Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
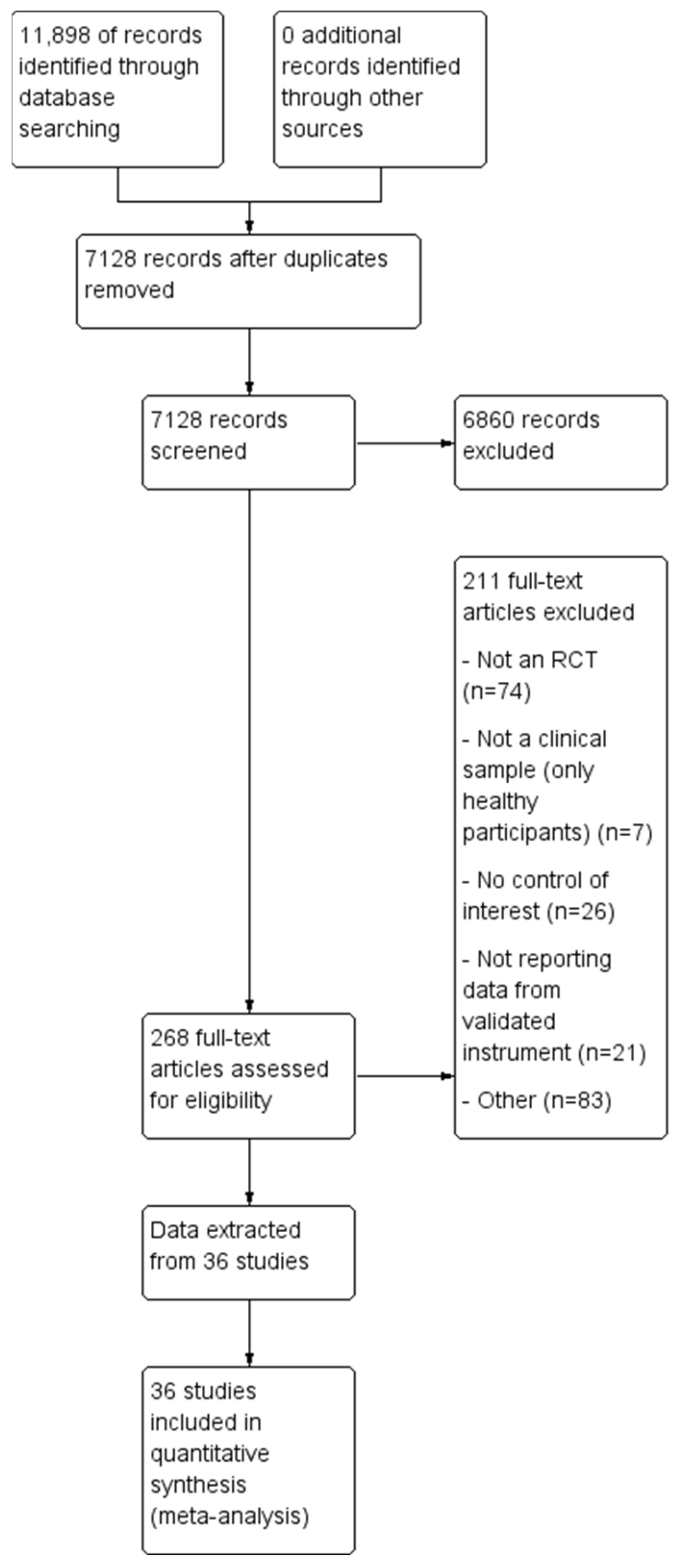
3.1. Study Selection
3.2. Study Characteristics
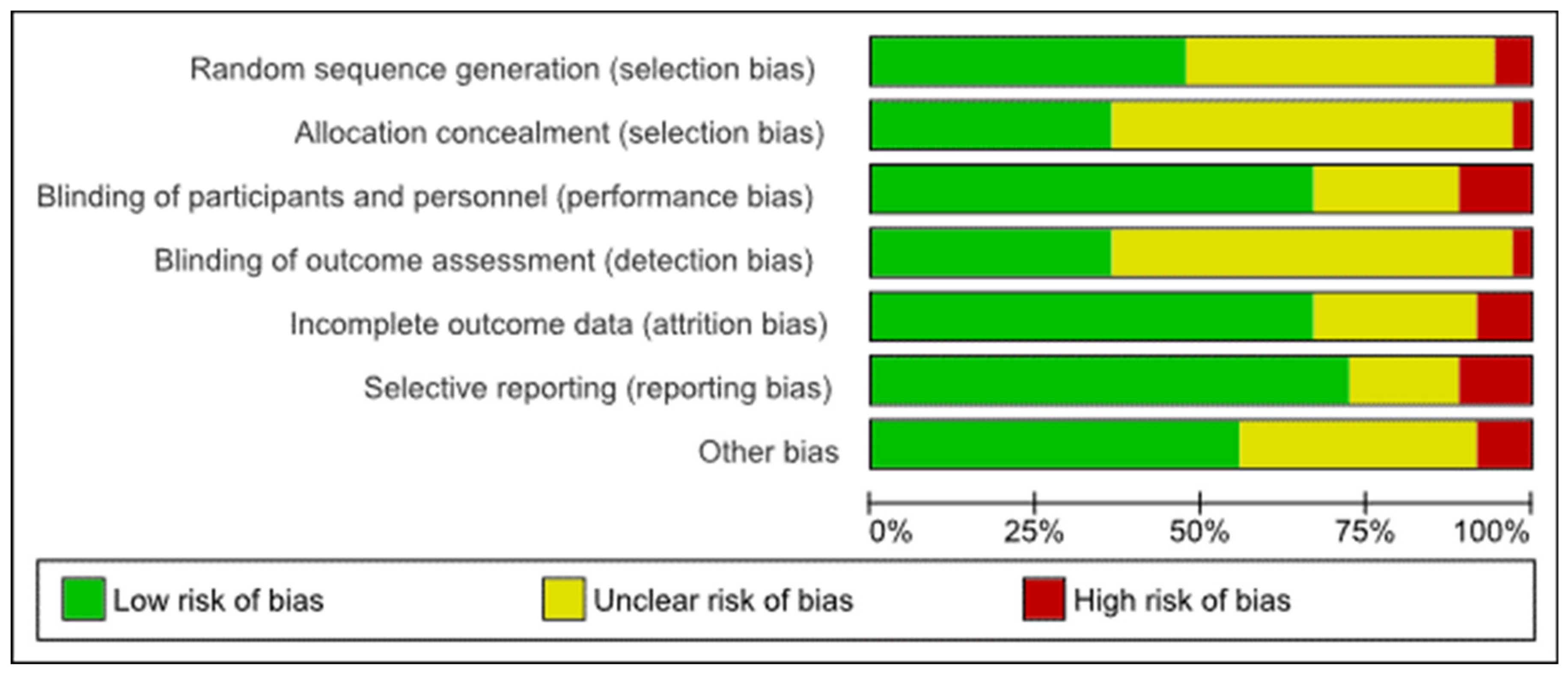
3.3. Risk of Bias
3.4. Results of Syntheses
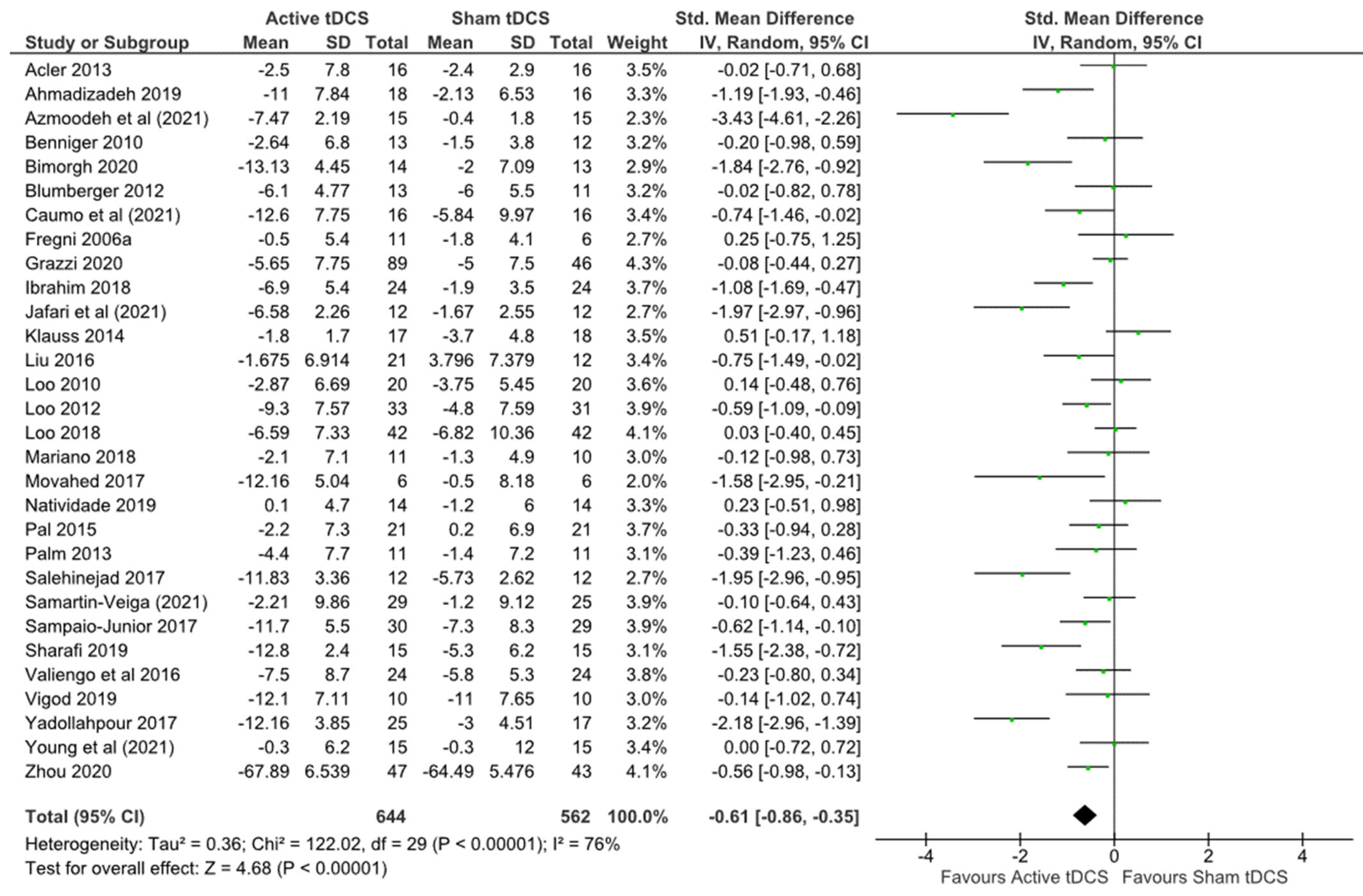
3.4.1. Primary Outcomes
3.4.2. Secondary Outcomes
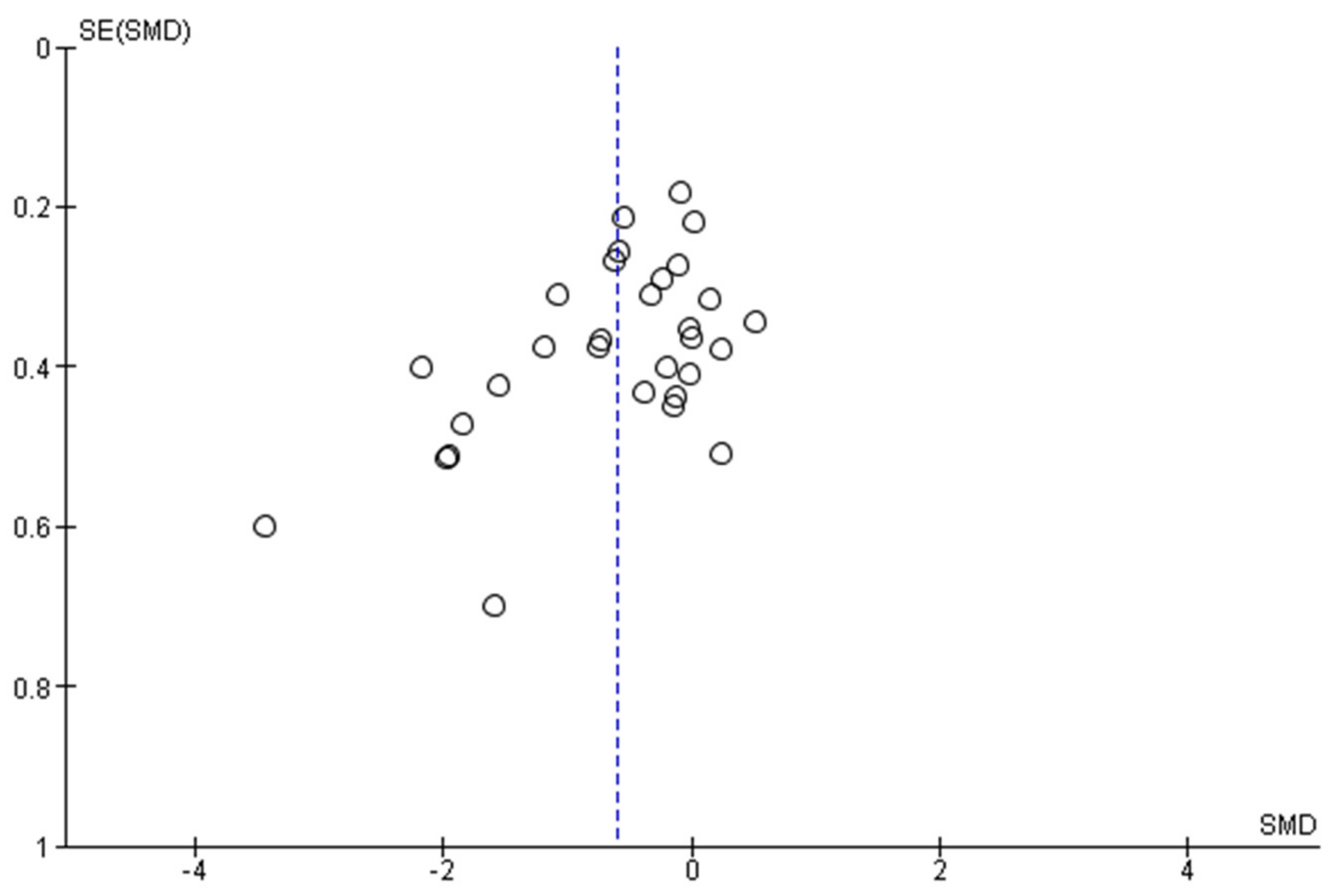
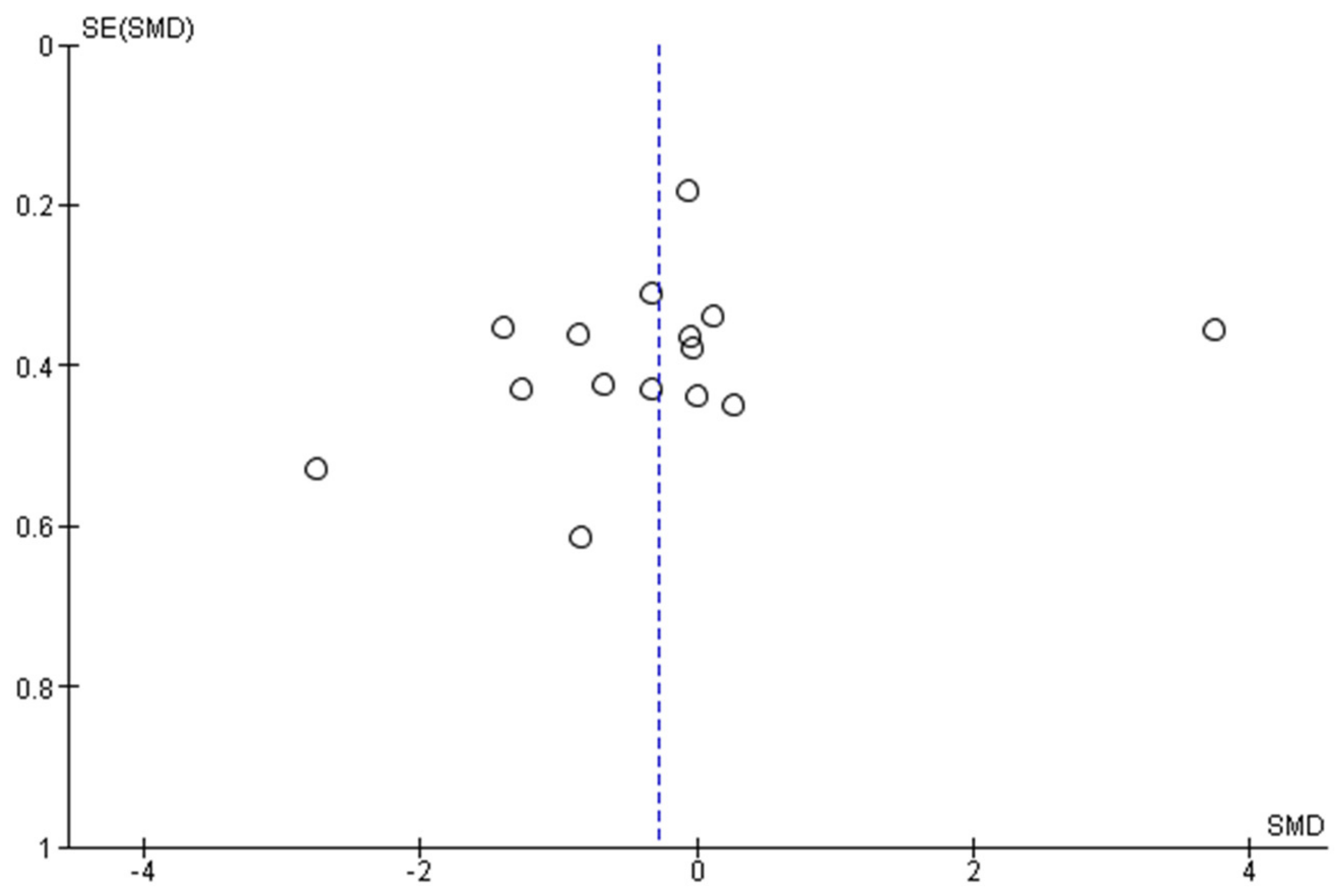
3.5. Reporting Bias
3.6. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
PROSPERO Registration Number
References
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.-J.; Andersson, G. Tinnitus and tinnitus disorder: Theoretical and Operational Definitions (An International Multidisciplinary Proposal), in Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2021; pp. 12–15. [Google Scholar]
- Biswas, R.; Lugo, A.; Akeroyd, M.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: A multi-country cross-sectional population study. Lancet 2022, 12, 100250. [Google Scholar] [CrossRef] [PubMed]
- Cima, R.F.F.; Kikidis, D.; Mazurek, B.; Haider, H.; Cederroth, C.R.; Noreña, A.; Lapira, A.; Bibas, A.; Hoare, D.J. Tinnitus healthcare: A survey revealing extensive variation in opinion and practices across Europe. BMJ Open 2020, 10, e029346. [Google Scholar] [CrossRef] [PubMed]
- Baldo, P.; Doree, C.; Molin, P.; McFerran, D.; Cecco, S. Antidepressants for patients with tinnitus. Cochrane Database Syst. Rev. 2012, 2012, CD003853. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.P.; Zimmermann, E.F.; Hunt, W.T. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2013, 28, CD003852. [Google Scholar] [CrossRef]
- Hoekstra, C.; Rynja, S.P.; Van Zanten, G.A.; Rovers, M. Anticonvulsants for tinnitus. Cochrane Database Syst. Rev. 2009, 6, CD007960. [Google Scholar]
- Wegner, I.; Hall, D.A.; Smit, A.L.; McFerran, D.; Stegeman, I. Betahistine for tinnitus. Cochrane Database Syst. Rev. 2018, 2018, CD013093. [Google Scholar] [CrossRef]
