Abstract
Multiple lines of evidence suggest that a deficiency of Fragile X Mental Retardation Protein (FMRP) mediates dysfunction of the metabotropic glutamate receptor subtype 5 (mGluR5) in the pathogenesis of fragile X syndrome (FXS), the most commonly known single-gene cause of inherited intellectual disability (ID) and autism spectrum disorder (ASD). Nevertheless, animal and human studies regarding the link between FMRP and mGluR5 expression provide inconsistent or conflicting findings about the nature of those relationships. Since multiple clinical trials of glutamatergic agents in humans with FXS did not demonstrate the amelioration of the behavioral phenotype observed in animal models of FXS, we sought measure if mGluR5 expression is increased in men with FXS to form the basis for improved clinical trials. Unexpectedly marked reductions in mGluR5 expression were observed in cortical and subcortical regions in men with FXS. Reduced mGluR5 expression throughout the living brains of men with FXS provides a clue to examine FMRP and mGluR5 expression in FXS. In order to develop the findings of our previous study and to strengthen the objective tools for future clinical trials of glutamatergic agents in FXS, we sought to assess the possible value of measuring both FMRP levels and mGluR5 expression in men with FXS. We aimed to show the value of measurement of FMRP levels and mGluR5 expression for the diagnosis and treatment of individuals with FXS and related conditions. We administered 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB), a specific mGluR5 radioligand for quantitative measurements of the density and the distribution of mGluR5s, to six men with the full mutation (FM) of FXS and to one man with allele size mosaicism for FXS (FXS-M). Utilizing the seven cortical and subcortical regions affected in neurodegenerative disorders as indicator variables, adjusted linear regression of mGluR5 expression and FMRP showed that mGluR5 expression was significantly reduced in the occipital cortex and the thalamus relative to baseline (anterior cingulate cortex) if FMRP levels are held constant (F(7,47) = 6.84, p < 0.001).These findings indicate the usefulness of cerebral mGluR5 expression measured by PET with [18F]FPEB and FMRP values in men with FXS and related conditions for assessments in community facilities within a hundred-mile radius of a production center with a cyclotron. These initial results of this pilot study advance our previous study regarding the measurement of mGluR5 expression by combining both FMRP levels and mGluR5 expression as tools for meaningful clinical trials of glutamatergic agents for men with FXS. We confirm the feasibility of this protocol as a valuable tool to measure FMRP levels and mGluR5 expression in clinical trials of individuals with FXS and related conditions and to provide the foundations to apply precision medicine to tailor treatment plans to the specific needs of individuals with FXS and related conditions.
1. Introduction
Fragile X syndrome (FXS), the leading monogenetic cause of intellectual disability (ID) and autism spectrum disorder (ASD), results from excessive trinucleotide cytosine–guanine–guanine (CGGn) repeats in the promotor region [1,2,3,4] of the fragile X mental retardation 1 (FMR1) gene. The FMR1 gene leads to the development in healthy humans with typical development (TD) of Fragile X Mental Retardation Protein (FMRP) [1,5,6,7], an RNA binding protein playing key roles in the protein synthesis of dendritic spines by controlling 4% of human cerebral mRNA translation [1,8,9,10]. The normal sequence of the FMR1 gene facilitating the growth of FMRP throughout the body is accomplished in healthy individuals with TD by means of a normal number of trinucleotide CGGn repeats in the promotor region [1,2,3,4] of the FMR1 gene. Healthy individuals with typical development (TD) have a CGG repeat sequence of approximately 6 to 54 [11] categorized as low zone (<24 CGG), normal (24–42 CGG), and gray zone (42–54 CGG) [12]. Individuals with 55 to 200 CGG repeats are given premutation (PM) or carrier status [13,14,15,16] leading to fragile X tremor and ataxia syndrome (FXTAS) or fragile X primary ovarian insufficiency (FXPOI) [12]. An intermediate zone of approximately 45 to 60 repeats characterizes individuals between the typical and PM status [17]. The full mutation (FM), clinical FXS, is characterized by more than 200 CGG repeats, resulting in epigenetic silencing of the FMR1 gene via hypermethylation leading to a marked deficiency of the gene’s product: FMRP. Studies with Fmr1 knockout (KO) rodent models demonstrate that FMRP is required for neuronal dynamics at multiple levels in the auditory [18], limbic [19], and sensory systems [20]. Furthermore, FMRP suppresses translation of the endoplasmic reticulum stress response augmented by amyloid beta (Aβ), a toxic peptide that accumulates in the brains of people with Alzheimer’s disease and other cognitive deficits [21].
Glutamate, another agent playing a role in stress responses throughout the body, is the major excitatory neurotransmitter. Glutamate is needed for the development and function of neurons throughout the nervous system. The vesicular glutamate transporter takes glutamate in presynaptic neurons to vesicles to be released into the synapse and to act on receptors in post-synaptic neurons to generate signals to activate the neurons [22]. Glutamine acts upon both (A) ionotropic receptors including N-methyl-d-aspartate (NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainic acid (KA) receptors, and (B) metabotropic glutamate receptors. Additionally, kyurenic acid, a noncompetitive antagonist of the N-methyl-d-aspartate (NMDA) glutamate receptor, plays a role in the release of glutamate, dopamine, and acetylcholine [22,23]. Dysfunction of glutamatergic neurotransmission plays a role in mood disorders [22,23], Alzheimer’s disease [24,25] and other neuropsychiatric disorders. The development of pharmacological agents for the glutamatergic systems that offer promise for multiple neuropsychiatric disorders [26] provide the motivation for continued efforts to develop glutamatergic agents to alleviate the symptoms and signs of FXS.
To utilize the key role of the glutamatergic system in the understanding of the pathogenesis of FXS, the mGluR theory was postulated on the observation that activation of group I metabotropic glutamate receptors (metabotropic glutamate receptors subtypes 1 and 5 (mGluR1/5)) [27] is associated with dysregulated downstream signaling cascades [28] based on the loss of the regulation of translation in the absence of FMRP. The affected signaling cascades include the mammalian target of rapamycin (mTOR) [29,30,31,32,33], the microtubule-associated protein kinase (MAPK) [34], the protein kinase A (PKA) [35], and the extracellular signal-regulated kinase (ERK) pathways, which may contribute to metabotropic glutamate receptor dependent long-term depression (mGluR-LTD) and to the neurobehavioral symptoms of FXS in Fmr1 knockout (KO) mouse models [7,15,16,36,37,38]. Although the mGluR theory hypothesizes a possible mechanism for FXS in Fmr1 KO mouse models [30,39], mGluR-based clinical trials have not yielded improvements in the behavioral phenotype of FXS in humans [39,40,41]. The addition of measurements of FMRP levels to the measurements of mGluR expression in the relevant regions of the brains of men with FXS may facilitate future clinical trials for FXS. The linkage of FMRP values and the expression of metabotropic glutamate receptors subtype 5 (mGluR5) in the living human brain could provide a tool to ameliorate cognitive and behavioral symptoms associated with FXS such as ASD [2,6,7,41,42].
Nevertheless, the role of metabotropic glutamate receptors subtype 5 (mGluR5) in the expression of the neurobehavioral phenotype of FXS [7,8,15,16] is unknown. Although human autopsy and animal imaging studies have reported inconsistent values for mGluR5 [41,42], mGluR5 expression was decreased in studies of KO mouse models of FXS [43]. Additionally, reduced protein synthesis in cerebral [44] and peripheral measurements [45] in FXS raises questions to the mGluR theory [9] that require further clarification.
Although the behavioral benefits of negative allosteric modulators (NAMs) in animal models of FXS [46] were not translated in multiple clinical trials of humans with FXS [40,41], there may be different mechanisms in animal models of FXS and humans with FXS [47]. Since NAMs bind allosterically to mGluR5s as noncompetitive antagonists, reduced post-synaptic excitation [48] may decrease mGluR5 expression in humans with FXS. Furthermore, decreased connectivity between mGluR5s and Homer proteins, the primary members of the post-synaptic density (PSD) connecting mGluR5s to their signaling complexes [49], may occur in FXS. The positive behavioral effects of deletion of the gene responsible for the irregular mGluR5–Homer scaffold interactions supports a relationship to the phenotype in FXS [50]. Tolerance following chronic treatment with NAMs, acquired treatment resistance downstream of glycogen synthetase kinase 3α (GSK3α) and upstream of protein synthesis [51,52,53], may play a role in the negligible response to NAMs in human clinical trials. Chronic treatment of Fmr1 KO mice with an inhibitor of GSK3α and GSK3β reversed the social discrimination deficit observed in the mice [54]. While some recent data in humans with FXS suggested decreased protein synthesis was observed in cerebral [44] and peripheral measurements [45], the majority of prior studies have found increased protein synthesis in the cells of people with FXS [5,55].
