Synapsin II Directly Suppresses Epileptic Seizures In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
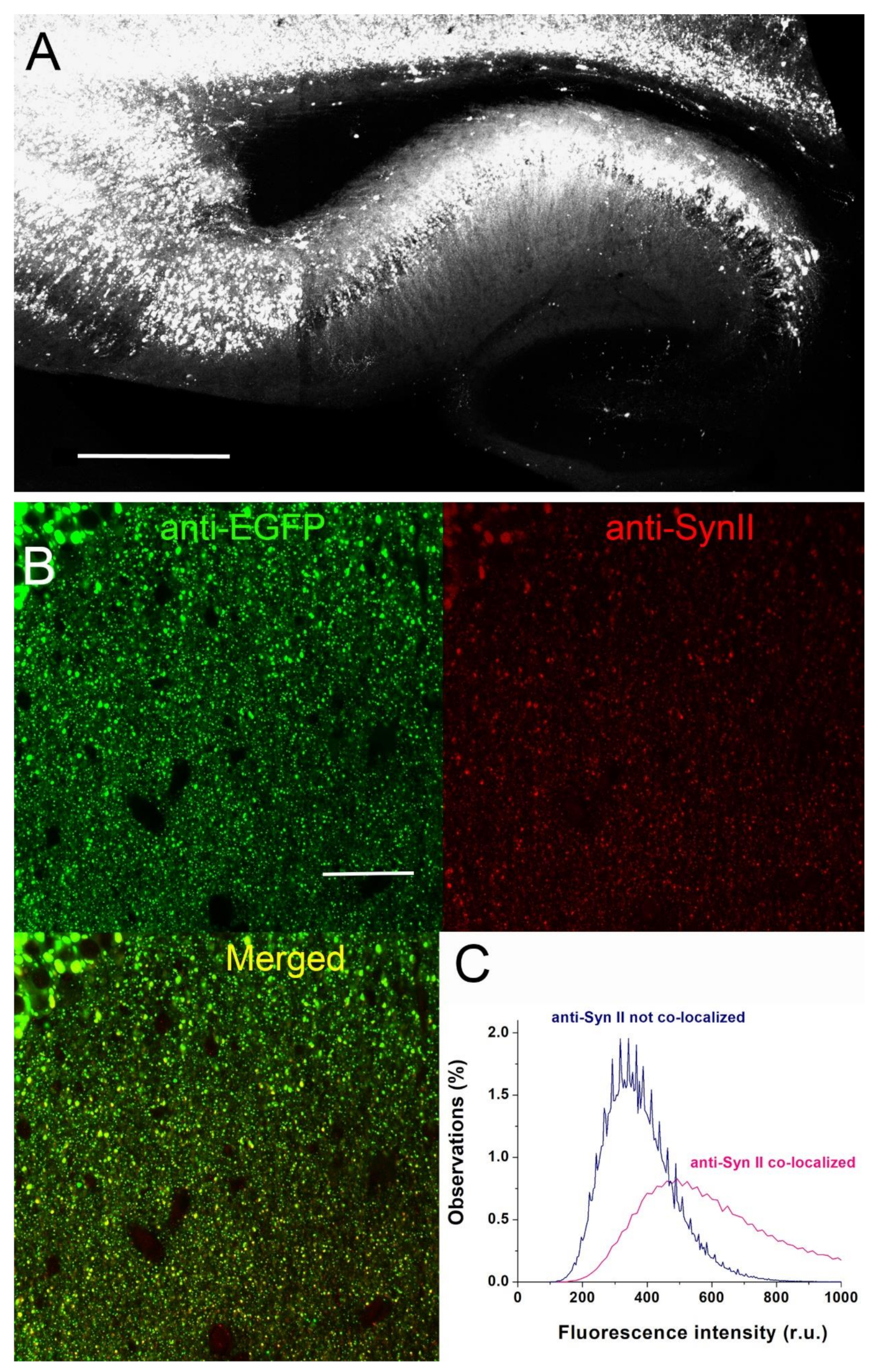
2.2. AAV Injections
2.3. Slice Preparation
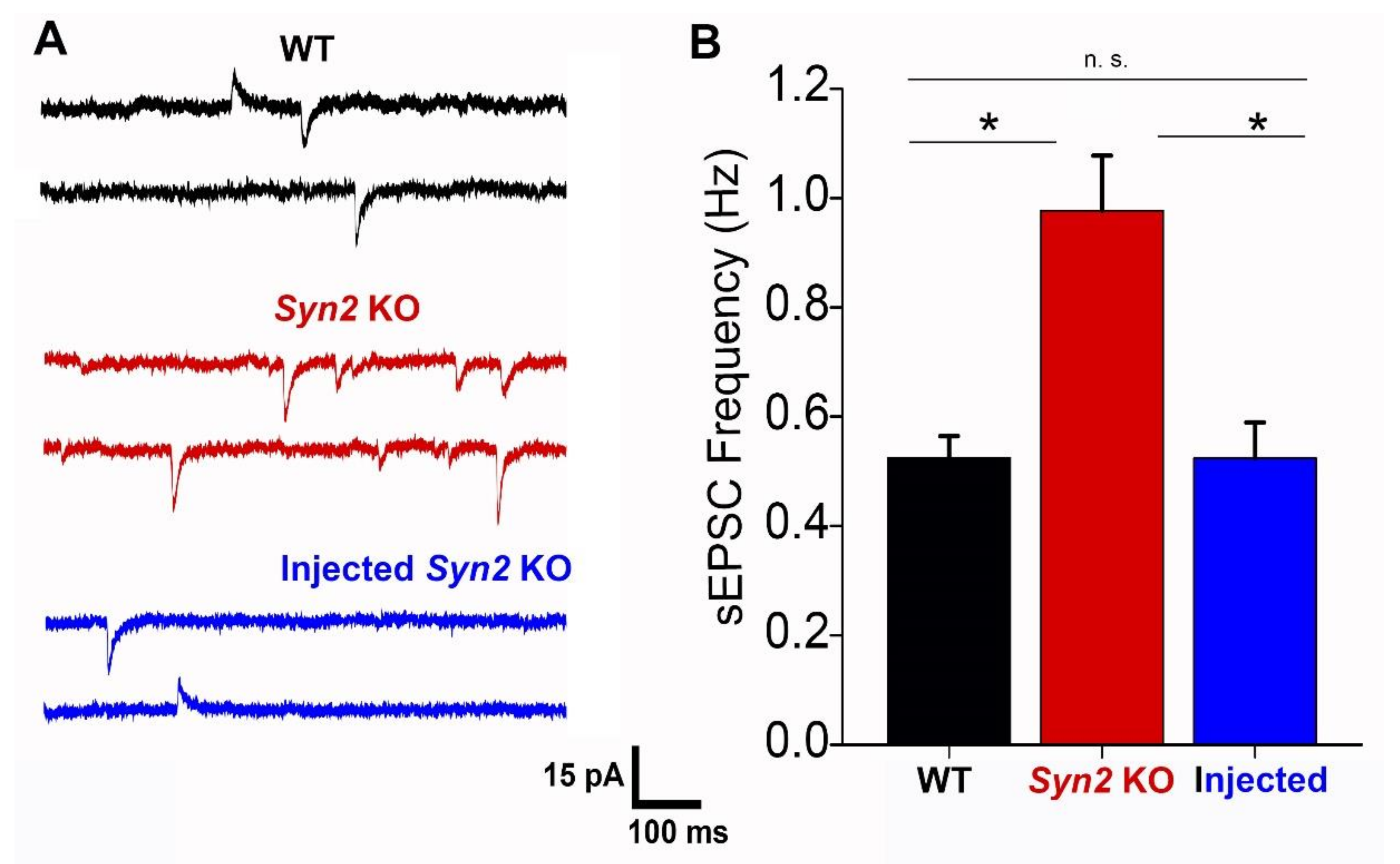
2.4. Electrophysiology
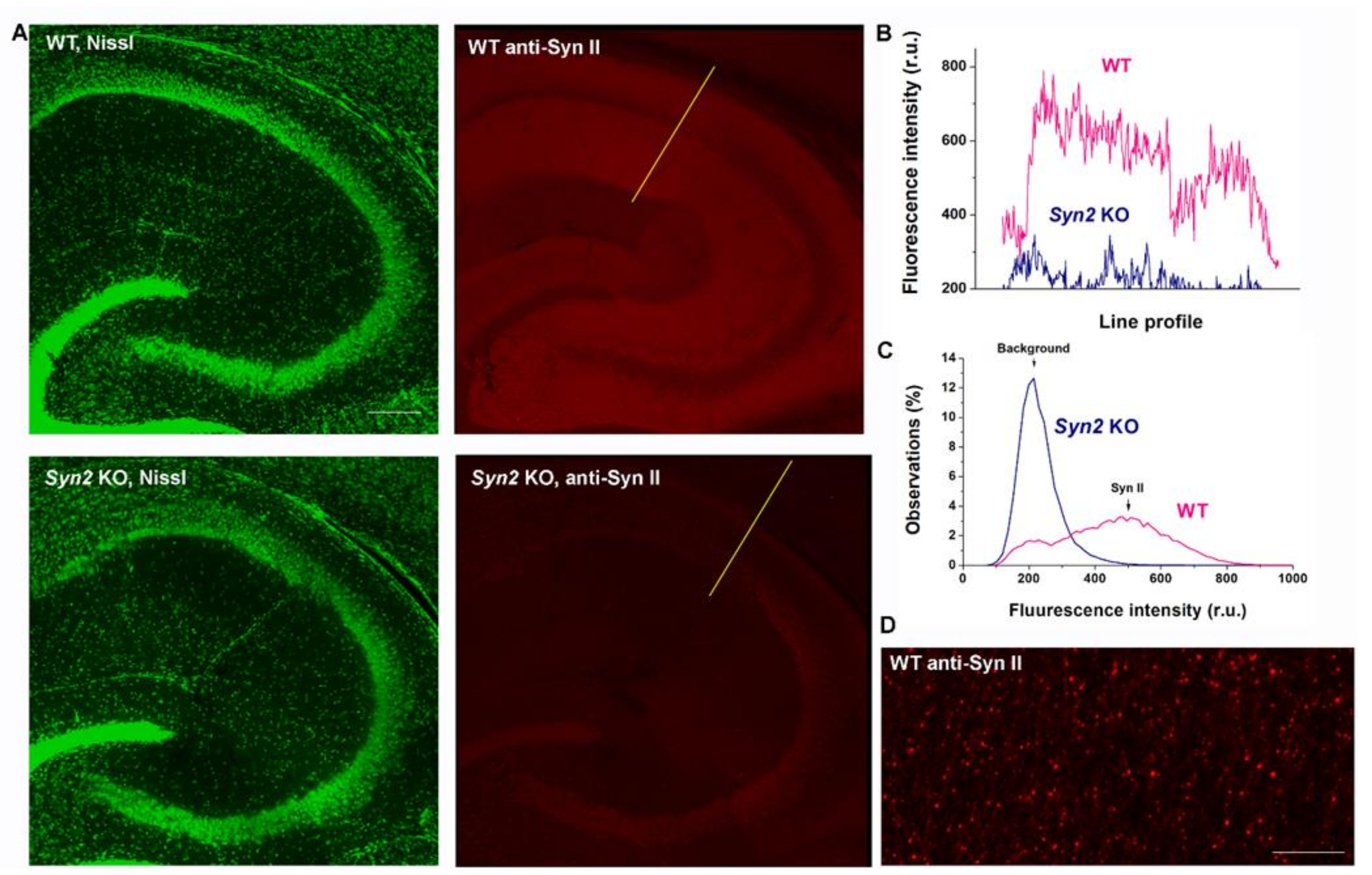
2.5. Immunohistochemistry
2.6. Behavioral Seizures
2.7. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casillas-Espinosa, P.M.; Powell, K.L.; O’Brien, T.J. Regulators of synaptic transmission: Roles in the pathogenesis and treatment of epilepsy. Epilepsia 2012, 53, 41–58. [Google Scholar] [CrossRef]
- Fassio, A.; Raimondi, A.; Lignani, G.; Benfenati, F.; Baldelli, P. Synapsins: From synapse to network hyperexcitability and epilepsy. Semin. Cell. Dev. Biol. 2011, 22, 408–415. [Google Scholar] [CrossRef] [PubMed]
- De Camilli, P.; Benfenati, F.; Valtorta, F.; Greengard, P. The synapsins. Annu. Rev. Cell Biol. 1990, 6, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Greengard, P.; Valtorta, F.; Czernik, A.J.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 259, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Bykhovskaia, M. Synapsin regulation of vesicle organization and functional pools. Semin. Cell Dev. Biol. 2011, 22, 387–392. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef]
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.T.; Augustine, G.J.; Greengard, P. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 269–279. [Google Scholar] [CrossRef]
- Shupliakov, O.; Haucke, V.; Pechstein, A. How synapsin I may cluster synaptic vesicles. Semin. Cell Dev. Biol. 2011, 22, 393–399. [Google Scholar] [CrossRef]
- Hosaka, M.; Hammer, R.E.; Sudhof, T.C. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 1999, 24, 377–387. [Google Scholar] [CrossRef]