- Person, O.C.; Puga, M.E.S.; da Silva, E.M.K.; Torloni, M.R. Zinc supplementation for tinnitus. Cochrane Database Syst. Rev. 2016, 11, CD009832. [Google Scholar] [CrossRef]
- Peter, N.; Kleinjung, T. Neuromodulation for tinnitus treatment: An overview of invasive and non-invasive techniques. J. Zhejiang Univ. Sci. B 2019, 20, 116–130. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef] [Green Version]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Hermann, C.S.; Kappenman, E.S. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Brunoni, A.R.; Ferrucci, R.; Fregni, F.; Boffio, P.S.; Priori, A. Transcranial direct current stimulation for the treatment of major depressive disorder: A summary of preclinical, clinical and translational findings. Prog. Neuro Psychopharmacol. Biol. Psych. 2012, 39, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanan, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; McIntyre, A.; Guy, S.; Teasell, R.W.; Loh, E. Effectiveness of transcranial direct current stimulation for the management of neuropathic pain after spinal cord injury: A meta-analysis. Spinal Cord 2015, 53, 780–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, F.; Codecà, C.; Kusayanagi, H.; Monteleone, F.; Buttari, F.; Fiore, S.; Bernardi, G. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J. Pain 2010, 11, 436–442. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. tDCS for the treatment of depression: A comprehensive review. Eur. Arch. Psych. Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef]
- Stein, D.J.; Medeiros, L.F.; Caumo, W.; Torres, I.L.S. Transcranial direct current stimulation in patients with anxiety: Current perspectives. Neuropsych. Dis. Treat. 2020, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Fregni, F.; El-Hagrassi, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, L.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int. J. Neuropsychopharmac. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Salazar, J.W.; Meisel, K.; Smith, E.R.; Quiggle, A.; McCoy, D.B.; Amans, M.R. Depression in patients with tinnitus: A systematic review. Otolaryngol. Head Neck Surg. 2019, 161, 28–35. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Geocze, L.; Mucci, S.; Abranches, D.C.; de Marco, M.A.; de Oliveira Penido, N. Systematic review on the evidences of an association between tinnitus and depression. Braz. J. Otorhinolaryngol. 2013, 79, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, W.T.; Ost, J.; Hart, J.; De Ridder, D.; Vanneste, S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J. Neural Trans. 2017, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yadollahpour, A.; Bayat, A.; Rashidi, S.; Saki, N.; Karimi, M. Dataset of acute repeated sessions of bifrontal transcranial direct current stimulation for treatment of intractable tinnitus: A randomized controlled trial. Data Brief 2017, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-C.; Tyler, R.S.; Chang, T.-Y.; Chen, J.-C.; Lin, C.-D.; Chung, H.-K.; Tsou, Y.-A. Effect of transcranial direct current stimulation in patients with tinnitus: A meta-analysis and systematic review. Ann. Otol. Rhinol. Larynigol. 2018, 127, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.d.H.M.; Santos, A.P.S.; Santos, H.S.; da Silva, A.C. The use of tDCS as a therapeutic option for tinnitus: A systematic review. Braz. J. Otorhinolaryngol. 2018, 84, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labree, B.; Hoare, D.J.; Gascoyne, L.E.; Sereda, M. Determining the effects of transcranial direct current stimulation on tinnitus and tinnitus-related outcomes: Protocol for a systematic review. BMJ Open 2021, 11, e047191. [Google Scholar] [CrossRef]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011; Available online: www.cochrane-handbook.org (accessed on 14 January 2022).
- Review Manager (RevMan). The Cochrane Collaboration. 2020. Available online: https://get.covidence.org/cochrane-v2?campaignid=14927826163&adgroupid=129805882033&gclid=CjwKCAjw0a-SBhBkEiwApljU0kokghaMa3ttbq96YLE1KLBCQ5A8aR1oqvqxMwRTaEMEY_tzk2ScOxoC_7UQAvD_BwE (accessed on 14 January 2022).