Clinical trials of mGluR5 NAMs for FXS were limited by the variable age groups of study participants, problematic outcome measurements, placebo effects, and potentially the absence of a tool to measure the expression of mGluR5 in the living brains of participants with FXS [2,42,43,44,46,56,57]. Our finding that mGluR5 expression measured by positron emission tomography (PET) with 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB), a potent and safe specific mGluR5 inhibitor (Figure 1 and Figure 2) [58,59], is reduced in all brain regions in men with FXS [2] compared to participants of both sexes with ASD and TD [2,42,60,61,62], was confirmed utilizing positron emission tomography and magnetic resonance (PET/MR) imaging on an unmedicated cohort of older men with FXS and age- and sex-matched participants with TD suggesting that the current protocol may be a valuable biomarker for mGluR5 expression in clinical trials of novel agents for humans with FXS and other subtypes of ASD [63]. We showed that [18F]FPEB may be a promising tool to obtain quantitative measurements of mGluR5 expression in individuals with FXS for clinical trials and other investigations [2,42,64,65,66,67]. Since our prior finding of reduced cerebral mGluR5 expression in cortical and subcortical regions of men with FXS has been confirmed [41,63], we sought expand the previous protocol of cerebral mGluR5 expression alone with a more refined measure tool to include simultaneous FRMP levels and cerebral mGluR5 expression for clinical trials of FXS. Here, we seek to investigate the relationship between FMRP [68] and mGluR5 expression in unmedicated men with the FM of FXS [15,16].
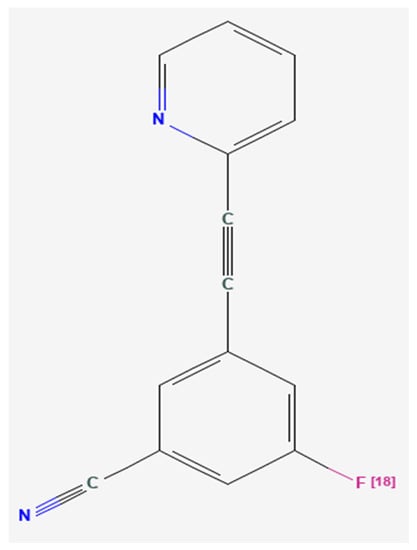
Figure 1.
Chemical structure of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) [58], a potent, specific inhibitor of the metabotropic glutamate receptor subtype 5 (mGluR5) [60].
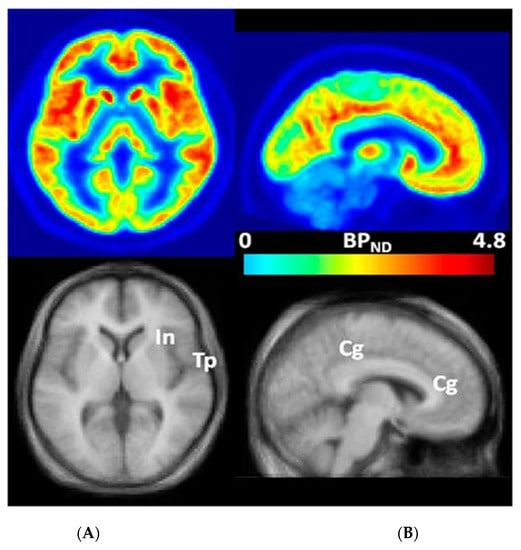
Figure 2.
Transaxial (A) and sagittal (B) non-displaceable binding potential (BPND) [69] images of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) (top) and matching magnetic resonance (MR) images (bottom) in statistical parametric mapping (SPM) [60,70] standard space. Regions with high BPND values, namely, insular (In), temporal (Tp), and cingulate (Cg) cortices, are indicated on co-registered MR images [60,70]. This research was originally published in JNM. Wong, D.F.; Waterhouse, R.; Kuwabara, H.; Kim, J.; Brašić, J.R.; Chamroonrat, W.; Stabins, M.; Holt, D.P.; Dannals, R.F.; Hamill, T.G.; Mozley, P.D. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: A first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J. Nucl. Med. 2013, 54, 388–396. © SNMMI [60].
Development of interventions to ameliorate the specific molecular deficits of individuals with FXS may facilitate the development of treatment plans to address the unique needs of each person [41,42,64,71,72,73]. We aimed to determine if (A) FMRP levels are correlated with mGluR5 expression in men with the FM of FXS [2,41,42] and (B) the measurement of FMRP and PET with ([18F]FPEB) is feasible in men with FXS [2,41,42,60,61]. The proposed protocol could then be utilized as a screening measure in pilot studies to optimize dosing for people with FXS and related conditions.
2. Materials and Methods
2.1. Participants
2.1.1. Recruiting Sites
The study is approved by Johns Hopkins Medicine Institutional Review Board IRB 169,249 [2]. The protocols for the study of humans were approved by the Institutional Review Boards of the Institute for Neurodegenerative Disorders (IND) in New Haven, Connecticut [74] and the Johns Hopkins University (JHU) in Baltimore, Maryland [75,76]. Since exposure to radioactivity in PET constitutes greater than minimal risk, this pilot study was restricted to adults [2,42]. Written informed consent was obtained from each participant at both locations.
Although we have reported the data of eight participants from IND and four participants from JHU [2,42,60,64,65,66,67,71,72], we currently report only the finding of six men with the FM of FXS and one man with FXS-M with a premutation allele of 181–189 CGG repeats close to the allele size of people with the FM (>200 CGG repeats) from IND. We excluded the data of men from JHU due to the uncertain effects of the markedly different protocols at IND and JHU. We excluded another participant from IND due to missing data. We now report the findings of a cohort at the IND of six Caucasian males with the FM of FXS (mean age 27.8 ± 4.7, range (22.3, 33.6) years) and a Caucasian man with an FXS-M (age 56.6 years) [2,42,60,62,64,65,66,67,71,72,73] (Table 1) [62].

Table 1.
Clinical characteristics of Caucasian male participants with the full mutation (FM) or allele size mosaicism (FXS-M) (age = 56.6) of fragile X syndrome (FXS) [62].
2.1.2. Inclusion Criteria
Inclusion criteria for all participants included age between 18 and 60 years [2,42,74]. Participants with FXS had a diagnosis of the FM or FXS-M of FXS based on FMR1 DNA gene testing by polymerase chain reaction (PCR)/Southern Blot [77] on peripheral venous blood samples [11,15,16], supplemented by clinical neurobehavioral profiling [2,7,42,62].
2.1.3. Exclusion Criteria
Exclusion criteria were clinically significant abnormal laboratory values and/or clinically significant unstable serious medical, neurological, or psychiatric illnesses [2,42,60,62,74].
2.2. Procedures
2.2.1. Fragile X Mental Retardation Protein (FMRP)
Enzyme-Linked Immunoassay (ELISA)
Primary lymphocytes or fibroblasts were quickly thawed and spun at 2000× g for 10 min. Pelleted cells were resuspended in phosphate-buffered saline (PBS) containing protease inhibitor tablet and washed two times more. Cells were lysed in the presence of protease inhibitors, rotated overnight at 4 °C and spun at 16,000× g for 15 min. Supernatant was removed and aliquoted for storage at −80 °C. Quantitation of total protein concentration was accomplished with a bicinchoninic acid protein assay kit. Then, 96-well plates were coated with a chicken antibody generated to the peptide sequence near the carboxy terminus, KDRNQKKEKPDSVD (Aves Labs, Inc. Tigard, OR, USA), and 100 uL of lymphocyte extract or FMRP was added to the prepared wells and incubated overnight at room temperature. Following extensive washing, detection antibody, 1:10,000 (v:v) mouse anti-FMRP (1C3, MilliporeSigma, Temecula, CA, USA), was added to each well and incubated for 8–10 h at room temperature. Wells were washed and incubated with peroxidase conjugated secondary antibody to mouse IgG. Detection was accomplished with a luminescent peroxidase substrate. All lymphocyte samples were assessed at multiple dilutions, and concentrations of FMRP were calculated relative to a reference sample of purified FMRP of known concentration using a 4-parameter fit logistics curve. Coefficients of variation were calculated to assess reliability of ELISA measurements [78].
