Eslicarbazepine, but Not Lamotrigine or Ranolazine, Shows Anticonvulsant Efficacy in Carbamazepine-Resistant Rats Developed by Window-Pentylenetetrazole Kindling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Development of the Window-PTZ Kindling Model
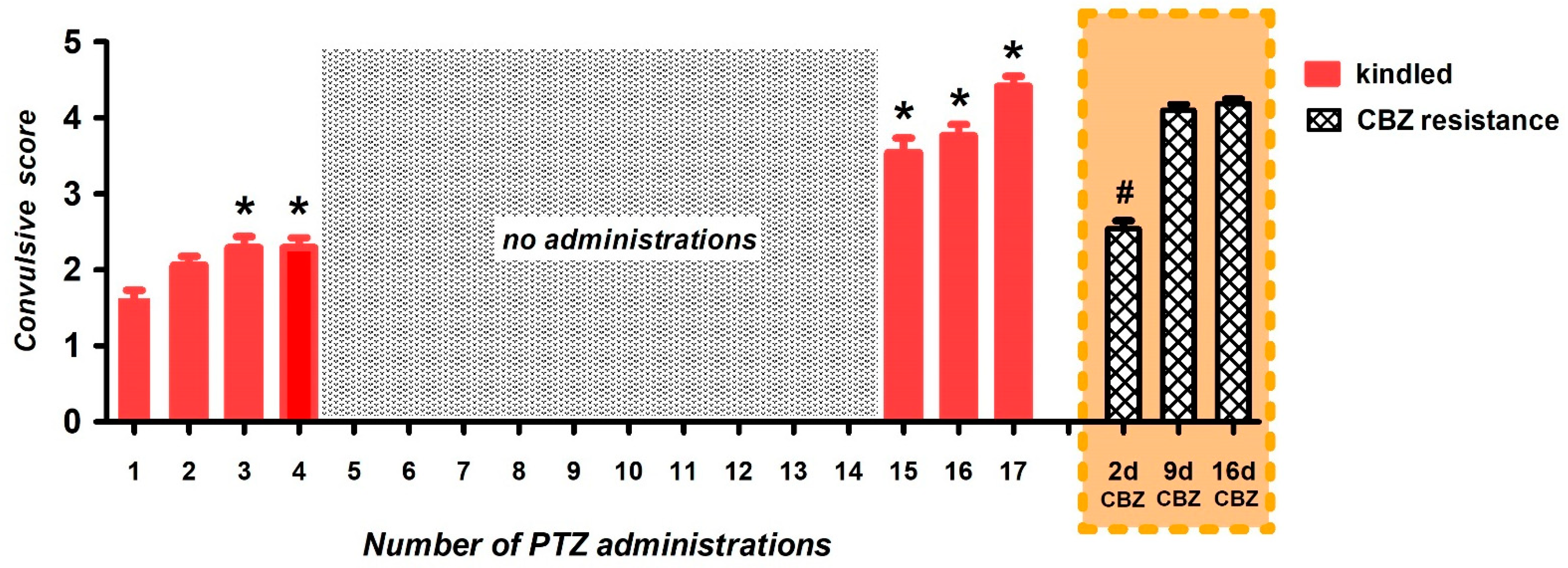
2.4. Generation of CBZ Resistance in Kindled Rats
2.5. Evaluation of Sodium Channel Blockers in CBZ-Resistant Rats
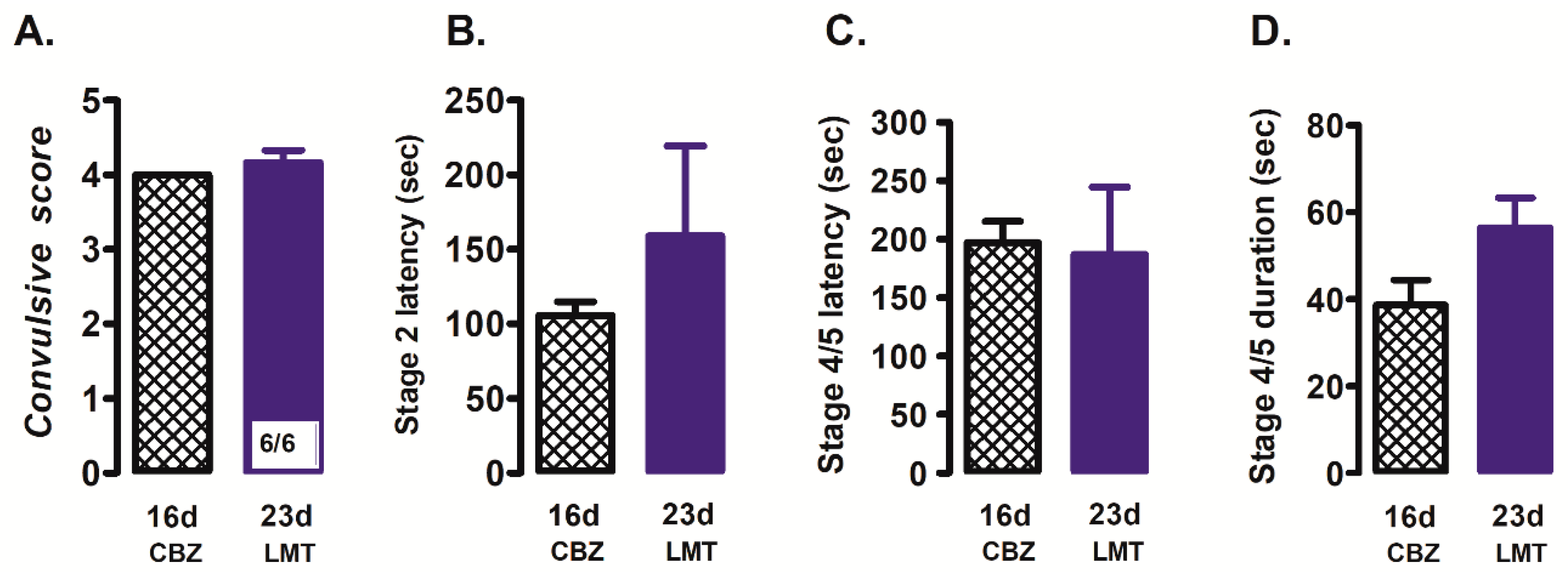
- Lamotrigine group (n = 6), rats received lamotrigine (30 mg/kg i.p.) 70 min prior to stimulation with PTZ (35 mg/kg i.p.);
- Eslicarbazepine group (n = 12), here we tested two doses of eslicarbazepine (150 or 300 mg/kg i.p.) each of which were administered 40 min prior to PTZ stimulation;
- Ranolazine group (n = 30), in this group we tested three doses of ranolazine (10, 20 or 40 mg/kg i.p.) that were administered 30 min prior to PTZ stimulation.
2.6. Statistical Analysis
3. Results
3.1. Generation of Carbamazepine-Resistant Fully Kindled Rats
3.2. Effect of Sodium Channel Blockers on Convulsions Generated by PTZ in CBZ-Resistant Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTZ | pentylenetetrazole |
| CBZ | carbamazepine |
References
- Devinsky, O.; Vezzani, A.; O’Brien, T.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2009, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Gabriel, S.; Urban, B.W.; Dietrich, D.; Lehmann, T.N.; Elger, C.E.; Heinemann, U.; Beck, H. A novel mechanism underlying drug resistance in chronic epilepsy. Ann. Neurol. 2003, 53, 469–479. [Google Scholar] [CrossRef]
- Remy, S.; Urban, B.W.; Elger, C.E.; Beck, H. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur. J. Neurosci. 2003, 17, 2648–2658. [Google Scholar] [CrossRef] [Green Version]
- Jandova, K.; Päsler, D.; Antonio, L.L.; Raue, C.; Ji, S.; Njunting, M.; Kann, O.; Kovács, R.; Meencke, H.J.; Cavalheiro, E.; et al. Carbamazepine-resistance in the epileptic dentate gyrus of human hippocampal slices. Brain 2006, 129, 3290–3306. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; White, H.S. Carbamazepine, but not valproate, displays pharmacoresistance in lamotrigine-resistant amygdala kindled rats. Epilepsy Res. 2013, 104, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Bazhanova, E.D.; Kozlov, A.A.; Litovchenko, A.V. Mechanisms of Drug Resistance in the Pathogenesis of Epilepsy: Role of Neuroinflammation. A Literature Review. Brain Sci. 2021, 11, 663. [Google Scholar] [CrossRef]