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Martin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Schunemann, H. Grade Handbook for Grading Quality of Evidence and Strength of Recommendation; Version 3.2; Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group: Hamilton, ON, Canada, 2008; Available online: http://www.cc-ims.net/gradepro (accessed on 14 January 2022).
- StataCorp. Stata Statistical Software, Release 17; StataCorp: College Station, TX, USA, 2021. [Google Scholar]
- Acler, M.; Bocci, T.; Valenti, D.; Turri, M.; Priori, A.; Bertolasi, L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor. Neurol. Neurosci. 2013, 31, 661–668. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.J.; Rezaei, M.; Fitzgerald, P.B. Transcranial direct current stimulation (tDCS) for post-traumatic stress disorder (PTSD): A randomized, double-blinded, controlled trial. Brain Res. Bull. 2019, 153, 273–278. [Google Scholar] [CrossRef]
- Azmoodeh, S.; Soleimani, E.; Issazadegan, A. The effects of transcranial direct current stimulation on depression, anxiety, and stress in patients with epilepsy: A randomized clinical trial. Iran. J. Med. Sci. 2021, 46, 272. [Google Scholar] [PubMed]
- Benninger, D.H.; Lomarev, M.; Lopez, G.; Wassermann, E.M.; Li, X.; Considine, E.; Hallett, M. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psych. 2010, 81, 1105–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi Bimorgh, M.; Omidi, A.; Goreishi, F.S.; Rezeai Ardani, A.; Ghaderi, A.; Banafshe, H.R.iI. The effect of transcranial direct current stimulation on relapse, anxiety, and depression in patients with opioid dependence under methadone maintenance treatment: A pilot study. Front. Pharmacol. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.; Tran, L.; Fitzgerald, P.; Hoy, K.B.B.N.S. DaskalakisA randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front. Psych. 2012, 3, 74. [Google Scholar]
- Caumo, W.; Alves, R.L.; Vicuña, P.; da Silveira Alves, C.F.; Ramalho, L.; Sanchez, P.R.S.; Silva, D.P., Jr.; da Dilva Torres, T.L.; Fregni, F. Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: A randomized, double-blind sham-controlled study. J. Pain 2021, 23, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, K.; Basil-Neto, J.P.; Allam, N.; Boechat-Barros, R. A double-blind, placebo-controlled study of the effects of daily tDCS sessions targeting the dorsolateral prefrontal cortex on tinnitus handicap inventory and visual analog scale scores. Brain Stimul. 2015, 8, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.R.D.V.; Pegado, R.; Silva, L.K.; da Silva Dantas, H.; Câmara, H.A.; Silva-Filho, E.M.; Correira, G.N.; Micussi, M.T.A.B.C. Modulating anxiety and functional capacity with anodal tDCS over the left dorsolateral prefrontal cortex in primary dysmenorrhea. Int. J. Women’s Health 2020, 12, 243. [Google Scholar]
- Forogh, B.; Mirshaki, Z.; Raissi, G.H.; Shirazi, A.; Mansoori, K.; Ahadi, T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: A pilot randomized controlled trial. Neurol. Sci. 2016, 37, 253–259. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Lima, M.C.; Ferreira, M.J.K.; Wagner Rigonetti, S.P.; Castro, W.A.; Souza, D.R.; Riberto, M.; Freedman, S.D. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef]
- Grazzi, L.; Usai, S.; Bolognini, N.; Grignani, E.; Sansinem, E. No efficacy of transcranial direct current stimulation on chronic migraine with medication overuse: A double blind, randomised clinical trial. Cephalalgia 2020, 40, 12021–12211. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Abdelhameed, K.M.; Kamal, S.M.M.; Khedr, E.M.H.; Kotb, H.I.M. Effect of transcranial direct current stimulation of the motor cortex on visceral pain in patients with hepatocellular carcinoma. Pain Med. 2018, 19, 5505–5560. [Google Scholar] [CrossRef] [Green Version]
- Jafari, E.; Alizadehgoradel, J.; Koluri, F.P.; Nikoozadehkordmirza, E.; Refahi, M.; Taherifard, M.; Nejati, V.; Hallajian, A.-H.; Ghanavati, E.; Vicario, C.M. Intensified electrical stimulation targeting lateral and medial prefrontal cortices for the treatment of social anxiety disorder: A randomized, double-blind, parallel-group, dose-comparison study. Brain Stimul. 2021, 14, 974–986. [Google Scholar] [CrossRef]
