Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer’s Disease
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Statistical Analysis
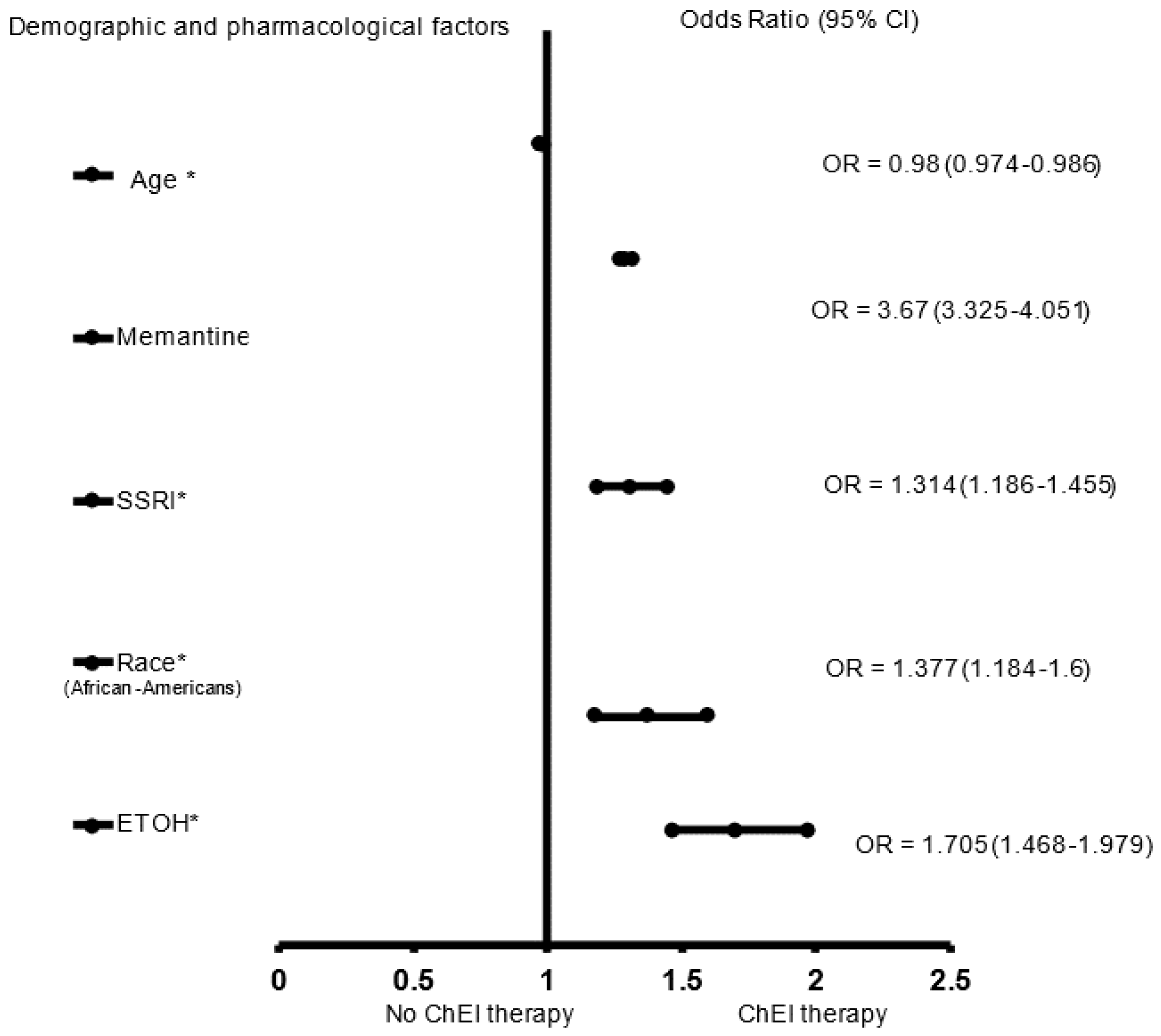
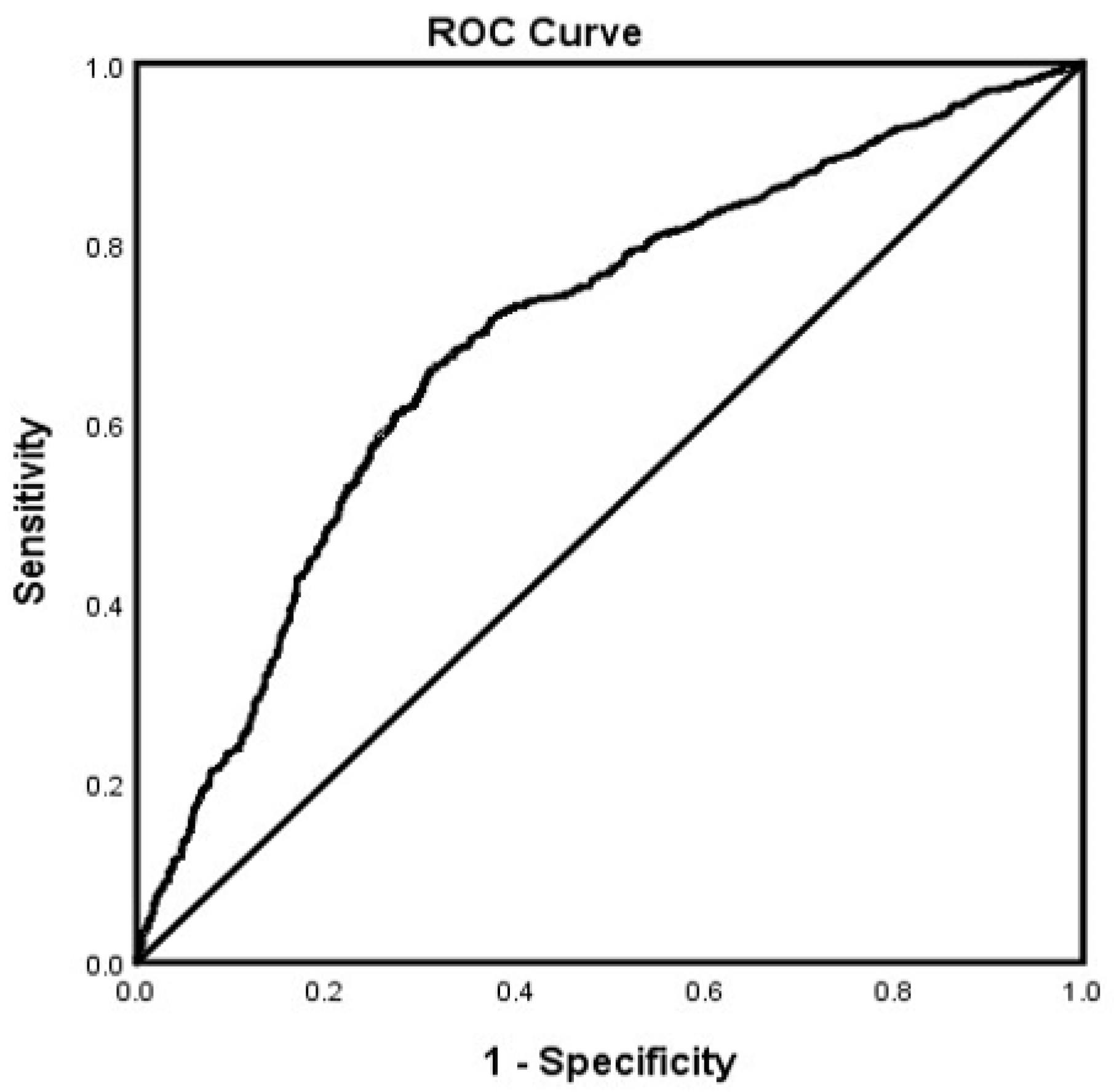
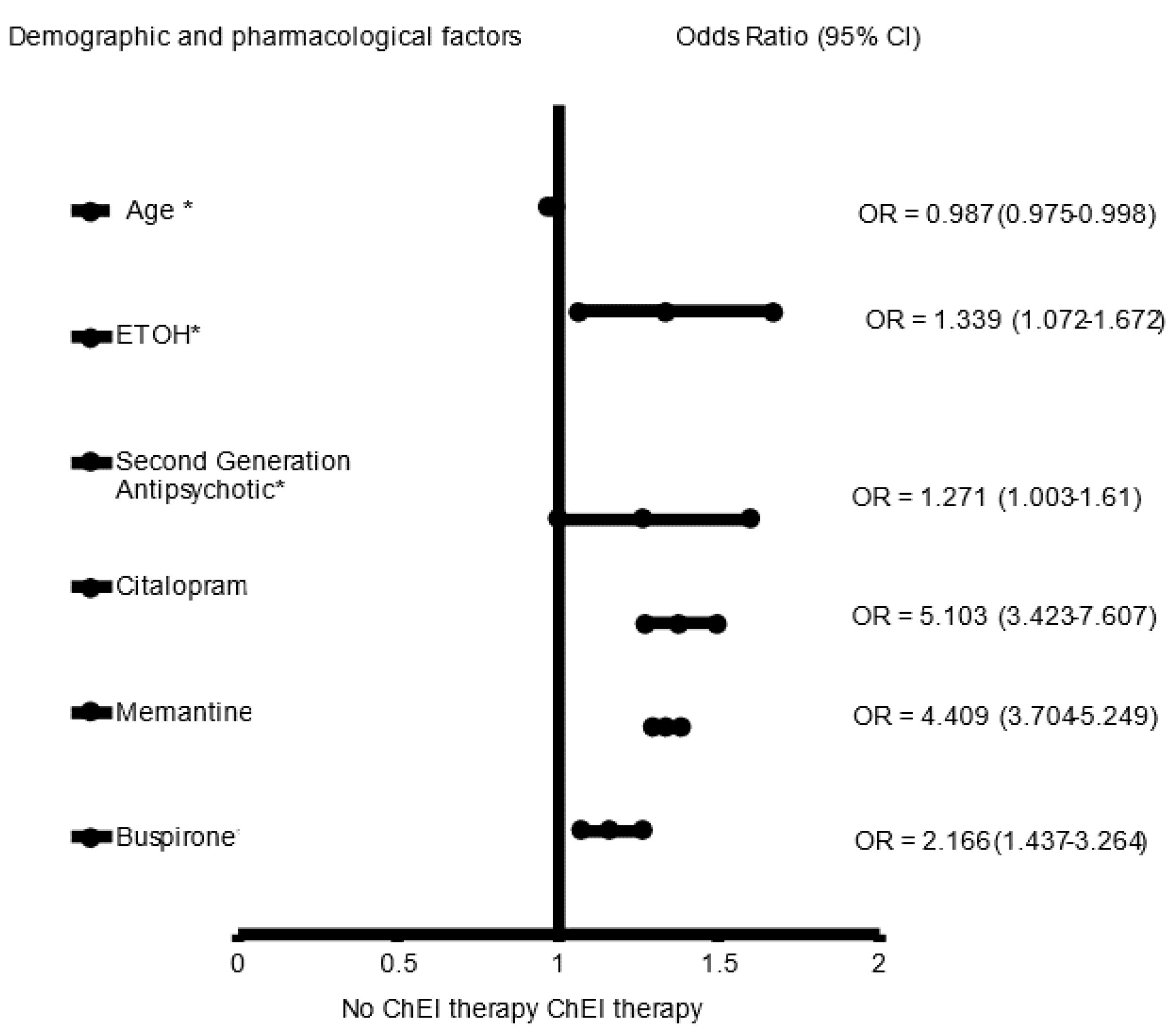
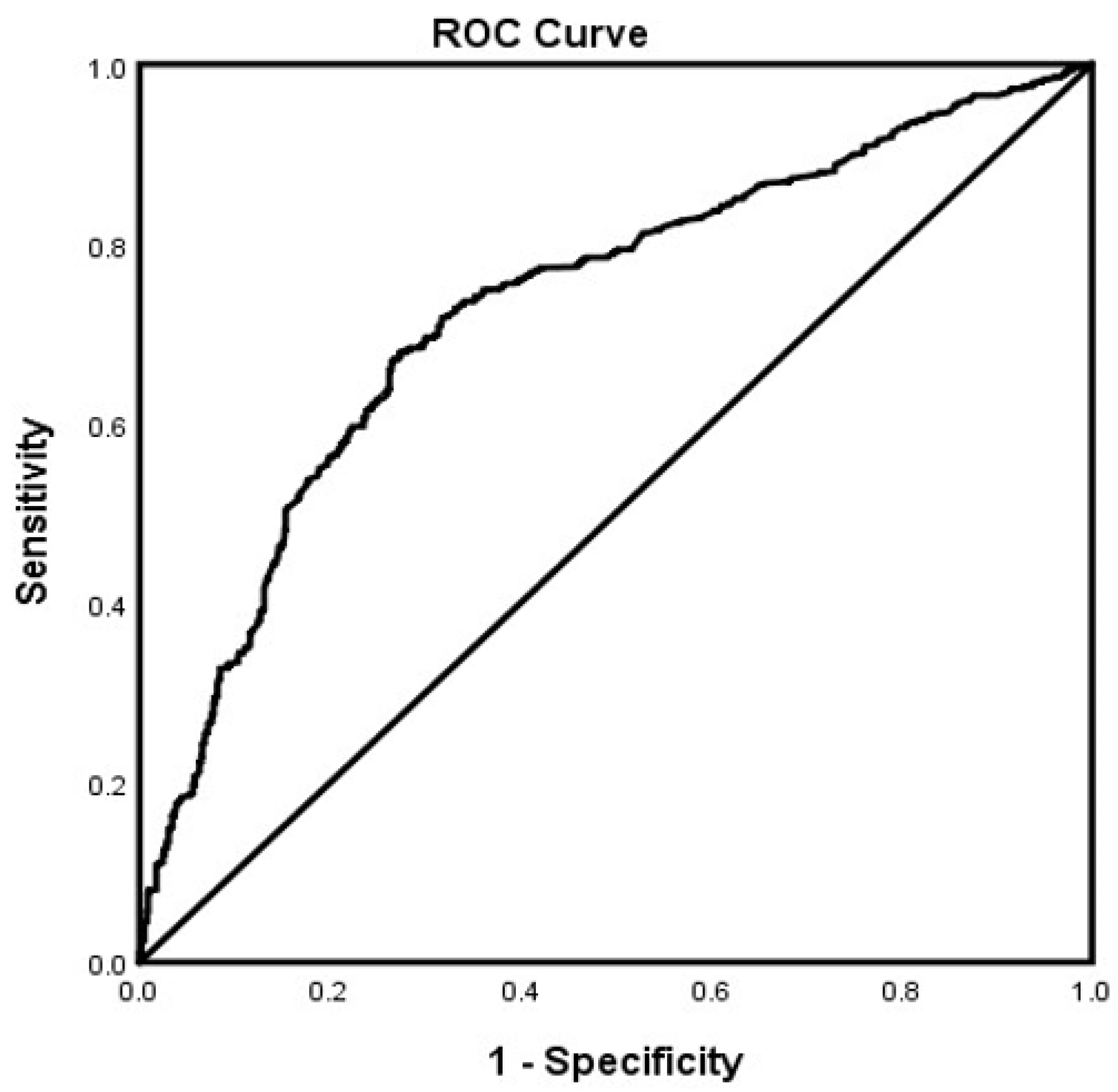
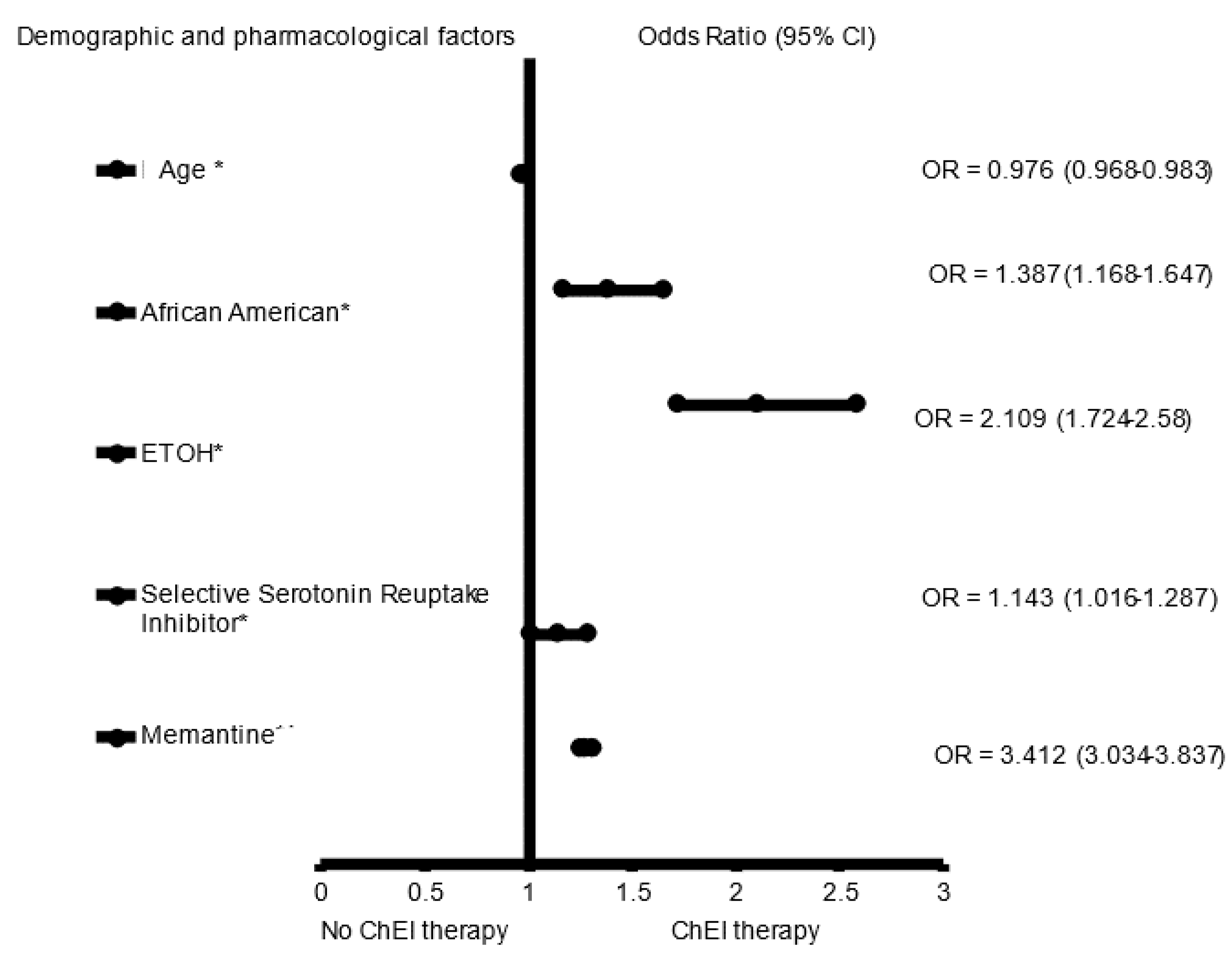
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haque, M.A.; Khalil, M.I.; Barman, N.; Islam, M.T.; Mannan, M.; Rob, M.A.; Saha, A.; Hossain, M.A. Gender variation in the risk factors with ischemic stroke: Bangladesh perspective. Mymensingh Med. J. MMJ 2015, 24, 710–716. [Google Scholar] [PubMed]
- Alzheimer‘s association report (2020) Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Alzheimer’s, A. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Gaugler, J.; James, B.; Johnson, T.; Marin, A.; Weuve, J.; Alzheimer’s, A. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical Epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef]
- Ott, B.R.; Lapane, K.L.; Gambassi, G.; Grp, S.S. Gender differences in the treatment of behavior problems in Alzheimer’s disease. Neurology 2000, 54, 427–432. [Google Scholar] [CrossRef]