- Li, L.; Chin, L.S.; Shupliakov, O.; Brodin, L.; Sihra, T.S.; Hvalby, O.; Jensen, V.; Zheng, D.; McNamara, J.O.; Greengard, P.; et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc. Natl. Acad. Sci. USA 1995, 92, 9235–9239. [Google Scholar] [CrossRef]
- Rosahl, T.W.; Spillane, D.; Missler, M.; Herz, J.; Selig, D.K.; Wolff, J.R.; Hammer, R.E.; Malenka, R.C.; Sudhof, T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 1995, 375, 488–493. [Google Scholar] [CrossRef]
- Garcia, C.C.; Blair, H.J.; Seager, M.; Coulthard, A.; Tennant, S.; Buddles, M.; Curtis, A.; Goodship, J.A. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J. Med. Genet. 2004, 41, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Patry, L.; Congia, S.; Onofri, F.; Piton, A.; Gauthier, J.; Pozzi, D.; Messa, M.; Defranchi, E.; Fadda, M.; et al. Syn1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum. Mol. Genet. 2011, 20, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, F.C.; Pozzi, D.; Raimondi, A.; Fesce, R.; Valente, M.M.; Delvecchio, V.S.; Van Esch, H.; Matteoli, M.; Benfenati, F.; D’Adamo, P.; et al. A novel Syn1 missense mutation in non-syndromic X-linked intellectual disability affects synaptic vesicle life cycle, clustering and mobility. Hum. Mol. Genet. 2017, 26, 4699–4714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J.; Xia, L.; Li, R.; Zhang, Q.; Pan, S. Syn1 Mutation Causes X-Linked Toothbrushing Epilepsy in a Chinese Family. Front. Neurol. 2021, 12, 736977. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, G.L.; Weale, M.E.; Shianna, K.V.; Singh, R.; Lynch, J.M.; Grinton, B.; Szoeke, C.; Murphy, K.; Kinirons, P.; O’Rourke, D.; et al. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: A case-control study. Lancet Neurol. 2007, 6, 970–980. [Google Scholar] [CrossRef]