Assay by Luminex Technology
Primary lymphocytes were quickly thawed and spun at 4000× g for 10 min. Pelleted cells were resuspended in phosphate-buffered saline (PBS) containing protease inhibitor tablet (Roche, Basel, Switzerland) and washed two times more. Cells were lysed in the presence of protease inhibitors, antipain, and chymostatin, rotated overnight at 4 °C and spun at 16,000× g for 20 min. Supernatant was removed and aliquoted for storage at −80 °C. Quantitation of total protein concentration was accomplished with a bicinchoninic acid protein assay kit. Then, 4 ug of cell lysate was suspended in Luminex buffer (PBS, 1% BSA, 0.05% Tween 20, 0.05% NaAzide) and approximately 3000 serological beads were coated with capture antibody. Mouse monoclonal 6B8 were loaded in 96-well filter plates and incubated on vortex shaker for 6 h. Rabbit anti-FMRP R477 antibody was added 1:625 (v:v) to each well and incubated overnight in 4 °C. Goat anti-rabbit IgG conjugated to phycoerythrin antibody (Thermo Scientific, Waltham, MA, USA) was added 1:250 (v:v) and incubated for 2 h. Samples were read on a Luminex 200 machine counting 50 events/bead region (region 33). All lymphocyte samples were assessed with duplicates and the concentration of FMRP was calculated relative to a standard reference sample of a recombinant fusion protein carrying short domains of FMRP, GST-SR7 at known concentration. Coefficients of variation were calculated to assess reliability of Luminex measurements [79].
2.2.2. Positron Emission Tomography (PET)
Participants received training by behavioral psychologists including mock scanner training before the procedure. All participants underwent scans conducted by an experienced research staff of Certified Nuclear Medicine Technologists (CNMT) who had attained certification by the Nuclear Medicine Technology Certification Board (NMTCB). Before conducting these scans, the technologists had conducted many PET scans with participants who experienced challenges to maintain stillness throughout scans. The technologists maintained the physical conditions of each scan optimally for the completion of the scans. Participants were positioned by the technologists in the most comfortable manner for scans. Heads were stabilized in the scanner by gauge strips [2,42,74]. In order to maintain a comfortable environment during the scans, technologists utilized blankets and pads to raise legs. The physical conditions of the scans were maintained in optimal manners for participants by outstanding technologists.
Positron emission tomography (PET) after the intravenous bolus injection 185 MBq (5 mCi) of [18F]FPEB [2,42,60,61,62,63,64,65,66,67,80] was conducted on an ECAT EXACT HR+ PET manufactured by Siemens/CTI (Knoxville, TN, USA) [81] for 90–120 min after injection. Injectors obtained measured doses of [18F]FPEB synthesized by radiochemists in the adjacent radiochemistry laboratory following the published methods [60] to be administered to participants in the scanning chambers.
2.2.3. Statistical Analyses
Statistical Parametric Mapping (SPM) [70] was applied to PET frames to obtain regional time activity (radioactivity) curves (TACs) in regions often affected in neurodegenerative disorders. Standard uptake values (SUVs) of ratios of radiotracer uptake in the region of interest to the cerebellum, a region with minimal radio tracer uptake [82] were generated (Table 2).

Table 2.
Fragile X Mental Retardation Protein (FMRP) in nanogram per microgram total protein and ratios of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) uptake in the regions of interest (ROIs) to uptake in the cerebellum of men with the full mutation (FM) or an allele size mosaicism (FXS-M) (age = 56.6) of fragile X syndrome (FXS) [62].
The Pearson correlation coefficient between the FMRP and the [18F]FPEB uptake in the ROIs was calculated with a significance level of 0.05 in six men with the FM and one man with the FXS-M of FXS [83].
Linear regression of [18F]FPEB uptake on FMRP was examined in men with the FM and FXS-M of FXS (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.).
3. Results
None of the regions demonstrated statistical significance for the Pearson correlation coefficient between the FMRP and the [18F]FPEB uptake in the ROIs of the six men with the FM of FXS (Table 3) [83].

Table 3.
Pearson correlation coefficient R between Fragile X Mental Retardation Protein (FMRP) in nanogram per microgram total protein and ratios of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) uptake in the regions of interest (ROIs) to uptake in the cerebellum of men with the full mutation (FM) of fragile X syndrome [83].
The addition of data from the man with an FXS-M yielded Pearson correlation coefficients that did not attain statistical significance (Table 4). However, the findings suggest that a larger sample may attain significance.

Table 4.
Pearson correlation coefficient R between Fragile X Mental Retardation Protein (FMRP) in nanogram per microgram total protein and ratios of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB) uptake in the regions of interest (ROIs) to uptake in the cerebellum of men with the full mutation (FM) or an allele size mosaicism (FXS-M) of fragile X syndrome [83].
In order to clarify the roles of individual brain regions on the association of FMRP and [18F]FPEB uptake, we performed a simple linear regression for brain regions leading to a significant result [p < 0.001] (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.) (Table 5). Simple linear regression demonstrated a significant association [F(1,47) = 9.87, p < 0.001] (Table 5). Utilizing the seven cortical and subcortical regions affected in neurodegenerative disorders as indicator variables, adjusted linear regression of mGluR5 expression and FMRP (Table 6) showed that mGluR5 expression was significantly reduced in the occipital cortex and the thalamus relative to baseline (Anterior cingulate cortex [ACC]) if FMRP levels are held constant [F(7,47) = 6.84, p < 0.001] (Table 5) (Figure 3).

Table 5.
Linear regression of 3-[18F]fluoro-5-(2)pyridinylethynyl)benzonitrile ([18F]FPEB) uptake on FMRP in men with a FM and an FXS-M of FXS with and without adjustment set to [18F]FPEB uptake in the anterior cingulate cortex (ACC) as the baseline with standard errors in parentheses (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.) (Figure 3).

Table 6.
Code to adjust linear regression of mGluR5 expression and FMRP utilizing seven cortical and subcortical regions affected in neurodegenerative disorders as indicator variables (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.)
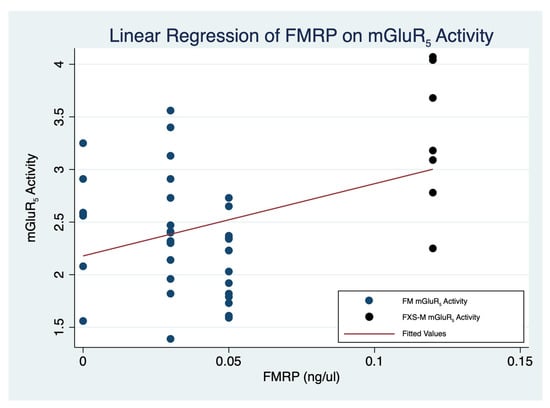
Figure 3.
Linear regression of 3-[18F]fluoro-5-(2)pyridinylethynyl)benzonitrile ([18F]FPEB) uptake on FMRP in men with a FM and an FXS-M of FXS (F(7,47) = 6.84, p < 0.001) (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.) (Table 5). FM: Full mutation; FMRP: Fragile X Mental Retardation Protein; FXS: Fragile X syndrome; FXS-M: FXS-M: Allele size mosaicism of fragile X syndrome.
4. Discussion
In order to generate a secure foundation to guage the effects of glutamatergic agents in clinical trials of men with FXS, we have demonstrated the value of simultaneous measurements of FMRP levels and mGluR5 expression in relevant brain regions. We have shown the benefit to include FMRP levels along with our previously reported protocol for mGluR5 expression in relevant brain regions of men with FXS. The current study expands and enhances the extent of our prior report using mGluR5 expression in relevant brain regions of men with FXS for clinical trials (2,42). Among a small sample of men with the FM and FXS-M of FXS there is a significant association between the FMRP levels and the mGluR5 expression. Additionally, the anterior cingulate cortex [2], the occipital cortex, and the thalamus modify this relationship by decreasing mGluR5 expression (Figure 3; Table 5). We anticipate that future studies with adequate sample sizes will have the power to confirm a positive association between FMRP levels and mGluR5 expression in more brain regions in men with the FM of FXS. Due to the negligible levels of FMRP in men with the FM, we included in our analyses data from a man with an FXS-M with a premutation allele of 181–189 CGG repeats close to the allele size of people with the FM (>200 CGG repeats) because he contributed a larger value of FMRP. We sought to avoid the confounding effects of the autopsy specimens of individuals with FMs and premutations (PMs) [84,85]. The current study advances the foundations for clinical trials of glutamatergic agents for FXS by adding the objective quantification of FMRP levels to the measurement of mGluR5 expression in relevant brain regions. We provide the tools for sound protocols of glutamatergic agents for FXS.