- Lin, K.A.; Choudhury, R.K.; Rathakrishnan, B.G.; Marks, D.M.; Petrella, J.R.; Doraiswamya, P.M. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 2015, 1, 103–110. [Google Scholar] [CrossRef]
- Chene, G.; Beiser, A.; Au, R.; Preis, S.R.; Wolf, P.A.; Dufouil, C.; Seshadri, S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015, 11, 310–320. [Google Scholar] [CrossRef]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 2016, 6, 54–65. [Google Scholar] [CrossRef]
- Ropacki, S.A.; Jeste, D.V. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am. J. Psychiatry 2005, 162, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Rosenheck, R.; Lyketsos, C.G.; Kaczynski, R.; Sultzer, D.L.; Schneider, L.S. Effect of second-generation antipsychotics on caregiver burden in Alzheimer’s disease. J. Clin. Psychiatry 2012, 73, 121–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sultzer, D.L.; Davis, S.M.; Tariot, P.N.; Dagerman, K.S.; Lebowitz, B.D.; Lyketsos, C.G.; Rosenheck, R.A.; Hsiao, J.K.; Lieberman, J.A.; Schneider, L.S. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: Phase 1 outcomes from the CATIE-AD effectiveness trial. Am. J. Psychiatry 2008, 165, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C. Atypical antipsychotics fail to improve functioning or quality of life in people with Alzheimer’s disease. Evid.-Based Ment. Health 2009, 12, 20. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef]