- Lakhan, R.; Kalita, J.; Misra, U.K.; Kumari, R.; Mittal, B. Association of intronic polymorphism rs3773364 A>G in synapsin-2 gene with idiopathic epilepsy. Synapse 2010, 64, 403–408. [Google Scholar] [CrossRef]
- Prasad, D.K.; Shaheen, U.; Satyanarayana, U.; Prabha, T.S.; Jyothy, A.; Munshi, A. Association of GABRA6 1519 T>C (rs3219151) and Synapsin II (rs37733634) gene polymorphisms with the development of idiopathic generalized epilepsy. Epilepsy Res. 2014, 108, 1267–1273. [Google Scholar] [CrossRef]
- Etholm, L.; Bahonjic, E.; Heggelund, P. Sensitive and critical periods in the development of handling induced seizures in mice lacking synapsins: Differences between synapsin I and synapsin II knockouts. Exp. Neurol. 2013, 247, 59–65. [Google Scholar] [CrossRef]
- Etholm, L.; Bahonjic, E.; Walaas, S.I.; Kao, H.T.; Heggelund, P. Neuroethologically delineated differences in the seizure behavior of synapsin 1 and synapsin 2 knock-out mice. Epilepsy Res. 2012, 99, 252–259. [Google Scholar] [CrossRef]
- Etholm, L.; Heggelund, P. Seizure elements and seizure element transitions during tonic-clonic seizure activity in the synapsin I/II double knockout mouse: A neuroethological description. Epilepsy Behav. 2009, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ketzef, M.; Kahn, J.; Weissberg, I.; Becker, A.J.; Friedman, A.; Gitler, D. Compensatory network alterations upon onset of epilepsy in synapsin triple knock-out mice. Neuroscience 2011, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, C.E.; Grier, B.D.; Belluscio, L. Bulk regional viral injection in neonatal mice enables structural and functional interrogation