These findings suggest that mGluR5 expression may be related to FMRP levels to play a role in the pathogenesis of FXS. The protocol for this investigation provides a feasibility tool that may facilitate the measurement of a biomarker of mGluR5 expression to conduct rigorously designed clinical trials of FXS [2,40,41] and perhaps other subtypes of ASD [42]. That said, the findings of this study merit replication in a larger sample of the groups studied here and other neurodevelopmental disorders [86]. The proposed protocol may provide a tool to assess the role of FMRP and mGluR5 expression in other subtypes of ASD [87], schizophrenia [88], and mood disorders [89]. Indeed, the current protocol may be expanded to promote knowledge about multiple neuromodulators in FXS, Rett syndrome [90,91], and other subtypes of ASD [92].
The proposed protocol is particularly important as a means to assess individuals with FXS and related conditions in community settings within a hundred miles of a production center with a cyclotron. Samples of blood can be drawn to be sent on dry ice for FMRP analysis at specialized research laboratories. Since [18F]FPEB is an [18F] compound with a half-life of two hours, it can be manufactured in specialized facilities for distribution two hours away. Thus, [18F]FPEB, like 2-deoxy-2 [18F]fluoro-D-glucose ([18F]FDG), the most widely used radiotracer, can be utilized for scans in community settings with portable PET scanners and nuclear medical scanners modified to conduct PET scans in proximity to production centers with cyclotrons. Unlike [11C] radiotracers with a half-life of around 20 min that require manufacture in a cyclotron adjacent to the scanner, [18F]FPEB, like [18F]FDG, can be commercially manufactured by central facilities with cyclotrons for distribution to community settings within a radius of a hundred miles [93]. Thus, the proposed protocol has the potential for commercial development to obtain data for sophisticated analyses by experts in tertiary centers.
The current status of glutamatergic interventions for FXS resembles the status of amyloid agents for Alzheimer’s disease years ago. Now three [18F] radiotracers for amyloid are commercially available for use before the administration of novel agents to reduce amyloid. The proposed protocol could be utilized for clinical trials of glutamatergic agents for FXS.
The current investigation is limited by the utilization of data from brain regions conjectured to be affected in men with the FM or an FXS-M of FXS utilizing SUV analysis of PET scans only with an established analytic technique for MR scan [70]. Since regions were selected for this study based on our experience with neurodegenerative disorders, key anatomical regions likely to be affected in FXS were not included. The neurobehavioral phenotype of FXS is associated with delayed socialization reflecting cognitive processes in cortical regions and avoidance reflecting limbic circuits [94]. Utilization of the same protocol to obtain MR and PET scans on all participants will enhance the accuracy of future investigations. Coregistration of PET and MR images will facilitate the analysis of future investigations. The regions chosen for this analysis were those commonly affected in neurodegenerative disorders. Future investigations will be enhanced by precise description of the delineation of each region (e.g., the entire caudate nucleus or just the head of the caudate nucleus, which part of each cortical region or the entire cortex). Comparing and contrasting of mGluR5 expression in participants with FXS, related conditions, and healthy participants with TD, particularly in insular, temporal, and cingulate cortices, the regions with high of mGluR5 expression in healthy men with TD [60], will greatly enhance anatomical localization of mGluR5 expression in FXS and related conditions. Future investigations to identify characteristic structures related to (A) higher cortical functions will include the prefrontal cortex and (B) learning and emotions will include the hippocampus and the amygdala. Future work will include measurement of inhibitory control [95], mediofrontal negativity [96], and other additional variables likely to be impaired in men with FXS.
Future investigations will be enhanced by contemporaneous conduct of all investigations at all participating institutions with identical protocols and analyses [2,42]. Since full maturation of the human brain may continue through the ages of 25 and 30, it is possible that expression of mGluR5 in these participants is still influenced by developmental processes. Therefore, future studies of older individuals may better identify the characteristics of mGluR5 expression in the brains of mature individuals. Additionally, the age differences may represent a confounding influence because increased age is associated with decreased cerebral expression of mGluR5 measured by PET with [18F]FPEB [97]. The variability of BMIs may represent another confounding influence [2,42,62].
Diurnal variation may have influenced mGluR5 expression in the different scans utilized for this study. The ranges of injection times were (1100, 1543). Since changes in mGluR5 expression vary as much as 30% in two hours [2,98], diurnal variation may have influenced the variability of mGluR5 expression in our cohort. Performing scans at exactly the same time in all participants is desirable. Another limitation is our use of ELISA in lymphocytes to estimate FMRP expression as a function of brain region. Peripheral FMRP expression may differ from FMRP expression in various brain regions. Utilization of only participants with the full mutation of FXS will eliminate the errors introduced with participants with PMs and other genetic variations [6,84].
Multicenter PET/MR studies on unmedicated individuals with FXS [99] with optimal qualification [100] provide a tool to obtain optimal target engagement measurements [67] without the unknown influences of concomitant medications [101]. In order to avoid the marked diurnal variations in mGluR5 expression [2,98], scans may be performed at exactly the same time in all participants. Since dysfunction of the amygdala and the rectus gyrus underlie anxiety in individuals with FXS [102,103], especially in females with FXS [104], future investigations of these regions in females and males with FXS and other subtypes of ASD may provide foundations for interventions to reduce anxiety in affected individuals. The proposed procedure may be developed to facilitate the diagnosis and treatment of individuals with FXS and other subtypes of ASD early in life [105,106,107]. Prompt procedures to rule out competing items of the differential diagnosis leading to confirmation of the diagnosis of FXS and other subtypes of ASD will facilitate the basis to institute optimal educational placement and other needed interventions [108]. This protocol will provide the foundations of trustworthy evidence [109] to disseminate widely with the general public [110].
The current investigation demonstrates the usefulness of the proposed protocol to measure FMRP levels and of mGluR5 expression in brain regions relevant for FXS to provide sound foundations for quantitation before, during, and after clinical trials of glutamatergic agents. We have demonstrated that the proposed protocol including challenging physical and psychological tasks in is feasible in individuals with FXS and other disabilities by means of training by behavioral psychologists before and during the tasks. The ultimate goal of this work is to utilize the proposed protocol for large-scale multicenter studies of potential interventions for FXS. We seek to expand this protocol to develop optimal means for the diagnosis, treatment, cure, and prevention of FXS and related conditions.
5. Conclusions
We showed an association of FMRP levels and cerebral mGluR5 expression measured by PET with [18F]FPEB in the anterior cingulate cortex, the occipital cortex, and the thalamus in men with the FM or FXS-M of FXS. Reduced cortical mGluR5 expression in these cortical regions may provide a basis for the severity of the neurobehavioral phenotype of cognitive deficits and delayed socialization of individuals with FXS [108]. Reduced limbic mGluR5 expression may provide a basis for the avoidance behaviors of individuals with FXS [7,94]. Since the effects of interventions on animal models of FXS may not produce similar effects in humans with FXS [111], current theories of FXS merit reexamination [44]. The proposed protocol may provide a crucial tool to measure mGluR5 expression in clinical trials of peptides [26] and other novel interventions in FXS. Our findings may provide the foundations to generate novel theories of humans with FXS.
The proposed protocol may provide a biomarker for measurement of FMRP and mGluR5 expression of relevance for clinical trials of FXS and other subtypes of ASD. The proposed protocol may provide a tool to foster diagnostic and therapeutic interventions for FXS and related conditions.