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: An evidence-based treatment review. Expert Opin. Pharmacother. 2018, 19, 1057–1070. [Google Scholar] [CrossRef]
- Mdawar, B.; Ghossoub, E.; Khoury, R. Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen. Res. 2020, 15, 41–46. [Google Scholar] [CrossRef]
- Todd, S.; Barr, S.; Roberts, M.; Passmore, A.P. Survival in dementia and predictors of mortality: A review. Int. J. Geriatr. Psychiatry 2013, 28, 1109–1124. [Google Scholar] [CrossRef]
- Tom, S.E.; Hubbard, R.A.; Crane, P.K.; Haneuse, S.J.; Bowen, J.; McCormick, W.C.; McCurry, S.; Larson, E.B. Characterization of Dementia and Alzheimer’s disease in an older population: Updated Incidence and life expectancy with and without dementia. Am. J. Public Health 2015, 105, 408–413. [Google Scholar] [CrossRef]
- Au, B.; Dale-McGrath, S.; Tierney, M.C. Sex differences in the prevalence and incidence of mild cognitive impairment: A meta-analysis. Ageing Res. Rev. 2017, 35, 176–199. [Google Scholar] [CrossRef] [PubMed]
- Liesinger, A.M.; Graff-Radford, N.R.; Duara, R.; Carter, R.E.; Hanna Al-Shaikh, F.S.; Koga, S.; Hinkle, K.M.; DiLello, S.K.; Johnson, M.F.; Aziz, A.; et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018, 136, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Kawas, C.H. The oldest old and the 90+ Study. Alzheimers Dement. 2008, 4, S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Fratiglioni, L.; Viitanen, M.; von Strauss, E.; Tontodonati, V.; Herlitz, A.; Winblad, B. Very old women at highest risk of dementia and Alzheimer’s disease: Incidence data from the Kungsholmen project, Stockholm. Neurology 1997, 48, 132–138. [Google Scholar] [CrossRef]
- Wu, L.; Rosa-Neto, P.; Hsiung, G.Y.; Sadovnick, A.D.; Masellis, M.; Black, S.E.; Jia, J.; Gauthier, S. Early-onset familial Alzheimer’s disease (EOFAD). Can. J. Neurol. Sci. 2012, 39, 436–445. [Google Scholar] [CrossRef]
- Stanley, K.; Walker, Z. Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int. Psychogeriatr. 2014, 26, 1945–1953. [Google Scholar] [CrossRef]
- Eriksson, H.; Fereshtehnejad, S.M.; Falahati, F.; Farahmand, B.; Religa, D.; Eriksdotter, M. Differences in Routine clinical practice between early and late onset Alzheimer’s Disease: Data from the Swedish Dementia Registry (SveDem). J. Alzheimers Dis. 2014, 41, 411–419. [Google Scholar] [CrossRef]