- Zavala-Tecuapetla, C.; Orozco-Suarez, S.; Manjarrez, J.; Cuellar-Herrera, M.; Vega-Garcia, A.; Buzoianu-Anguiano, V. Activation of adenosine receptors modulates the efflux transporters in brain capillaries and restores the anticonvulsant effect of carbamazepine in carbamazepine resistant rats developed by window-pentylenetetrazole kindling. Brain Res. 2020, 1726, 146516. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Davoudi, M.; Shojaei, A.; Palizvan, M.R.; Javan, M.; Mirnajafi-Zadeh, J. Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res. 2013, 106, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Alex, A.B.; Wilcox, K.S.; White, H.S. Rapid loss of efficacy to the antiseizure drugs lamotrigine and carbamazepine: A novel experimental model of pharmacoresistant epilepsy. Epilepsia 2013, 54, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Hebeisen, S.; Pires, N.; Loureiro, A.I.; Bonifácio, M.J.; Palma, N.; Whyment, A.; Spanswick, D.; Soares-Da-Silva, P. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: A comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology 2015, 89, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Kahlig, K.M.; Lepist, I.; Leung, K.; Rajamani, S.; George, A.L. Ranolazine selectively blocks persistent current evoked by epilepsy-associated NaV1.1 mutations. Br. J. Pharmacol. 2010, 161, 1414–1426. [Google Scholar] [CrossRef] [Green Version]
- Kahlig, K.M.; Hirakawa, R.; Liu, L.; George, A.L.; Belardinelli, L.; Rajamani, S. Ranolazine Reduces Neuronal Excitability by Interacting with Inactivated States of Brain Sodium Channels. Mol. Pharmacol. 2014, 85, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Elger, C.; Bialer, M.; Cramer, J.A.; Maia, J.; Almeida, L.; Soares-da-Silva, P. Eslicarbazepine Acetate: A Double-blind, Add-on, Placebo-controlled Exploratory Trial in Adult Patients with Partial-onset Seizures. Epilepsia 2007, 48, 497–504. [Google Scholar] [CrossRef]
- Elger, C.; Halász, P.; Maia, J.; Almeida, L.; Soares-da-Silva, P. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: A randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia 2009, 50, 454–463. [Google Scholar] [CrossRef]
- Potschka, H.; Soerensen, J.; Pekcec, A.; Loureiro, A.; Soares-da-Silva, P. Effect of eslicarbazepine acetate in the corneal kindling progression and the amygdala kindling model of temporal lobe epilepsy. Epilepsy Res. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Doeser, A.; Dickhof, G.; Reitze, M.; Uebachs, M.; Schaub, C.; Pires, N.M.; Bonifácio, M.J.; Soares-Da-Silva, P.; Beck, H. Targeting pharmacoresistant epilepsy and epileptogenesis with a dual-purpose antiepileptic drug. Brain 2015, 138, 371–387. [Google Scholar] [CrossRef] [Green Version]
- Ben-Menachem, E.; Gabbai, A.A.; Hufnagel, A.; Maia, J.; Almeida, L.; Soares-da-Silva, P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010, 89, 278–285. [Google Scholar] [CrossRef]