of defined neuronal populations throughout targeted brain areas. Front. Neural. Circuits 2015, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ash, R.T.; Ceballos-Diaz, C.; Levites, Y.; Golde, T.E.; Smirnakis, S.M.; Jankowsky, J.L. Viral transduction of the neonatal brain delivers controllable genetic mosaicism for visualising and manipulating neuronal circuits in vivo. Eur. J. Neurosci. 2013, 37, 1203–1220. [Google Scholar] [CrossRef]
- Kim, J.Y.; Grunke, S.D.; Levites, Y.; Golde, T.E.; Jankowsky, J.L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J. Vis. Exp. 2014, 91, 51863. [Google Scholar] [CrossRef]
- Weber, E.T.; Andrade, R. Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front. Neurosci. 2010, 4, 36. [Google Scholar]
- Coleman, W.L.; Bykhovskaia, M. Cooperative regulation of neurotransmitter release by Rab3a and synapsin II. Mol. Cell. Neurosci. 2010, 44, 190–200. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Bykhovskaia, M. Making quantal analysis more convenient, fast, and accurate: User-friendly software QUANTAN. J. Neurosci. Methods 2008, 168, 500–513. [Google Scholar] [CrossRef]
- Gitler, D.; Xu, Y.; Kao, H.T.; Lin, D.; Lim, S.; Feng, J.; Greengard, P.; Augustine, G.J. Molecular determinants of synapsin targeting to presynaptic terminals. J. Neurosci. 2004, 24, 3711–3720. [Google Scholar] [CrossRef]
- Feliciano, P.; Andrade, R.; Bykhovskaia, M. Synapsin II and Rab3a cooperate in the regulation of epileptic and synaptic activity in the CA1 region of the hippocampus. J. Neurosci. 2013, 33, 18319–18330. [Google Scholar] [CrossRef] [PubMed]
- Boido, D.; Farisello, P.; Cesca, F.; Ferrea, E.; Valtorta, F.; Benfenati, F.; Baldelli, P. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: Age-dependency and response to the antiepileptic drug levetiracetam. Neuroscience 2010, 171, 268–283. [Google Scholar] [CrossRef]