- Tellechea, P.; Pujol, N.; Esteve-Belloch, P.; Echeveste, B.; Garcia-Eulate, M.R.; Arbizu, J.; Riverol, M. Early- and late-onset Alzheimer disease: Are they the same entity? Neurologia 2018, 33, 244–253. [Google Scholar] [CrossRef]
- Lee, W.J.; Yoon, C.W.; Kim, S.W.; Jeong, H.J.; Seo, S.; Na, D.L.; Noh, Y.; Seong, J.K. Effects of Alzheimer’s and Vascular Pathologies on Structural Connectivity in Early- and Late-Onset Alzheimer’s Disease. Front. Neurosci. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Delrieu, J.; Piau, A.; Caillaud, C.; Voisin, T.; Vellas, B. Managing cognitive dysfunction through the continuum of Alzheimer’s disease role of pharmacotherapy. CNS Drugs 2011, 25, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Shahrivar-Gargari, M.; Hamzeh-Mivehroud, M.; Hemmati, S.; Mojarrad, J.S.; Notash, B.; Kucukkilinc, T.T.; Ayazgok, B.; Dastmalchi, S. Design, synthesis, and biological evaluation of novel indanone-based hybrids as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J. Mol. Struct. 2021, 1229, 129787. [Google Scholar] [CrossRef]
- Miles, J.A.; Ng, J.H.; Sreenivas, B.Y.; Courageux, C.; Igert, A.; Dias, J.; McGeary, R.P.; Brazzolotto, X.; Ross, B.P. Discovery of drug-like acetylcholinesterase inhibitors by rapid virtual screening of a 6.9 million compound database. Chem. Biol. Drug Des. 2021, 97, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.A.; Ross, B.P. Recent advances in virtual screening for cholinesterase inhibitors. ACS Chem. Neurosci. 