- Halász, P.; Cramer, J.A.; Hodoba, D.; Członkowska, A.; Guekht, A.; Maia, J.; Elger, C.; Almeida, L.; Soares-Da-Silva, P. Long-term efficacy and safety of eslicarbazepine acetate: Results of a 1-year open-label extension study in partial-onset seizures in adults with epilepsy. Epilepsia 2010, 51, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Gil-Nagel, A.; Lopes-Lima, J.; Almeida, L.; Maia, J.; Soares-da-Silva, P. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol. Scand. 2009, 120, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gil-Nagel, A.; Elger, C.; Ben-Menachem, E.; Halász, P.; Lopes-Lima, J.; Gabbai, A.A.; Nunes, T.; Falcão, A.; Almeida, L.; Soares-Da-Silva, P. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: Integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia 2013, 54, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Sokolov, S.; Rajamani, S.; Ruben, P. Effects of the antianginal drug, ranolazine, on the brain sodium channel Na V 1.2 and its modulation by extracellular protons. Br. J. Pharmacol. 2013, 169, 704–716. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.L.; Thompson, C.H.; Hawkins, N.A.; Nath, R.D.; Petersohn, A.A.; Rajamani, S.; Bush, W.; Frankel, W.N.; Vanoye, C.G.; Kearney, J.; et al. Antiepileptic activity of preferential inhibitors of persistent sodium current. Epilepsia 2014, 55, 1274–1283. [Google Scholar] [CrossRef]
- Corda, M.G.; Orlandi, M.; Lecca, D.; Carboni, G.; Frau, V.; Giorgi, O. Pentylenetetrazol-induced kindling in rats: Effect of GABA function inhibitors. Pharmacol. Biochem. Behav. 1991, 40, 329–333. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Corda, M.G.; Giorgi, O.; Longoni, B.; Orlandi, M.; Biggio, G. Decrease in the Function of the ?-Aminobutyric Acid-Coupled Chloride Channel Produced by the Repeated Administration of Pentylenetetrazol to Rats. J. Neurochem. 1990, 55, 1216–1221. [Google Scholar] [CrossRef]
- Cunningham, M.O.; Jones, R.S.G. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacoly 2000, 39, 2139–2146. [Google Scholar] [CrossRef]
- Metcalf, C.S.; Huff, J.; Thomson, K.E.; Johnson, K.; Edwards, S.F.; Wilcox, K.S. Evaluation of antiseizure drug efficacy and tolerability in the rat lamotrigine-resistant amygdala kindling model. Epilepsia Open. 2019, 4, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koneval, Z.; Knox, K.M.; White, H.S.; Barker-Haliski, M. Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia 2018, 59, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Benes, J.; Parada, A.; Figueiredo, A.A.; Alves, P.C.; Freitas, A.P.; Learmonth, D.A.; Cunha, R.A.; Garrett, J.; Soares-Da-Silva, P. Anticonvulsant and Sodium Channel-Blocking Properties of Novel 10,11-Dihydro-5H-dibenz[b, f]azepine-5-carboxamide Derivatives. J. Med. Chem. 1999, 42, 2582–2587. [Google Scholar] [CrossRef] [PubMed]
- Doeser, A.; Soares-da-Silva, P.; Beck, H.; Uebachs, M. The effects of eslicarbazepine on persistent Na+ current and the role of the Na+ channel β subunits. Epilepsy Res. 2014, 108, 202–211. [Google Scholar] [CrossRef]