- Etholm, L.; Linden, H.; Eken, T.; Heggelund, P. Electroencephalographic characterization of seizure activity in the synapsin I/II double knockout mouse. Brain Res. 2011, 1383, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Etholm, L.; Arabadzisz, D.; Lipp, H.P.; Heggelund, P. Seizure logging: A new approach to synchronized cable-free EEG and video recordings of seizure activity in mice. J. Neurosci. Methods 2010, 192, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Ali, I.; Bakochi, A.; Bahonjic, E.; Etholm, L.; Ekdahl, C.T. Alterations in Brain Inflammation, Synaptic Proteins, and Adult Hippocampal Neurogenesis during Epileptogenesis in Mice Lacking Synapsin2. PLoS ONE 2015, 10, e0132366. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Takagishi, Y.; Feng, J.; Ren, Y.; Rodriguiz, R.M.; Wetsel, W.C.; Greengard, P.; Augustine, G.J. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci. 2004, 24, 11368–11380. [Google Scholar] [CrossRef]
- Chiappalone, M.; Casagrande, S.; Tedesco, M.; Valtorta, F.; Baldelli, P.; Martinoia, S.; Benfenati, F. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb. Cortex 2009, 19, 1422–1439. [Google Scholar] [CrossRef] [PubMed]
- Medrihan, L.; Cesca, F.; Raimondi, A.; Lignani, G.; Baldelli, P.; Benfenati, F. Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat. Commun. 2013, 4, 1512. [Google Scholar] [CrossRef]
- Medrihan, L.; Ferrea, E.; Greco, B.; Baldelli, P.; Benfenati, F. Asynchronous GABA Release Is a Key Determinant of Tonic Inhibition and Controls Neuronal Excitability: A Study in the Synapsin II-/- Mouse. Cereb. Cortex 2015, 10, 3356–3368. [Google Scholar] [CrossRef]