Author Contributions
Conceptualization, J.R.B., D.S.R., E.M.B.-K., D.F.W. and D.B.B.; data curation, J.R.B., J.A.G., J.A.G., A.N., D.S.R., D.J., O.B., S.D.M., A.K.M., E.M.B.-K., D.F.W. and D.B.B.; formal analysis, J.R.B., J.A.G., A.N., O.B., E.M.B.-K., D.F.W. and D.B.B.; funding acquisition, J.R.B., D.S.R., E.M.B.-K., D.F.W. and D.B.B.; investigation, J.R.B., A.N., D.S.R., D.J., S.D.M., K.S., T.S., J.P.S., D.F.W. and D.B.B.; methodology, J.R.B., J.A.G., A.N., D.S.R., D.J., O.B., S.D.M., K.S., T.S., J.P.S., D.F.W. and D.B.B.; project administration, J.R.B., A.N., D.S.R., D.J., A.K.M., J.P.S., E.M.B.-K., D.F.W. and D.B.B.; resources, J.R.B., A.N., D.S.R., O.B., K.S., T.S., J.P.S., E.M.B.-K., D.F.W. and D.B.B.; software, J.R.B., J.A.G., A.N. and O.B.; supervision, J.R.B., D.S.R., K.S., T.S., J.P.S., E.M.B.-K., D.F.W. and D.B.B.; validation, J.R.B., J.A.G., A.N., D.S.R., D.J., O.B., S.D.M., K.S., T.S., A.K.M., J.P.S., E.M.B.-K., D.F.W. and D.B.B.; visualization, J.R.B., J.A.G., A.N., D.S.R., D.J., O.B., J.P.S., E.M.B.-K., D.F.W. and D.B.B.; and editing, J.R.B., J.A.G., A.N., D.S.R., E.M.B.-K. and D.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible by a Radiology BRidge/Development Funding Initiative to STimulate and Advance Research (RAD BriteStar Bridge) Award (80046104) from the Johns Hopkins University School of Medicine, Baltimore, Maryland to J.R.B. with the assistance of D.F.W.; and the Intellectual & Developmental Disabilities Research Center (U54 HD079123), Kennedy Krieger Institute, and Johns Hopkins Medical Institutions, Baltimore, Maryland, to J.R.B.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki [76] and approved by the Institutional Review Board of the Johns Hopkins School of Medicine in Baltimore, Maryland (Protocol Number: IRB00169249 and Initial Approval Date: 11 July 2018).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data presented in this study are openly available in [62]. Available online: https://doi.org/10.5281/zenodo.5792324 (accessed on 22 December 2021) [62].
Acknowledgments
The authors thank the patients and families for their participation and dedication to these studies; they are the inspiration for our efforts at improving treatments. The authors thank the FORWARD Database and Registry of the National Fragile X Foundation (NFXF) funded by the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, for referral of participants. The authors thank the teams of the Institute of Neurodegenerative Disorders, the Positron Emission Tomography (PET) Radiotracer Service Center, and the Research Magnetic Resonance Imaging (MRI) Service Center of the Johns Hopkins University School of Medicine for conducting the scans. The authors thank Hiroto Kuwabara for PET analysis. The authors thank Emw for permission to reproduce the structure of FMRP (https://commons.wikimedia.org/wiki/File:Protein_FMR1_PDB_2bkd.png, accessed on 22 December 2021) and Brian Hwang for permission to reproduce his image in the Graphical Abstract. Earlier versions of this article were presented at the American Academy of Neurology Annual Meeting, Virtual, 17–22 April 2021 [66], the Society of Nuclear Medicine and Molecular Imaging 2021 Annual Meeting, Virtual, 11–15 June 2021, accessed on 22 December 2021 [67], the Undergraduate Research Symposium, Johns Hopkins University, Baltimore, Maryland, 28 October 2020, the World Molecular Imaging Congress 2020, the Society for Neuroscience Global Connectome, Virtual, 12 January 2021 [16], the American Academy of Child and Adolescent Psychiatry Annual Meeting, 27 October 2021, accessed on 22 December 2021 [72], and the Society for Neuroscience 50th Annual Meeting 2021, 10 November 2021 [73].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| [18F]FDG | 2-deoxy-2-[18F]fluoro-D-glucose |
| [18F]FPEB | 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ASD | Autism spectrum disorder |
| BMI | Basal metabolic index |
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence interval |
| CGG | Cytosine-guanine-guanine |
| CN | Caudate nucleus |
| CNMT | Certified Nuclear Medicine Technologist |
| df | Degrees of freedom |
| DNA | Deoxyribonucleic aced |
| ERK | Extracellular signal-regulated kinase |
| FM | Full mutation |
| FMR1 | Fragile X mental retardation 1 gene |
| FMRP | Fragile X Mental Retardation Protein |
| FORWARD | Fragile X Online Registry With Accessible Research Database of the National Fragile X Foundation (NFXF) |
| FXS | Fragile X syndrome |
| ICMJE | International Committee of Medical Journal Editors |
| ID | Intellectual disability |
| IND | Institute for Neurodegenerative Disorders |
| IRB | Institutional review board |
| JHU | Johns Hopkins University |
| KA | Jainic acid |
| KO | Knockout |
| LTD | Long-term depression protein kinase |
| MAPK | Microtubule-associated protein kinase |
| MBq | Megabequerel |
| mCi | Millicurie |
| mGluR | Metabotropic glutamate receptor |
| mGluR1/5 | Metabotropic glutamate receptors subtypes 1 and 5 |
| mGluR5 | Metabotropic glutamate receptor subtype 5 |
| mGluR-LTD | Metabotropic glutamate receptor dependent long-term depression |
| MR | Magnetic resonance |
| MS | Mean square |
| mRNA | Messenger ribonucleic acid |
| mTOR | Mammalian target of rapamycin |
| N | Number |
| NAM | Negative allosteric modulator |
| NFXF | National Fragile X Foundation |
| NMDA | N-methyl-d-aspartate |
| NMTCB | Nuclear Medicine Technology Certification Board |
| OC | Occipital cortex |
| p | Probability |
| PBS | Phosphate-buffered saline |
| PC | Parietal cortex |
| pCC | Posterior cingulate cortex |
| PCR | Polymerase chain reaction |
| PET | Positron emission tomography |
| PET/MR | Positron emission tomography and magnetic resonance |
| PM | Premutation |
| PSD | Post-synaptic density |
| Pu | Putamen |
| RNA | Ribonucleic acid |
| S | Striatum |
| SE | Standard error |
| SPM | Statistical Parametric Mapping [68] |
| SS | Sum of squares |
| SUVR | Standard uptake value ratio |
| T | Thalamus |
| TC | Temporal cortex |
| TD | Typical development |
References
- Bardoni, B.; Schenck, A.; Mandel, J.-L. The Fragile X mental retardation protein. Brain Res. Bull. 2001, 56, 375–382. [Google Scholar] [CrossRef]
- Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, T.; Martin, S.D.; Brinson, Z.; et al. Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome. Brain Sci. 2020, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.; Kaufmann, W.E. What Can We Learn about Autism from Studying Fragile X Syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Subramanian, M. Neurobiology of autism and intellectual disability: Fragile X syndrome. In Neurobiology of Disease, 2nd ed.; Johnston, M.V., Ed.; Oxford University Press: New York, NY, USA, 2016; pp. 375–384. [Google Scholar]
- Bagni, C.; Tassone, F.; Neri, G.; Hagerman, R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. J. Clin. Investig. 2012, 122, 4314–4322. [Google Scholar] [CrossRef]
- Kim, K.; Hessl, D.; Randol, J.L.; Espinal, G.M.; Schneider, A.; Protic, D.; Aydin, E.Y.; Hagerman, R.J.; Hagerman, P.J. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS ONE 2019, 14, e0226811. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Schlageter, A.; Filopovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainnen, J.; et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in fragile X patients with neurobehavioral assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef]
- Kurosaki, T.; Imamachi, N.; Pröschel, C.; Mitsutomi, S.; Nagao, R.; Akimitsu, N.; Maquat, L.E. Loss of the fragile X syndrome protein FMRP results in misregulation of nonsense-mediated mRNA decay. Nat. Cell Biol. 2021, 23, 40–48. [Google Scholar] [CrossRef]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Hoffmann, A.; Berry-Kravis, E. Fragile X syndrome. In Neuronal and Synaptic Dysfunction in Autism Spectrum Disorder and Intellectual Disability; Sala, C., Verpelli, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 325–346. [Google Scholar]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Brown, W.T. Detection and Quantification of the Fragile X Mental Retardation Protein 1 (FMRP). Genes 2016, 7, 121. [Google Scholar] [CrossRef]
- Sirois, C.L.; Li, M.; Guo, Y.; Korabelnikov, T.; Xing, Y.; Levesque, B.; Bhattacharyya, A.; Zhao, X. Function of the trinucleotide (CGG) repeats within the FMR1 gene in human neurons. In Proceedings of the Society for Neuroscience 50th Annual Meeting, 12–16 November 2021; Available online: https://www.astractsonline.com/pp8/#!/10485/presentation/16188 (accessed on 22 December 2021).