- Klauss, J.; Penido Pinheiro, L.C.; Silva Merlo, B.L.; de Correira Santos, G.A.; Fregni, F.; Nitsche, M.A.L.; Miyuki Nakamura-Palacios, E. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int. J. Neuropsychopharmacol. 2014, 17, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Bryant, A.; Jefferson, A.; Friedmann, D.; Minhas, P.; Barnard, S.; Barr, W.; Thesen, T.; O’Connor, M.; Shafi, M. Exploring the efficacy of a 5-day course of transcranial direct current stimulation (TDCS) on depression and memory function in patients with well-controlled temporal lobe epilepsy. Epilepsy Behav. 2016, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.K.; Sachdev, P.; Martin, D.; Pigot, M.; Alonzo, A.; Malhi, G.S.; Lagopoulos, J.; Mitchell, P. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int. J. Neuropsychopharmacol. 2010, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.K.; Alonzo, A.; Martin, D.; Mitchell, P.B.; Glavez, V.; Sachdev, P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br. J. Psych. 2012, 200, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Loo, C.K.; Husain, M.M.; McDonald, J.P.; Aaronson, S.; O’Rearden, J.P.; Alonzo, A.; Weickert, C.S.; Mohan, A. International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul. 2018, 11, 125–133. [Google Scholar] [CrossRef]
- Mariano, T.Y.; Burgess, F.W.; Bowker, M.; Kirschner, J.; van’t Wout-Frank, M.; Jones, R.N.; Halladay, C.W.; Stein, M.; Greenberg, B.D. eTranscranial direct current stimulation for affective symptoms and functioning in chronic low back pain: A pilot double-blinded, randomized, placebo-controlled trial. Pain Med. 2019, 20, 1166–1177. [Google Scholar] [CrossRef]
- Movahed, F.S.; Goradel, J.A.; Poucesmali, A.; Mowlaie, M. Effectiveness of transcranial direct current stimulation on worry, anxiety, and depression in generalized anxiety disorder: A randomized, single-blind pharmacotherapy and sham-controlled clinical trial. Iran. J. Psych. Behav. Sci. 2018, 12, e11071. [Google Scholar]
- Natividade, G.R.; De Aranjo, G.R.; Fitz, R.C.; Brietzke, E.; Schestatsky, P.; Gerchmann, F. Psychiatric profile and quality of life of subjects with excess weight treated with transcranial direct current stimulation combined with a hypocaloric diet. Nutr. Neurosci. 2021, 24, 919–926. [Google Scholar] [CrossRef]
- Pal, N.; Maire, R.; Stephan, M.A.; Herrmann, F.R.; Benninger, D.H. Transcranial direct current stimulation for the treatment of chronic tinnitus: A randomized controlled study. Brain Stimul. 2015, 8, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Fintescu, Z.; Obermeier, M.; Schiller, C.; Reisinger, E.; Keeser, D.; Pogarell, O.; Bondy, B.; Zill, P.; Padberg, F. Serum levels of brain-derived neurotrophic factor are unchanged after transcranial direct current stimulation in treatment-resistant depression. J. Affect. Disord. 2013, 150, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Pegado, R.; Silve, L.K.; da Silva Dantas, H.; Andrade Câmara, H.; Andrade Mesconto, K.; Silva Filho, M.E.; Lopes, J.M.; Micussi, M.T.A.B.C.; Correira, G.N. Effects of transcranial direct current stimulation for treatment of primary dysmenorrhea: Preliminary results of a randomized sham-controlled trial. Pain Med. 2020, 21, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Ghanavai, M.A.; Rostenami, R.; Nejati, V. Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): Evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC). J. Affect. Disord. 2017, 210, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Samartin-Veiga, N.; Pidal-Miranda, M.; González-Villar, A.J.; Bradley, C.; Garcia-Larrea, L.; O’Brien, A.T.L.; Carillio-de-la-Peña, M.T. Transcranial direct current stimulation of three cortical targets is no more effective than placebo as treatment for fibromyalgia: A double-blind sham-controlled clinical trial. Pain 2021. [Google Scholar] [CrossRef]
- Sharafi, E.; Tagvha, A.; Arbabi, M.; Dadarkhah, A.; Ghaderi, J. Transcranial direct current stimulation for treatment-resistant major depression: A double-blind randomized sham-controlled trial. Clinical EEG Neurosci. 2019, 50, 375–382. [Google Scholar] [CrossRef]
- Da Silva Souza, D.; Almeida, A.A.; dos Santos Andrade, S.M.; da Silva Machado, D.G.; Leitão, M.; Sanchez, T.G.; da Rosa, M.R.D. Transcranial direct current stimulation improves tinnitus perception and modulates cortical electrical activity in patients with tinnitus: A randomized clinical trial. Neurophysiol. Clin. 2020, 50, 289–300. [Google Scholar] [CrossRef]
- Valiengo, L.; Casati, R.; Bolognini, N.; Lotufo, P.A.; Senseñor, I.M.; Goulart, A.C.; Brunoni, A.R. Transcranial direct current stimulation for the treatment of post-stroke depression in aphasic patients: A case series. Neurocase 2016, 22, 225–228. [Google Scholar] [CrossRef]