- Matos, H.; Quiles, R.; Andrade, R.; Bykhovskaia, M. Growth and excitability at synapsin II deficient hippocampal neurons. Mol. Cell. Neurosci. 2019, 96, 25–34. [Google Scholar] [CrossRef]
- Ketzef, M.; Gitler, D. Epileptic synapsin triple knockout mice exhibit progressive long-term aberrant plasticity in the entorhinal cortex. Cereb. Cortex 2014, 24, 996–1008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilfiker, S.; Benfenati, F.; Doussau, F.; Nairn, A.C.; Czernik, A.J.; Augustine, G.J.; Greengard, P. Structural domains involved in the regulation of transmitter release by synapsins. J. Neurosci. 2005, 25, 2658–2669. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.L.; Bill, C.A.; Simsek-Duran, F.; Lonart, G.; Samigullin, D.; Bykhovskaia, M. Synapsin II and calcium regulate vesicle docking and the cross-talk between vesicle pools at the mouse motor terminals. J. Physiol. 2008, 586, 4649–4673. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwark, R.; Andrade, R.; Bykhovskaia, M. Synapsin II Directly Suppresses Epileptic Seizures In Vivo. Brain Sci. 2022, 12, 325. https://doi.org/10.3390/brainsci12030325
Schwark R, Andrade R, Bykhovskaia M. Synapsin II Directly Suppresses Epileptic Seizures In Vivo. Brain Sciences. 2022; 12(3):325. https://doi.org/10.3390/brainsci12030325
Chicago/Turabian StyleSchwark, Ryan, Rodrigo Andrade, and Maria Bykhovskaia. 2022. "Synapsin II Directly Suppresses Epileptic Seizures In Vivo" Brain Sciences 12, no. 3: 325. https://doi.org/10.3390/brainsci12030325
APA StyleSchwark, R., Andrade, R., & Bykhovskaia, M. (2022). Synapsin II Directly Suppresses Epileptic Seizures In Vivo. Brain Sciences, 12(3), 325. https://doi.org/10.3390/brainsci12030325