- Hagerman, R.J. Fragile X syndrome and permutation-associated disorders. In Cassidy and Allanson’s Management of Genetic Syndromes, 4th ed.; Carey, J.C., Battaglia, A., Viskochil, D., Cassidy, S.B., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2021; pp. 443–457. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Kaufmann, W.E.; Ono, M.Y.; Tartaglia, N.; Lachiewicz, A.; Kronk, R.; Delahunty, C.; Hessl, D.; Visootsak, J.; et al. Advances in the treatment of fragile X syndrome. Pediatrics 2009, 123, 378–390. [Google Scholar] [CrossRef]
- Martin, S.D. Correlation between fragile X mental retardation protein and metabotropic glutamate receptor subtype 5 in fragile X syndrome. Hopkins Undergrad. Res. J. 2021, 25, 16–21. [Google Scholar]
- Martin, S.D.; Berry-Kravis, E.; Russel, D.; Budimirovic, D.; Brasic, J. Correlation between the Fragile X Mental Retardation Protein (FMRP) and the cerebral expression of the metabotropic glutamate receptor subtype 5 (mGluR5) in fragile X syndrome. Program No. 027-24. In Proceedings of the 2021 Neuroscience Meeting Planner, Society for Neuroscience, Chicago, IL, USA, 12 January 2021. [Google Scholar]
- Coleman, J.; Riley, K. Fragile X syndrome. In Encyclopedia of Infant and Early Childhood Development, 2nd ed.; Benson, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 647–654. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y. The role of fragile X mental retardation protein in cellular and synaptic dynamics of developing auditory circuits. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/19499 (accessed on 22 December 2021).
- Fernandes, G.; Mishra, P.K.; Nawaz, M.S.; Donlin-Asp, P.G.; Rahman, M.M.; Hazra, A.; Kedia, S.; Kayenaat, A.; Songara, D.; Wyllie, D.J.A.; et al. Correction of amygdalar dysfunction in a rat model of fragile X syndrome. Cell Rep. 2021, 37, 109805. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.A.; Kind, P.C. Mechanisms regulating input-output function and plasticity of neurons in the absence of FMRP. Brain Res. Bull. 2021, 175, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, S.; Yook, Y.; Tsai, N.-P. Role of Fragile X Mental Retardation Protein in amyloid beta induced translational suppression. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/20529 (accessed on 22 December 2021).
- Małgorzata, P.; Paweł, K.; Iwona, M.L.; Brzostek, T.; Andrzej, P. Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opin. Ther. Targets 2020, 24, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Calmodulin Binding Proteins and Alzheimer’s Disease: Biomarkers, Regulatory Enzymes and Receptors That Are Regulated by Calmodulin. Int. J. Mol. Sci. 2020, 21, 7344. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in microglia-neuron cross-talk in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef]
- Romagnoli, A.; Di Marino, D. The Use of Peptides in the Treatment of Fragile X Syndrome: Challenges and Opportunities. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef]
- Di Marco, B.; Dell’Albani, P.; D’Antoni, S.; Spatuzza, M.; Bonaccorso, C.; Musumeci, S.; Drago, F.; Bardoni, B.; Catania, M. Fragile X mental retardation protein (FMRP) and metabotropic glutamate receptor subtype 5 (mGlu5) control stress granule formation in astrocytes. Neurobiol. Dis. 2021, 154, 105338. [Google Scholar] [CrossRef]
- Yook, Y.; Liu, D.; Soriano, S.; Lizarazo, S.; Eagleman, D.E.; Tsai, N. Chronic activation of Gp1 mGluRs leads to distinct refinement of neural network activity through non-canonical p53 and Akt signaling. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/19993 (accessed on 22 December 2021).
- LiCausi, F.; Hartman, N.W. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef]
- Magdalon, J.; Sánchez-Sánchez, S.M.; Griesi-Oliveira, K.; Sertié, A.L. Dysfunctional mTORC1 Signaling: A Convergent Mechanism between Syndromic and Nonsyndromic Forms of Autism Spectrum Disorder? Int. J. Mol. Sci. 2017, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Limanaqi, F.; Frati, A.; Busceti, C.L.; Fornai, F. mTOR-Related Brain Dysfunctions in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2018, 19, 2226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mehan, S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021, 147, 105067. [Google Scholar] [CrossRef] [PubMed]
- Garro-Martínez, E.; Fullana, M.N.; Florensa-Zanuy, E.; Senserrich, J.; Paz, V.; Ruiz-Bronchal, E.; Adell, A.; Castro, E.; Díaz, Á; Pazos, Á; et al. mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. Int. J. Mol. Sci. 2021, 22, 8671. [Google Scholar] [CrossRef]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef]
- Ojha, P.; Pal, S.; Bhattacharyya, S. Regulation of Metabotropic Glutamate Receptor Internalization and Synaptic AMPA Receptor Endocytosis by the Postsynaptic Protein Norbin. J. Neurosci. 2021, 42, 731–748. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Bagni, C.; Zukin, R.S. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef]
- Soong, H.K.; Markham, J.A.; Weiler, I.V.; Greenough, W.T. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 4429–4434. [Google Scholar]
- Yau, S.S.Y.; Bettio, L.; Vetrici, M.; Truesdell, A.; Chiu, C.; Chiu, J.; Christie, B. Chronic minocycline treatment improves hippocampal neuronal structure, NMDA receptor function, and memory processing in Fmr1 knockout mice. Neurobiol. Dis. 2018, 113, 11–22. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Knox, A.; Hervey, C. Targeted treatments for fragile X syndrome. J. Neurodev. Disord. 2011, 3, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Berry-Kravis, E.; Erickson, C.A.; Hall, S.S.; Hessl, D.; Reiss, A.L.; King, M.K.; Abbeduto, L.; Kaufmann, W.E. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 2017, 9, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.; Nandi, A.; Russell, D.; Jennings, D.; Barret, O.; Martin, S.; Slifer, K.; Sedlak, T.; Seibyl, J.; Wong, D.; et al. Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 2863. [Google Scholar] [CrossRef] [PubMed]
- Dahlhaus, R. Of Men and Mice: Modeling the Fragile X Syndrome. Front. Mol. Neurosci. 2018, 11, 41. [Google Scholar] [CrossRef]
- Schmidt, K.C.; Loutaev, I.; Quezado, Z.; Sheeler, C.; Smith, C.B. Regional rates of brain protein synthesis are unaltered in dexmedetomidine sedated young men with fragile X syndrome: A L-[1-11C]leucine PET study. Neurobiol. Dis. 2020, 143, 104978. [Google Scholar] [CrossRef]
- Dionne, O.; Lortie, A.; Gagnon, F.; Corbin, F. Rates of protein synthesis are reduced in peripheral blood mononuclear cells (PBMCs) from fragile X individuals. PLoS ONE 2021, 16, e0251367. [Google Scholar] [CrossRef]
- Zerbi, V.; Markicevic, M.; Gasparini, F.; Schroeter, A.; Rudin, M.; Wenderoth, N. Inhibiting mGluR5 activity by AFQ056/Mavoglurant rescues circuit-specific functional connectivity in Fmr1 knockout mice. NeuroImage 2019, 191, 392–402. [Google Scholar] [CrossRef]
- Telias, M. Molecular Mechanisms of Synaptic Dysregulation in Fragile X Syndrome and Autism Spectrum Disorders. Front. Mol. Neurosci. 2019, 12, 51. [Google Scholar] [CrossRef]
- Emmitte, K.A. mGlu5 negative allosteric modulators: A patent review (2010–2012). Expert Open. Ther. Pat. 2013, 23, 393–408. [Google Scholar] [CrossRef]
- Giuffrida, R.; Musumeci, S.A.; D’Antoni, S.; Bonaccorso, C.M.; Giuffrida-Stella, A.M.; Oostra, B.A.; Catania, M.V. A Reduced Number of Metabotropic Glutamate Subtype 5 Receptors Are Associated with Constitutive Homer Proteins in a Mouse Model of Fragile X Syndrome. J. Neurosci. 2005, 25, 8908–8916. [Google Scholar] [CrossRef]
- Ronesi, J.A.; Collins, K.A.; Hays, S.A.; Tsai, N.-P.; Guo, W.; Birnbaum, S.G.; Hu, J.-H.; Worley, P.F.; Gibson, J.R.; Huber, K.M. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat. Neurosci. 2012, 15, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.J.; Stoppel, D.C.; Weng, F.-J.; McCamphill, P.K.; Senter, R.K.; Bear, M.F. Protein synthesis-dependent hyperexcitability of Fmr1-KO visual cortex layer 5 pyramidal cells offers path to identify novel therapeutic targets for the treatment of fragile X syndrome. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/19361 (accessed on 22 December 2021).
- Heynen, A.J.; Stoppel, D.C.; McCamphill, P.K.; Senter, R.K.; Bear, M.F. Mglur5 negative modulators for Fragile X: Resistance and persistance. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/19362 (accessed on 22 December 2021).
