Tohoku J. Exp. Med. 2016, 239, 73–79. [Google Scholar] [CrossRef][Green Version]
- Mizoguchi, A.; Nakanishi, H.; Kimura, K.; Matsubara, K.; Ozaki-Kuroda, K.; Katata, T.; Honda, T.; Kiyohara, Y.; Heo, K.; Higashi, M.; et al. Nectin: An adhesion molecule involved in formation of synapses. J. Cell Biol. 2002, 156, 555–565. [Google Scholar] [CrossRef]
- Gao, Y.; Hong, X.; Wang, H. Role of Nectin-1/c-Src Signaling in the Analgesic Effect of GDNF on a Rat Model of Chronic Constrictive Injury. J. Mol. Neurosci. 2016, 60, 258–266. [Google Scholar] [CrossRef]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef]
- Guo, H.; Qiu, X.; Deis, J.; Lin, T.; Chen, X. Pentraxin 3 deficiency exacerbates lipopolysaccharide-induced inflammation in adipose tissue. Int. J. Obes. 2020, 44, 525–538. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, C.; Tang, X.; Chen, Y.; Wang, X.; Su, L.; Hu, N.; Xie, K.; Yu, Y.; et al. Sevoflurane-induced learning deficits and spine loss via nectin-1/corticotrophin- releasing hormone receptor type 1 signaling. Brain Res. 2019, 1710, 188–198. [Google Scholar] [CrossRef]
- Zhang, L.; Terrando, N.; Xu, Z.Z.; Bang, S.; Jordt, S.E.; Maixner, W.; Serhan, C.N.; Ji, R.R. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain after Bone Fracture in Mice. Front. Pharmacol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Peirs, C.; Seal, R.P. Neural circuits for pain: Recent advances and current views. Science 2016, 354, 578–584. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, R.; Wang, H.; Yu, Y.; Wang, C.; Yang, M.; Wang, M.; Wang, G. Hydrogen-rich saline prevents remifentanil-induced hyperalgesia and inhibits MnSOD nitration via regulation of NR2B-containing NMDA receptor in rats. Neuroscience 2014, 280, 171–180. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Yu, H.; Sun, Y.; Yu, W. Pentraxin-3 in the Spinal Dorsal Horn Upregulates Nectin-1 Expression in Neuropathic Pain after Spinal Nerve Damage in Male Mice. Brain Sci. 2022, 12, 648. https://doi.org/10.3390/brainsci12050648
Zhu M, Yu H, Sun Y, Yu W. Pentraxin-3 in the Spinal Dorsal Horn Upregulates Nectin-1 Expression in Neuropathic Pain after Spinal Nerve Damage in Male Mice. Brain Sciences. 2022; 12(5):648. https://doi.org/10.3390/brainsci12050648
Chicago/Turabian StyleZhu, Min, Hongli Yu, Ying Sun, and Wenli Yu. 2022. "Pentraxin-3 in the Spinal Dorsal Horn Upregulates Nectin-1 Expression in Neuropathic Pain after Spinal Nerve Damage in Male Mice" Brain Sciences 12, no. 5: 648. https://doi.org/10.3390/brainsci12050648
APA StyleZhu, M., Yu, H., Sun, Y., & Yu, W. (2022). Pentraxin-3 in the Spinal Dorsal Horn Upregulates Nectin-1 Expression in Neuropathic Pain after Spinal Nerve Damage in Male Mice. Brain Sciences, 12(5), 648. https://doi.org/10.3390/brainsci12050648