- Fattorusso, A.; Matricardi, S.; Mencaroni, E.; Dell’Isola, G.B.; Di Cara, G.; Striano, P.; Verrotti, A. The Pharmacoresistant Epilepsy: An Overview on Existant and New Emerging Therapies. Front. Neurol. 2021, 12, 674483. [Google Scholar] [CrossRef]
- Gastaldi, M.; Robaglia-Schlupp, A.; Massacrier, A.; Planells, R.; Cau, P. mRNA coding for voltage-gated sodium channel β2 subunit in rat central nervous system: Cellular distribution and changes following kainate-induced seizures. Neurosci. Lett. 1998, 249, 53–56. [Google Scholar] [CrossRef]
- Ellerkmann, R.; Remy, S.; Chen, J.; Sochivko, D.; Elger, C.; Urban, B.; Becker, A.; Beck, H. Molecular and functional changes in voltage-dependent na+ channels following pilocarpine-induced status epilepticus in rat dentate granule cells. Neuroscience 2003, 119, 323–333. [Google Scholar] [CrossRef]
- Uebachs, M.; Opitz, T.; Royeck, M.; Dickhof, G.; Horstmann, M.-T.; Isom, L.L.; Beck, H. Efficacy Loss of the Anticonvulsant Carbamazepine in Mice Lacking Sodium Channel Subunits via Paradoxical Effects on Persistent Sodium Currents. J. Neurosci. 2010, 30, 8489–8501. [Google Scholar] [CrossRef] [Green Version]
- Brady, K.; Hebeisen, S.; Konrad, D.P.; Soares-da-Silva, P. Poster Sessions. Epilepsia 2011, 52, 260. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.L.; Hawkins, N.A.; Thompson, C.H.; Kearney, J.A.; George, A.L. Unexpected Efficacy of a Novel Sodium Channel Modulator in Dravet Syndrome. Sci. Rep. 2017, 7, 1682. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.M.; Thompson, C.H.; Hawkins, N.A.; Wagnon, J.L.; Wengert, E.R.; Patel, M.K.; George, A.L., Jr.; Meisler, M.H.; Kearney, J.A. The novel sodium channel modulator GS-458967 (GS967) is an effective treatment in a mouse model of SCN8A encephalopathy. Epilepsia 2018, 59, 1166–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Cho, J.H.; Shin, H.; Jang, I.S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019, 855, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.R.; Cummins, T.R. Differential Inhibition of Human Nav1.2 Resurgent and Persistent Sodium Currents by Cannabidiol and GS967. Int. J. Mol. Sci. 2020, 21, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Shryock, J.C.; Song, Y.; Li, Y.; Antzelevitch, C.; Belardinelli, L. Antiarrhythmic Effects of Ranolazine in a Guinea Pig in Vitro Model of Long-QT Syndrome. J. Pharmacol. Exp. Ther. 2004, 310, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scirica, B.M.; Morrow, D.A.; Hod, H.; Murphy, S.A.; Belardinelli, L.; Hedgepeth, C.M.; Molhoek, P.; Verheugt, F.W.; Gersh, B.J.; McCabe, C.H.; et al. Effect of Ranolazine, an Antianginal Agent with Novel Electrophysiological Properties, on the Incidence of Arrhythmias in Patients with Non–ST-Segment–Elevation Acute Coronary Syndrome. Circulation 2007, 116, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.; Donner, E.J.; McLean, B.; Nashef, L.; Tomson, T. Sudden unexpected death in epilepsy (SUDEP): What every neurologist should know. Epileptic Disord. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi, J.; Buchholz, B.; Salguero, J.; Cañellas, C.; Kelly, J.; Men, P.; Zubillaga, M.; Rossi, A.; Merelli, A.; Gelpi, R.J.; et al. Pilocarpine-Induced Status Epilepticus Is Associated with P-Glycoprotein Induction in Cardiomyocytes, Electrocardiographic Changes, and Sudden Death. Pharmaceuticals 2018, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Akyuz, E.; Doganyigit, Z.; Eroglu, E.; Moscovicz, F.; Merelli, A.; Lazarowski, A.; Auzmendi, J. Myocardial Iron Overload in an Experimental Model of Sudden Unexpected Death in Epilepsy. Front. Neurol. 2021, 12, 609236. [Google Scholar] [CrossRef]
- Tomson, T.; Surges, R.; Delamont, R.; Haywood, S.; Hesdorffer, D.C. Who to target in sudden unexpected death in epilepsy prevention and how? Risk factors, biomarkers, and intervention study designs. Epilepsia 2016, 57, 4–16. [Google Scholar] [CrossRef]
- Potschka, H.; Fedrowitz, M.; Löscher, W. P-Glycoprotein-mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood-brain barrier: Evidence from microdialysis experiments in rats. Neurosci. Lett. 2002, 327, 173–176. [Google Scholar] [CrossRef]
- Zhang, C.; Zuo, Z.; Kwan, P.; Baum, L. In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia 2011, 52, 1894–1904. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavala-Tecuapetla, C.; Manjarrez-Marmolejo, J.; Ramírez-Jarquín, J.O.; Rivera-Cerecedo, C.V. Eslicarbazepine, but Not Lamotrigine or Ranolazine, Shows Anticonvulsant Efficacy in Carbamazepine-Resistant Rats Developed by Window-Pentylenetetrazole Kindling. Brain Sci. 2022, 12, 629. https://doi.org/10.3390/brainsci12050629
Zavala-Tecuapetla C, Manjarrez-Marmolejo J, Ramírez-Jarquín JO, Rivera-Cerecedo CV. Eslicarbazepine, but Not Lamotrigine or Ranolazine, Shows Anticonvulsant Efficacy in Carbamazepine-Resistant Rats Developed by Window-Pentylenetetrazole Kindling. Brain Sciences. 2022; 12(5):629. https://doi.org/10.3390/brainsci12050629
Chicago/Turabian StyleZavala-Tecuapetla, Cecilia, Joaquín Manjarrez-Marmolejo, Josué Orlando Ramírez-Jarquín, and Claudia Verónica Rivera-Cerecedo. 2022. "Eslicarbazepine, but Not Lamotrigine or Ranolazine, Shows Anticonvulsant Efficacy in Carbamazepine-Resistant Rats Developed by Window-Pentylenetetrazole Kindling" Brain Sciences 12, no. 5: 629. https://doi.org/10.3390/brainsci12050629
APA StyleZavala-Tecuapetla, C., Manjarrez-Marmolejo, J., Ramírez-Jarquín, J. O., & Rivera-Cerecedo, C. V. (2022). Eslicarbazepine, but Not Lamotrigine or Ranolazine, Shows Anticonvulsant Efficacy in Carbamazepine-Resistant Rats Developed by Window-Pentylenetetrazole Kindling. Brain Sciences, 12(5), 629. https://doi.org/10.3390/brainsci12050629