- Stoppel, D.C.; McCamphill, P.K.; Senter, R.K.; Heynen, A.J.; Bear, M.F. mGluR5 negative modulators for fragile X: Resistance and persistence. Front. Psychiatry 2021, 12, 718953. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Thornton, A.M.; Freeburn, A.; Greene, G.; Garrone, B.; Milanese, C.; Di Giorgio, F.P.; Callaghan, C.K.; Bianchi, M. Chronic treatment with the selective GSK-3 inhibitor SB216763 reverses behavioral impairments in the Fmr1 knock out mouse model of fragile X syndrome. In Proceedings of the Society for Neuroscience 50th Annual Meeting, New Orleans, LA, USA, 8 February 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/13956 (accessed on 22 December 2021).
- Gross, C.; Bhattacharya, A. Intracellular signaling networks in fragile X syndrome: Approaches to drug discovery and therapeutics. In Fragile X Syndrome: From Genetics to Targeted Treatment; Willemsen, R., Kooy, R.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–239. [Google Scholar]
- Díaz-Caneja, C.; State, M.; Hagerman, R.; Jacquemont, S.; Marín, O.; Bagni, C.; Umbricht, D.; Simonoff, E.; de Andrés-Trelles, F.; Kaale, A.; et al. A white paper on a neurodevelopmental framework for drug discovery in autism and other neurodevelopmental disorders. Eur. Neuropsychopharmacol. 2021, 48, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Duy, P.Q.; Budimirovic, D.B. Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials. Transl. Neurosci. 2017, 8, 7–8. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6857731, F-Peb F-18. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/F-Peb-F-18 (accessed on 22 December 2021).
- Kessler, R.M.; Seibyl, J.; Cowan, R.L.; Zald, D.; Young, J.S.; Ansari, M.S.; Stabin, M.G. Radiation Dosimetry of 18F-FPEB in Humans. J. Nucl. Med. 2014, 55, 1119–1121. [Google Scholar] [CrossRef][Green Version]
- Wong, D.F.; Waterhouse, R.; Kuwabara, H.; Kim, J.; Brašić, J.R.; Chamroonrat, W.; Stabins, M.; Holt, D.P.; Dannals, R.F.; Hamill, T.G.; et al. 18F-FPEB, a PET Radiopharmaceutical for Quantifying Metabotropic Glutamate 5 Receptors: A First-in-Human Study of Radiochemical Safety, Biokinetics, and Radiation Dosimetry. J. Nucl. Med. 2013, 54, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Wong, D.F.; Brašić, J.R.; Kuwabara, H.; Mathur, A.; Folsom, T.D.; Jacob, S.; Realmuto, G.M.; Pardo, J.V.; Lee, S. Metabotropic glutamate receptor 5 tracer [18F]-FPEB displays increased binding potential in postcentral gyrus and cerebellum of male individuals with autism: A pilot PET study. Cerebellum Ataxias 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Brasic, J.R.; Goodman, J.A.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Martin, S.D.; Slifer, K.; Sedlak, T.; Mathur, A.K.; et al. Fragile X Mental Retardation Protein and cerebral expression of metabotropic glutamate receptor subtype 5 in men with fragile X syndrome: A pilot study. Zenodo 2022, 1. [Google Scholar] [CrossRef]
- Mody, M.; Petibon, Y.; Han, P.; Kuruppu, D.; Ma, C.; Yokell, D.; Neelamegam, R.; Normandin, M.D.; El Fakhri, G.; Brownell, A.-L. In vivo imaging of mGlu5 receptor expression in humans with Fragile X Syndrome towards development of a potential biomarker. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Brašić, J.R.; Mathur, A.K.; Budimirovic, D.B. The urgent need for molecular imaging to confirm target engagement for clinical trials of fragile X syndrome and other subtypes of autism spectrum disorder. Arch. Neurosci. 2019, 6, e91831. [Google Scholar] [CrossRef]
- Brasic, J.; Mishra, C.; Mathur, A.; Sweeney, K.; Folsom, T.; Kitzmiller, K.; Mellinger-Pilgrim, R.; Wong, D.; Fatemi, S. Microdose PET for the metabotropic glutamate receptor type 5 (mGluR5). J. Nucl. Med. 2018, 59 (Suppl. 1), 1774. [Google Scholar]
- Brasic, J.; Nandi, A.; Russell, D.; Jennings, D.; Barret, O.; Mathur, A.; Slifer, K.; Sedlak, T.; Martin, S.; Brinson, Z.; et al. Reduced metabotropic glutamate receptor subtype 5 in fragile X syndrome. In Proceedings of the American Academy of Neurology Annual Meeting, New Orleans, LA, USA, 17 April 2021; Available online: https://index.mirasmart.com/AAN2021/PDFfiles/AAN2021-004378.html (accessed on 22 December 2021).
- Brasic, J.R.; Nandi, A.; Russell, D.; Jennings, D.; Barret, O.; Martin, S.; Slifer, K.; Seibyl, J.; Berry-Kravis, E.; Wong, D.; et al. Cerebral expression of metabotropic glutamate receptor subtype 5 in autism spectrum disorder and fragile X syndrome. J. Nucl. Med. 2021, 62 (Suppl. 1), 1057. [Google Scholar]
- Dionne, O.; Corbin, F. An “Omic” Overview of Fragile X Syndrome. Biology 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.-C.; Ichise, M.; et al. Consensus Nomenclature for in vivo Imaging of Reversibly Binding Radioligands. J. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef]
- The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, London, UK. Statistical Parametric Mapping (SPM). 2021. Available online: http://www.fil.ion.ucl.ac.uk/spm/ (accessed on 22 December 2021).
- Brasic, J.R.; Mathur, A.K.; Budimirovic, D.B. Clinical trials of pharmacological agents for developmental disabilities: Potential tools to demonstrate target engagement in children and adolescents. Md. Reg. Counc. Child Adolesc. Psychiatry (MRCCAP) 2020, 1, 2. [Google Scholar]
- Brasic, J.; Budimirovic, D.B.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Martin, S.D.; Slifer, K.; Berry-Kravis, E.; Wong, D.F. Measurement of cerebral expression of metabotropic glutamate receptor subtype 5 in autism spectrum disorder and fragile X syndrome. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60 (Suppl. 105), S257. [Google Scholar]
- Goodman, J.A.; Martin, S.D.; Russell, D.S.; Jennings, D.; Barret, O.; Nandi, A.; Slifer, K.J.; Mathur, A.K.; Sedlak, T.W.; Siebyl, J.P.; et al. Association of Fragile X Mental Retardation Protein (FMRP) and metabotropic glutamate receptor subtype 5 (mGluR5) in fragile X syndrome (FXS). In Proceedings of the Society for Neuroscience 50th Annual Meeting, July 2021; Available online: https://www.abstractsonline.com/pp8/#!/10485/presentation/19364 (accessed on 22 December 2021).
- Russell, D. A PET Brain Imaging Study of mGluR5 in Subjects with Neuropsychiatric Conditions (FPEB). ClinicalTrials.gov Identifier: NCT00870974 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT00870974 (accessed on 22 December 2021).
- International Committee of Medical Journal Editors (ICMJE). Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. 2021. Available online: http://www.icmje.org/recommendations/ (accessed on 22 December 2021).
- World Medical Association. Declaration of Helsinki: Medical Research Involving Human Subjects. 2013. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 22 December 2021).