- Vigod, S.N.; Murphy, K.E.; Dennis, C.L.; Oberlander, T.F.; Ray, J.G.; Daskalakis, Z.J.; Blumberger, D.M. Transcranial direct current stimulation (tDCS) for depression in pregnancy: A pilot randomized controlled trial. Brain Stimul. 2019, 12, 1475–1483. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Mayo, M.; Saki, N.; Rashidi, S.; Bayat, A. A chronic protocol of bilateral transcranial direct current stimulation over auditory cortex for tinnitus treatment: Dataset from a double-blinded randomized controlled trial. F1000Research 2018, 7, 733. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Zoghi, M.; Khan, F.; Galea, M.P. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: Randomized controlled trial. Pain Med. 2020, 21, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, C.; Yu, H.; Zhang, Y.; Liu, Z.; Hu, Z.; Yuan, T.-F.; Zhou, D. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020, 70, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, M.; Muwton, L.; Slade, T.; Baillie, A.J. Investigating differential symptom profiles in major depressive episode with and without generalized anxiety disorder: True co-morbidity or symptom similarity? Psychol. Med. 2010, 40, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Reference | Type of Study | Condition Studied | Primary Outcomes Measured | Instrument | Sample Size | Electrode Montage | Current Intensity | Session Duration | Number of Sessions |
|---|---|---|---|---|---|---|---|---|---|
| Acler et al., (2013) [34] | Parallel RCT | Post-Polio Syndrome | Depression | HDRS | 32 | two anode 2 cm ahead of C3 and C4, cathode over left shoulder | 1.5 mA | 15 min | 15 |
| Ahmadizadeh et al., (2019) [35] | Parallel RCT | PTSD | Depression, anxiety | BDI-II, BAI | 34 | anode over F3, cathode over F4; | 2 mA | 20 min | 10 |
| Azmoodeh et al., (2021) [36] | Parallel RCT | Epilepsy | Depression, anxiety | DASS-21 | 30 | anode over F3, cathode over F4; | 1.5 mA | 20 min | 10 |
| Benninger et al., (2010) [37] | Parallel RCT | Parkinson’s Disease | Depression | BDI | 25 | anode 10 mm anterior to Cz, two 25 cathodes over mastoids | 2 mA | 20 min | 8 |
| Bimorgh et al., (2020) [38] | Parallel RCT | Opioid dependence | Depression, anxiety | DASS-21 | 27 | anode over F4, cathode over F3 | 2 mA | 20 min | 7 |
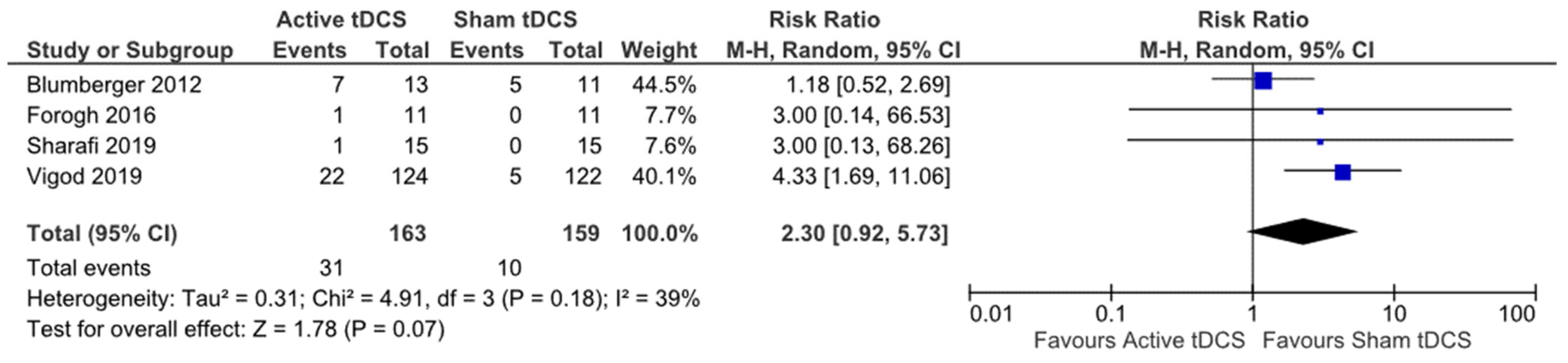
| Blumberger et al., (2012) [39] | Parallel RCT | Depression | Depression | MADRS, HDRS-17, BDI-II | 24 | anode over F3, cathode over F4 | 2 mA | 20 min | 15 |
| Caumo et al., (2021) [40] | Parallel RCT | Fibromyalgia | Depression | BDI-II | 32 | anode over F3, cathode over Fpz | 2 mA | 20 min | 20 |
| Cavalcanti et al., (2015) [41] | Parallel RCT | Tinnitus | Tinnitus | THI | 18 | anode over F4, cathode over F3 | 2 mA | 20 min | 5 |
| Dantas et al., (2020) [42] | Parallel RCT | Dysmenorrhea | Anxiety | HAS | 24 | anode over F3, cathode over Fp2 | 2 mA | 20 min | 5 |
| Forogh et al., (2016) [43] | Parallel RCT | Tinnitus | Tinnitus | THI | 22 | anode electrode halfway between C3 and T5, cathode over Fp2 | 2 mA | 20 min | 5 |
| Fregni et al., (2006) [44] | Parallel RCT | Central pain following spinal injury | Depression | BDI | 17 | anode electrode over C3 or C4, cathode electrode over the contralateral supraorbital area | 12 mA | 20 min | 5 |
| Grazzi et al., (2020) [45] | Parallel RCT | Chronic migraine with medication overuse | Depression, anxiety | BDI, STAIT | 135 | anode over C4, cathode over contralateral supraorbital area or cathode over C4, anode over contralateral supraorbital area | 2 mA | 20 min | 5 |
| Ibrahim et al., (2018) [46] | Parallel RCT | Visceral pain in hepatocellular carninoma | Depression | HDRS | 48 | anode over the primary motor cortex of the contralateral hemisphere of the most painful abdominal area was used to determine the primary motor area (M1) of the patient, cathode over the opposite supraorbital region cathode F3, anode over F4 | 2 mA | 30 min | 10 |
| Jafari et al., (2021) [47] | Parallel RCT | Social anxiety disorder | Depression | BDI-II | 24 | anode over F3, cathode over F4; | 1 or 2 mA | 20 min | 10 |
| Klauss et al., (2014) [48] | Parallel RCT | Alcohol dependance | Depression, anxiety | HDRS, HARS | 35 | cathode over F3, anode over F4 | 2 mA | 13 min | 5 |
| Liu et al., (2016) [49] | Parallel RCT | Temporal lobe epilepsy | Depression | BDI | 33 | anode over F3, cathode over Fp2 | 2 mA | 30 min | 5 |