- Cai, X.; Arifm, M.; Want, H.; Kornreich, R.; Edelmann, L.J. Clinical genetic testing for fragile X syndrome by polymerase chain reaction amplification and Southern Blot Analyses. In Fragile-X Syndrome; Ben-Yosef, D., Mayshar, Y., Eds.; Humana Press: New York, NY, USA, 2019; Volume 1942, pp. 11–27. [Google Scholar] [CrossRef]
- Iwahashi, C.; Tassone, F.; Hagerman, R.J.; Yasui, D.; Parrott, G.; Nguyen, D.; Mayeur, G.; Hagerman, P.J. A Quantitative ELISA Assay for the Fragile X Mental Retardation 1 Protein. J. Mol. Diagn. 2009, 11, 281–289. [Google Scholar] [CrossRef]
- LaFauci, G.; Adayev, T.; Kascsak, R.; Kascsak, R.; Nolin, S.; Mehta, P.; Brown, W.T.; Dobkin, C. Fragile X Screening by Quantification of FMRP in Dried Blood Spots by a Luminex Immunoassay. J. Mol. Diagn. 2013, 15, 508–517. [Google Scholar] [CrossRef]
- Kim, J.-H.; Marton, J.; Ametamey, S.M.; Cumming, P. A Review of Molecular Imaging of Glutamate Receptors. Molecules 2020, 25, 4749. [Google Scholar] [CrossRef]
- Brix, G.; Zaers, J.; Adam, L.E.; Bellemann, M.E.; Ostertag, H.; Trojan, H.; Haberkorn, U.; Doll, J.; Oberdorfer, F.; Lorenz, W.J. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J. Nucl. Med. 1997, 38, 1614–1623. [Google Scholar]
- Sullivan, J.M.; Lim, K.; Labaree, D.; Lin, S.-F.; McCarthy, T.J.; Seibyl, J.P.; Tamagnan, G.; Huang, Y.; Carson, R.E.; Ding, Y.-S.; et al. Kinetic Analysis of the Metabotropic Glutamate Subtype 5 Tracer [18F]FPEB in Bolus and Bolus-Plus-Constant-Infusion Studies in Humans. J. Cereb. Blood Flow Metab. 2012, 33, 532–541. [Google Scholar] [CrossRef]
- Stangroom, J. Social Sciences Statstics. 2021. Available online: https://www.socscistatistics.com/tests/pearson/default2.aspx (accessed on 14 December 2021).
- Lohith, T.G.; Osterweil, E.K.; Fujita, M.; Jenko, K.J.; Bear, M.F.; Innis, R.B. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol. Autism 2013, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kaufmann, W.E.; Frye, R.E.; Ong, K.; Kaminski, J.W.; Velinov, M.; Berry-Kravis, E. The association between mosaicism type and cognitive and behavioral functioning among males with fragile X syndrome. Am. J. Med Genet. Part A 2021. [Google Scholar] [CrossRef] [PubMed]
- Chugani, H.T. Positron Emission Tomography in Pediatric Neurodegenerative Disorders. Pediatr. Neurol. 2019, 100, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Bezzi, P. mGlu5-mediated signalling in developing astrocyte and the pathogenesis of autism spectrum disorders. Curr. Opin. Neurobiol. 2018, 48, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Santuy, A.; Merchan-Perez, A.; Sprengel, R. Glutamatergic Dysfunction and Synaptic Ultrastructural Alterations in Schizophrenia and Autism Spectrum Disorder: Evidence from Human and Rodent Studies. Int. J. Mol. Sci. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D. GABA receptor subunit distribution and FMRP–mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr. Res. 2014, 167, 42–56. [Google Scholar] [CrossRef]
- Brašić, J.R.; Bibat, G.; Kumar, A.; Zhou, Y.; Hilton, J.; Yablonski, M.E.; Dogan, A.S.; Guevara, M.R.; Stephane, M.; Johnston, M.; et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with rett syndrome. Synapse 2011, 66, 471–482. [Google Scholar] [CrossRef]
- Wong, D.F.; Blue, M.E.; Brašić, J.R.; Nandi, A.; Valentine, H.; Stansfield, K.H.; Rousset, O.; Bibat, G.; Yablonski, M.E.; Johnston, M.V.; et al. Are dopamine receptor and transporter changes in Rett syndrome reflected in Mecp2-deficient mice? Exp. Neurol. 2018, 307, 74–81. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- Brašić, J.R. Neurotransmitter visualization in schizophrenia. J. Biomed. Graph. Comput. 2013, 3, 30. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Bukelis, I.; Cox, C.; Gray, R.M.; Tierney, E.; Kaufman, W.E. Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. Am. J. Med. Genet. Part A 2006, 140A, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Timmermann, C.; Battaglia, S.; Maier, M.E.; di Pellegrino, G. Mediofrontal Negativity Signals Unexpected Timing of Salient Outcomes. J. Cogn. Neurosci. 2017, 29, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Mecca, A.P.; Rogers, K.; Jacobs, Z.; McDonald, J.W.; Michalak, H.R.; DellaGioia, N.; Zhao, W.; Hillmer, A.T.; Nabulsi, N.; Lim, K.; et al. Effect of age on brain metabotropic glutamate receptor subtype 5 measured with [18F]FPEB PET. NeuroImage 2021, 238, 118217. [Google Scholar] [CrossRef]
- DeLorenzo, C.; Gallezot, J.-D.; Gardus, J.; Yang, J.; Planeta, B.; Nabulsi, N.; Ogden, R.T.; Labaree, D.C.; Huang, Y.H.; Mann, J.J.; et al. In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. J. Cereb. Blood Flow Metab. 2016, 37, 2716–2727. [Google Scholar] [CrossRef]
- Gade, S.; Hjørnevik, T.; Park, J.H.; Shen, B.; Gu, M.; Tseng, J.; Barbosa, D.; Leuze, C.; Pitteri, S.; Jung, J.; et al. The first awake simultaneous PET-MR study of an adult with fragile X syndrome: A case report. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Catana, C.; Laforest, R.; An, H.; Boada, F.; Cao, T.; Faul, D.; Jakoby, B.; Jansen, F.P.; Kemp, B.J.; Kinahan, P.E.; et al. A path to qualification of PET/MR scanners for multicenter brain imaging studies: Evaluation of MR-based attenuation correction methods using a patient phantom. J. Nucl. Med. 2021, 63. [Google Scholar] [CrossRef]
- Dominick, K.C.; Andrews, H.F.; Kaufmann, W.E.; Berry-Kravis, E.; Erickson, C.A. Psychotropic drug treatment patterns in persons with fragile X syndrome. J. Child Adolesc. Psychopharmacol 2021, 31, 659–669. [Google Scholar] [CrossRef]
- Bartholomay, K.L.; Lee, C.H.; Bruno, J.L.; Lightbody, A.A.; Reiss, A.L. Closing the gender gap in fragile X syndrome: Review of females with fragile X syndrome and preliminary research findings. Brain Sci. 2019, 9, 11. [Google Scholar] [CrossRef]
- Miller, J.G.; Bartholomay, K.L.; Lee, C.H.; Bruno, J.L.; Lightbody, A.A.; Reiss, A.L. Empathy and Anxiety in Young Girls with Fragile X Syndrome. J. Autism Dev. Disord. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bartholomay, K.L.; Marzelli, M.J.; Miller, J.G.; Bruno, J.L.; Lightbody, A.A.; Reiss, A.L. Neuroanatomic profiles of young females with fragile X syndrome. Cereb. Cortex 2021. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.W.; Adams, C.; Nishino, T.; Hazlett, H.C.; Wolff, J.J.; Zwaigenbaum, L.; Constantino, J.N.; Shen, M.D.; Swanson, M.R.; Elison, J.T.; et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Hazlett, H.C.; Gu, H.; Munsell, B.C.; Kim, S.H.; Styner, M.; Wolff, J.J.; Elison, J.T.; Swanson, M.R.; Zhu, H.; Botteron, K.N.; et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017, 542, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Kraan, C.M.; Baker, E.K.; Arpone, M.; Bui, M.; Ling, L.; Gamage, D.; Bretherton, L.; Rogers, C.; Field, M.J.; Wotton, T.L.; et al. DNA Methylation at Birth Predicts Intellectual Functioning and Autism Features in Children with Fragile X Syndrome. Int. J. Mol. Sci. 2020, 21, 7735. [Google Scholar] [CrossRef]
- Heyman, M.; Galligan, M.L.; Salinas, G.B.; Baker, E.; Blacher, J.; Stavropoulos, K. Differential diagnosis of autism spectrum disorder, intellectual disability and attention-deficit hyperactivity disorder (ADHD). Adv. Autism 2021, 8, 89–103. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef]
- Lacruz-Pérez, I.; Sanz-Cervera, P.; Pastor-Cerezuela, G.; Gómez-Marí, I.; Tárraga-Mínguez, R. Is It Possible to Educate, Intervene or “Cure” Autism Spectrum Disorder? A Content Analysis of YouTube Videos. Int. J. Environ. Res. Public Health 2021, 18, 2350. [Google Scholar] [CrossRef]
- Qin, M.; Schmidt, K.C.; Zametkin, A.J.; Bishu, S.; Horowitz, L.M.; Burlin, T.V.; Xia, Z.; Huang, T.; Quezado, Z.; Smith, C.B. Altered Cerebral Protein Synthesis in Fragile X Syndrome: Studies in Human Subjects and Knockout Mice. J. Cereb. Blood Flow Metab. 2013, 33, 499–507. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).