| Loo et al., (2010) [50] | Parallel RCT | Depression | Depression | MADRS | 40 | anode over the left dorsolateral prefrontal cortex (DLPFC), identified as pF3 | 1 mA | 20 min | 5 |
| Loo et al., (2012) [51] | Parallel RCT | Depression | Depression | MADRS | 64 | anode over pF3, cathode over F8 | 2 mA | 20 min | 15 |
| Loo et al., (2018) [52] | Parallel RCT | Depression | Depression | MADRS | 84 | anode over F3, Cathode over F8 | 2.5 mA | 30 min | 20 |
| Mariano et al., (2018) [53] | Parallel RCT | Chronic lower back pain | Anxiety | GADS | 21 | cathode over FC1, anode over contralateral (right) mastoid | 2 mA | 30 min | 10 |
| Movahed et al., (2017) [54] | Parallel RCT | Anxiety | Depression, anxiety | HDRS, HARS | 12 | anode on the left deltoid and cathode over F4 | 2 mA | 20 min | 10 |
| Natividade et al., (2019) [55] | Parallel RCT | Obesity | Depression | BDI | 28 | anode electrode over F4, cathode over F3 | 2 mA | 20 min | 20 |
| Pal et al., (2015) [56] | Parallel RCT | Tinnitus | Tinnitus, depression, anxiety | THI, HADS | 42 | anode at F3-Fz-F4 and two cathodes 35.75 cm2 each at T3 | 2 mA | 20 min | 5 |
| Palm et al., (2013) [57] | Crossover RCT | Depression | Depression | HDRS | 22 | anode over F3, cathode over Fp2 | 1 or 2 mA | 20 min | 10 |
| Pegado et al., (2019) [58] | Parallel RCT | Dysmenorrhea | Anxiety | HARS | 22 | anode over C3, cathode over Fp2 | Not reported | 20 min | 5 |
| Salehinjad et al., (2017) [59] | Parallel RCT | Depression | Depression | BDI | 24 | anode over F3, cathode over F4 | 2 mA | 20 min | 10 |
| Samartin-Veiga et al., (2021) [60] | Parallel RCT | Fibromyalgia | Depression | HADS | 54 | C3 and Fp2 or F3 and Fp2 | 2 mA | 20 min | 15 |
| Sampaio-Junior et al., (2017) | Parallel RCT | Bipolar depression | Depression | HDRS, MADRS | 59 | anode right dlPFC, cathode left dlPF | 2 mA | 30 min | 12 |
| Sharafi et al., (2019) [61] | Parallel RCT | Depression | Depression | HDRS | 30 | anode F3 cathode F4 | 2 mA | 20 min | 10 |
| Souza et al., (2020) [62] | Parallel RCT | Tinnitus | Tinnitus | THI | 24 | anode over CP5, cathode over F4 | 2 mA | 20 min | 5 |
| Valiengo et al., (2016) [63] | Parallel RCT | Post-stroke depression | Depression | HDRS, MADRS | 48 | anode over F3, cathode over F4 | 2 mA | 30 min | 12 |
| Vigod et al., (2019) [64] | Parallel RCT | Antenatal depression | Depression | MADRS, Edinburgh Postnatal Depression Scale | 20 | anode over F3, cathode over F4 | 2 mA | 30 min | 15 |
| Yadollahpour et al., (2017) [24] | Parallel RCT | Tinnitus | Tinnitus, depression, anxiety | THI, BDI-II, BAI | 42 | anode over F4, cathode over F3 | 2 mA | 20 min | 5 |
| Yadollahpour et al., (2018) [65] | Parallel RCT | Tinnitus | Tinnitus | THI | 40 | anode halfway between T3 and F7, halfway between T4–F8 | 2 mA | 20 min | 10 |
| Young et al., (2021) [66] | Parallel RCT | Chronic neuropathic pain in MS | Depression, anxiety | DASS-21 | 30 | anodal electrode applied to the C3 or C4 contralateral to the side of pain; if both sides were affected, the side with higher pain level was selected, cathode over the supraorbital area contralateral to the stimulated motor cortex | 2 mA | 20 min | 5 |
| Zhou et al., (2020) [67] | Parallel RCT | Insomnia | Depression | SDS | 90 | anode over left DLPFC and cathode over right DLPFC | 2 mA | 30 min | 24 |
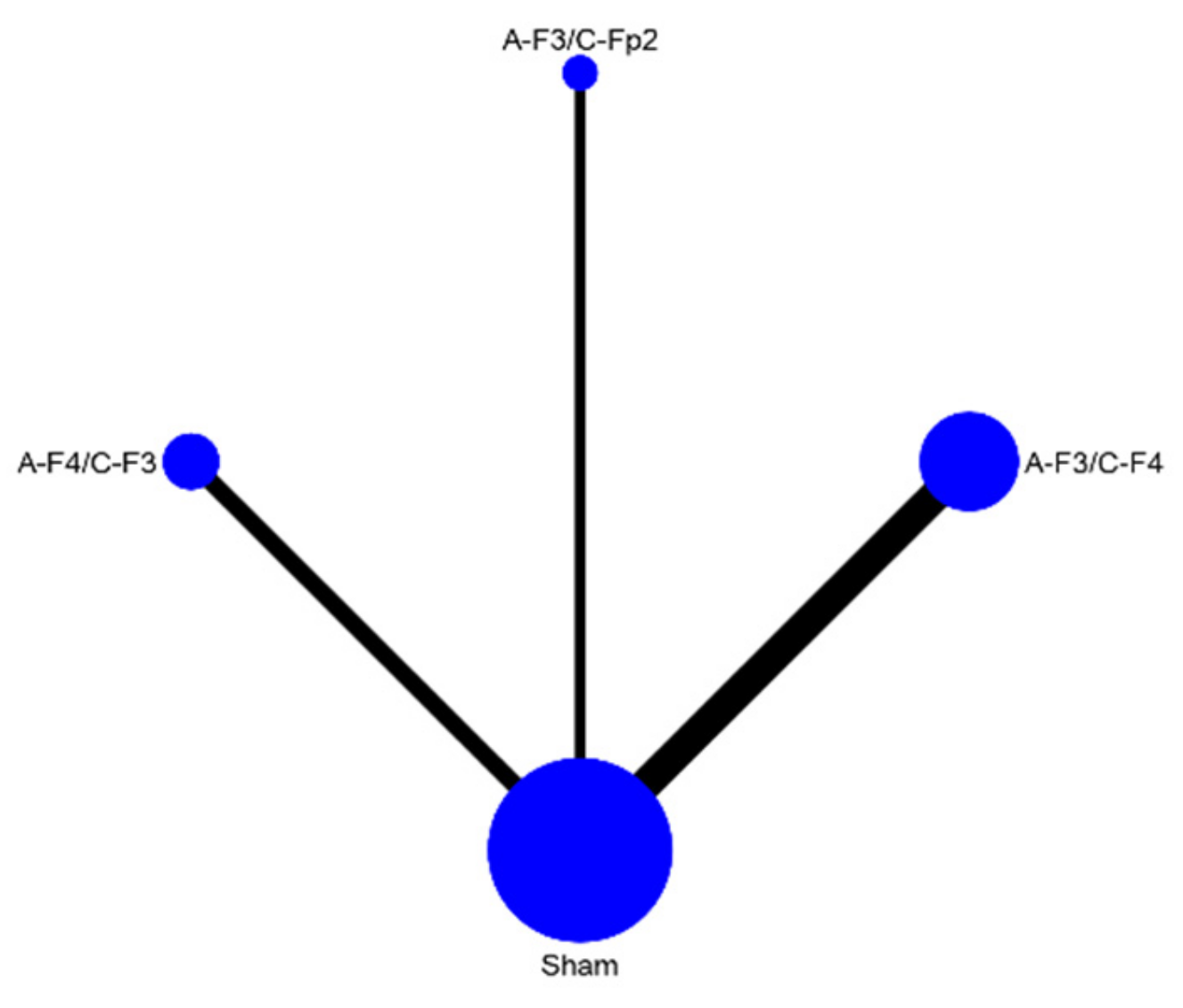
| A-F3/C-F4 | A-F3/C-Fp2 | A-F4/C-F3 | Sham | |||||
|---|---|---|---|---|---|---|---|---|
| Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | |
| Best | 38.9 | −0.591, 1.369 | 38 | −0.6, 1.36 | 23.2 | −0.552, 1.016 | 0 | 0 |
| Second | 40 | −0.58, 1.38 | 26.1 | −0.523, 1.045 | 33.9 | −0.641, 1.319 | 0 | 0 |
| Third | 21.1 | −0.573, 0.995 | 33 | −0.65, 1.31 | 42.1 | −0.559, 1.401 | 3.8 | −0.354, 0.43 |
| Worst | 0 | 0 | 2.9 | −0.363, 0.421 | 0.9 | −0.106, 0.286 | 96.2 | 0.57, 1.354 |
| Mean rank | 1.8 | 0 | 2 | 0 | 2.2 | 0 | 4 | 0 |
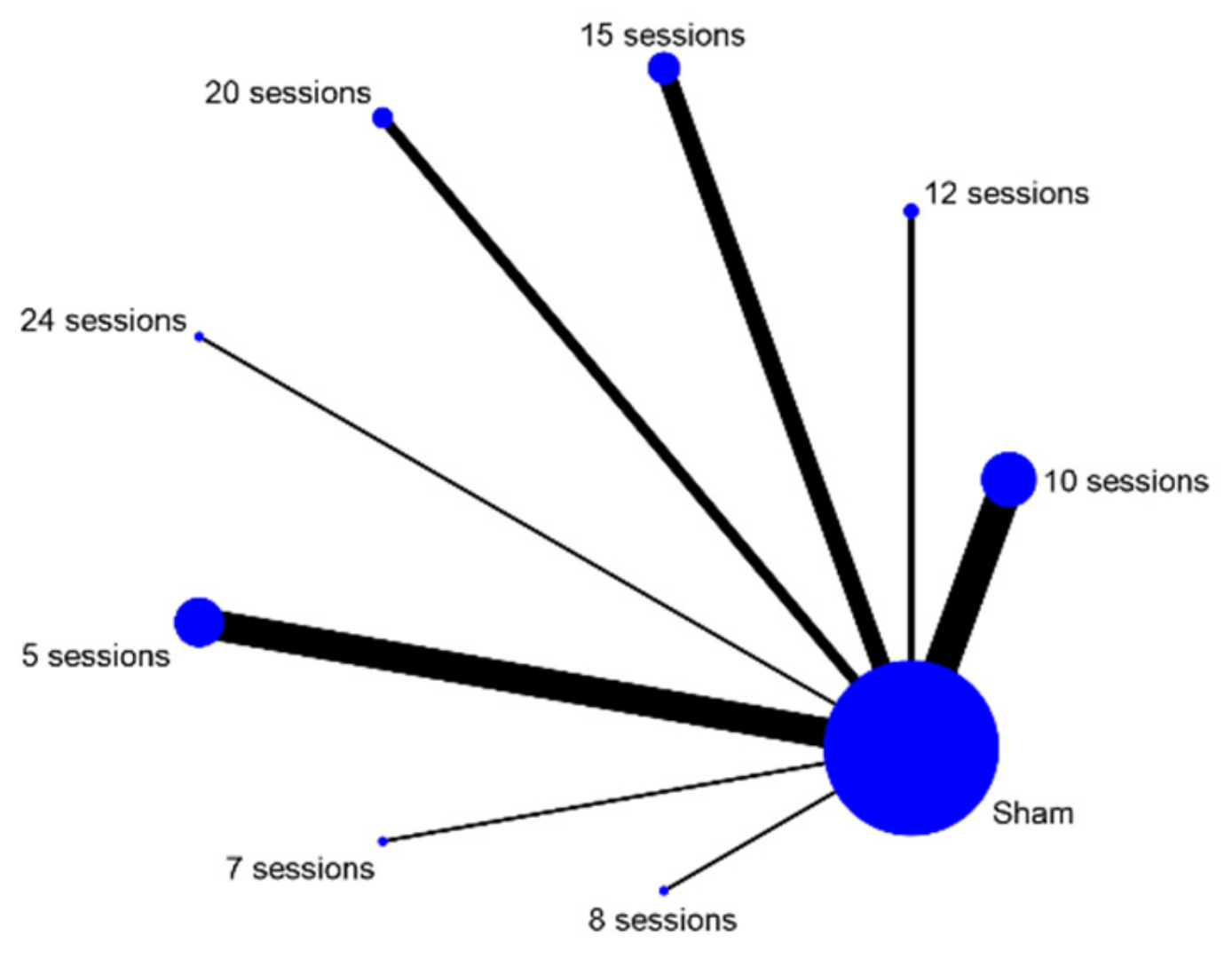
| 5 Sessions | 7 Sessions | 8 Sessions | 10 Sessions | 12 Sessions | |||||
|---|---|---|---|---|---|---|---|---|---|
| Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | |
| Best | 0 | 0 | 89.3 | 0.305, 1.481 | 1.1 | −0.185, 0.207 | 5.5 | −0.337, 0.447 | 1.4 |
| Second | 0.2 | 0.02, 0.02 | 6.7 | −0.521, 0.655 | 6.5 | −0.327, 0.457 | 61.2 | −0.368, 1.592 | 7.9 |
| Third | 6.5 | −0.327, 0.457 | 2.2 | −0.174, 0.218 | 10.9 | −0.479, 0.697 | 27.2 | −0.512, 1.056 | 14.1 |
| Fourth | 18.8 | −0.596, 0.972 | 1.1 | −0.185, 0.207 | 12.9 | −0.459, 0.717 | 5.4 | −0.338, 0.446 | 14.8 |
| Fifth | 27.6 | −0.508, 1.06 | 0.4 | −0.192, 0.2 | 11.1 | −0.477, 0.699 | 0.6 | −0.19, 0.202 | 11.7 |
| Sixth | 23.7 | −0.547, 1.021 | 0.2 | 0.002, 0.002 | 10.1 | −0.487, 0.684 | 0.1 | 0.001, 0.001 | 10.2 |
| Seventh | 14.5 | −0.639, 0.929 | 0.1 | 0.001, 0.001 | 9.6 | −0.492, 0.684 | 0 | 0 | 8.8 |
| Eighth | 6.5 | −0.327, 0.457 | 0.1 | 0.001, 0.001 | 11 | −0.478, 0.698 | 0 | 0 | 10.5 |
| Worst | 2 | −0.176, 0.216 | 0 | 0 | 26.9 | −0.515, 1.053 | 0 | 0 | 20.6 |
| Mean rank | 5.5 | 0 | 1.2 | 0 | 6.1 | 0 | 2.3 | 0 | 5.7 |
| 15 Sessions | 20 Sessions | 24 Sessions | Sham | ||||||
| 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | |
| Best | −0.182, 0.21 | 0 | 0 | 0 | 0 | 2.7 | 0.027, 0.027 | 0 | 0 |
| Second | −0.509, 0.667 | 0.5 | −0.191, 0.201 | 1.1 | −0.185, 0.207 | 15.9 | −0.625, 0.943 | 0 | 0 |
| Third | −0.447, 0.729 | 6.7 | −0.521, 0.655 | 6.9 | −0.519, 0.657 | 25.4 | −0.53, 1.038 | 0 | 0 |
| Fourth | −0.44, 0.736 | 15.9 | −0.625, 0.943 | 12.2 | −0.466, 0.71 | 18.8 | −0.596, 0.972 | 0.1 | 0.001, 0.001 |
| Fifth | −0.471, 0.705 | 20.3 | −0.581, 0.987 | 15.6 | −0.628, 0.94 | 11 | −0.478, 0.698 | 1.6 | −0.18, 0.212 |
| Sixth | −0.486, 0.69 | 20.7 | −0.577, 0.991 | 16.9 | −0.615, 0.953 | 8 | −0.508, 0.668 | 10.1 | −0.487, 0.689 |
| Seventh | −0.5, 0.676 | 16.7 | −0.617, 0.951 | 15.7 | −0.627, 0.941 | 5.9 | 0.333, 0.451 | 28.8 | 0.692, 1.268 |
| Eighth | −0.483, 0.693 | 11.9 | −0.469, 0.707 | 15.5 | −0629, 0.939 | 6 | −0.332, 0.452 | 38.5 | −0.595, 1.365 |
| Worst | −0.578, 0.99 | 7.1 | −0.517, 0.659 | 16.2 | −0.622, 0.946 | 6.3 | −0.329, 0.455 | 20.9 | −0.575, 0.993 |
| Mean rank | 0 | 5.9 | 0 | 6.3 | 0 | 4.4 | 0 | 7.7 | 0 |
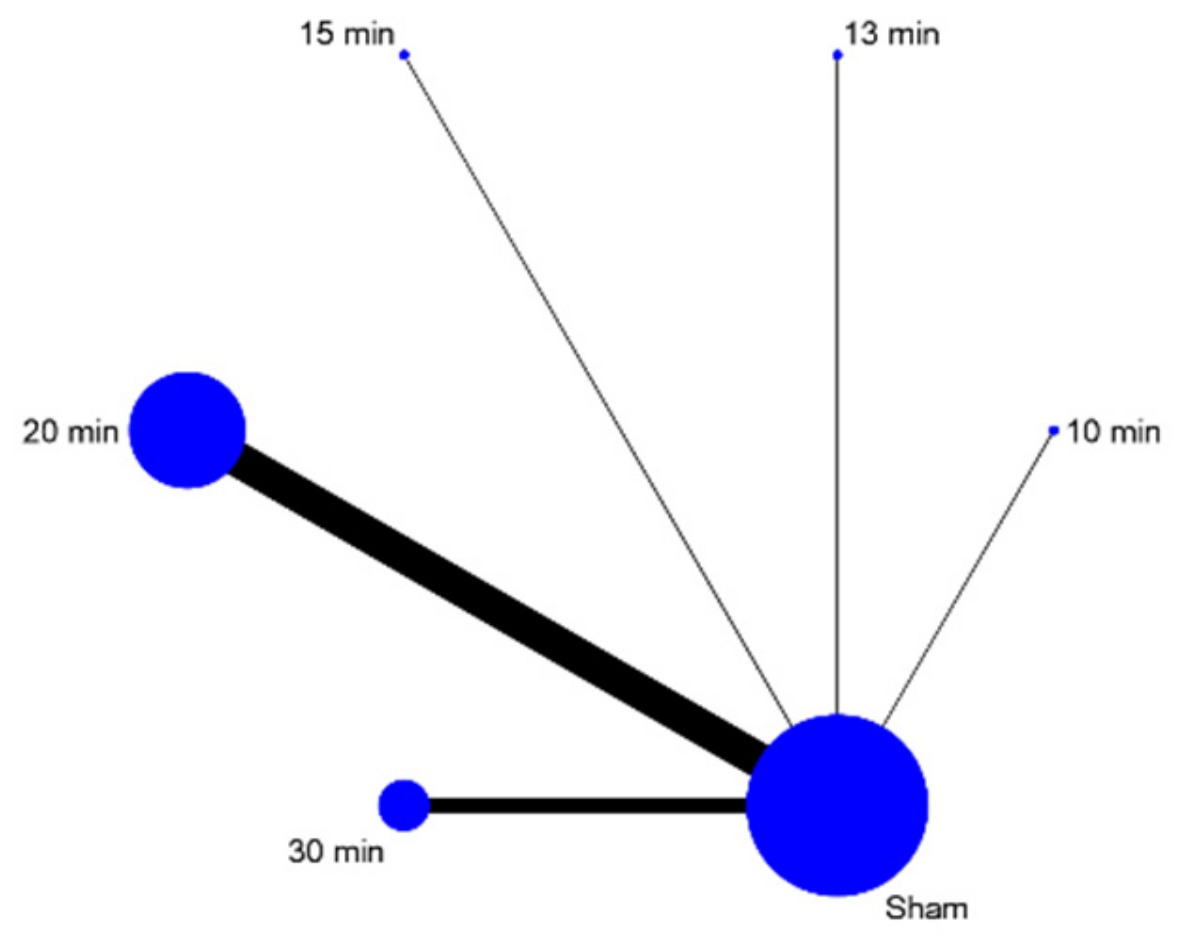
| 10 min | 13 min | 15 min | 20 min | 30 min | Sham | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | Probability | 95% CI | |
| Best | 16.1 | −0.623, 0.945 | 2.5 | −0.367, 0.417 | 10.5 | −0.483, 0.693 | 58.6 | −0.394, 1.566 | 12.3 | −0.465, 0.711 | 0 | 0 |
| Second | 10.3 | −0.485, 0.691 | 3.6 | −0.356, 0.428 | 9.6 | −0.492, 0.684 | 32.3 | −0.657, 1.303 | 44 | −0.54, 1.42 | 0.3 | −0.193, 0.199 |
| Third | 14.4 | −0.64, 0.928 | 9.6 | −0.492, 0.684 | 17 | −0.614, 0.954 | 8.1 | −0.507, 0.669 | 33.1 | −0.649, 1.311 | 17.9 | −0.605, 0.963 |
| Fourth | 14.2 | −0.446, 0.73 | 13.6 | −0.452, 0.724 | 18.6 | −0.598, 0.97 | 1.1 | −0.185, 0.207 | 9.3 | −0.495, 0.681 | 43.2 | −0.548, 1.412 |
| Fifth | 18.7 | −0.597, 0.971 | 26.4 | −0.52, 1.048 | 22.1 | −0.563, 1.005 | 0 | 0 | 1.2 | −0.184, 0.208 | 31.6 | −0.664, 1.296 |
| Worst | 26.3 | −0.521, 1.047 | 44.4 | −0.536, 1.424 | 22.2 | −0.562, 1.006 | 0 | 0 | 0.2 | 0.002, 0.002 | 7 | −0.518, 0.658 |
| Mean rank | 3.9 | 0 | 4.9 | 0 | 4 | 0 | 1.5 | 0 | 2.4 | 0 | 4.3 | 0 |
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Active | Sham Tinnitus | Relative (95% CI) | Absolute (95% CI) | |
| 6 | randomised trials | serious a | serious b | not serious | not serious | strong association | 103 | 85 | - | MD 11.62 lower (18.94 lower to 4.31 lower) | ⨁⨁⨁◯ Moderate |
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Active | Sham Depression | Relative (95% CI) | Absolute (95% CI) | |
| 30 | randomised trials | serious a | very serious b | not serious | not serious | strong association | 644 | 562 | - | SMD 0.61 lower (0.86 lower to 0.35 lower) | ⨁⨁◯◯ Low |
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Active | Sham Anxiety | Relative (95% CI) | Absolute (95% CI) | |
| 15 | randomised trials | serious a | very serious b | not serious | serious c | none | 326 | 266 | - | SMD 0.28 lower (0.93 lower to 0.37 higher) | ⨁◯◯◯ Very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labree, B.; Hoare, D.J.; Gascoyne, L.E.; Scutt, P.; Del Giovane, C.; Sereda, M. Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review. Brain Sci. 2022, 12, 484. https://doi.org/10.3390/brainsci12040484
Labree B, Hoare DJ, Gascoyne LE, Scutt P, Del Giovane C, Sereda M. Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review. Brain Sciences. 2022; 12(4):484. https://doi.org/10.3390/brainsci12040484
Chicago/Turabian StyleLabree, Bas, Derek J. Hoare, Lauren E. Gascoyne, Polly Scutt, Cinzia Del Giovane, and Magdalena Sereda. 2022. "Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review" Brain Sciences 12, no. 4: 484. https://doi.org/10.3390/brainsci12040484
APA StyleLabree, B., Hoare, D. J., Gascoyne, L. E., Scutt, P., Del Giovane, C., & Sereda, M. (2022). Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review. Brain Sciences, 12(4), 484. https://doi.org/10.3390/brainsci12040484