2021, 12, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Riswanto, F.D.O.; Rawa, M.S.A.; Murugaiyah, V.; Salin, N.H.; Istyastono, E.P.; Hariono, M.; Wahab, H.A. Anti-Cholinesterase activity of chalcone derivatives: Synthesis, in vitro assay and molecular docking study. Med. Chem. 2021, 17, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Wattmo, C.; Wallin, A.K. Early- versus Late-Onset Alzheimer disease: Long-Term functional outcomes, nursing home placement, and risk factors for rate of progression. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Wattmo, C.; Wallin, A.K.; Londos, E.; Minthon, L. Predictors of long-term cognitive outcome in Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3, 1–13. [Google Scholar] [CrossRef]
- Wattmo, C.; Wallin, A.K. Early- versus late-onset Alzheimer’s disease in clinical practice: Cognitive and global outcomes over 3 years. Alzheimers Res. Ther. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Xu, J.; Xia, L.L.; Song, N.; Chen, S.D.; Wang, G. Testosterone, estradiol, and sex hormone-binding globulin in Alzheimer’s Disease: A meta-analysis. Curr. Alzheimer Res. 2016, 13, 215–222. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef]
- Wattmo, C.; Londos, E.; Minthon, L. Risk factors that affect life expectancy in Alzheimer’s Disease: A 15-year follow-up. Dement. Geriatr. Cogn. Disord. 2014, 38, 286–299. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2018. [Google Scholar]
- Zhang, S.; Wu, Y.H.; Zhang, Y.; Zhang, Y.; Cheng, Y. Preliminary study of the validity and reliability of the Chinese version of the Saint Louis University Mental Status Examination (SLUMS) in detecting cognitive impairment in patients with traumatic brain injury. Appl. Neuropsychol.-Adult 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wattmo, C.; Londos, E.; Minthon, L. Response to cholinesterase inhibitors affects lifespan in Alzheimer’s disease. BMC Neurol. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Konig, T.; Stogmann, E. Genetics of Alzheimer’s disease. Wiener Med. Wochenschr. 2021, 171, 249–256. [Google Scholar] [CrossRef]
- Larson, E.B.; Shadlen, M.F.; Wang, L.; McCormick, W.C.; Bowen, J.D.; Teri, L.; Kukull, W.A. Survival after initial diagnosis of Alzheimer disease. Ann. Intern. Med. 2004, 140, 501–509. [Google Scholar] [CrossRef]
- Marvanova, M. Antipsychotic use in elderly patients with dementia: Efficacy and safety concerns. Ment. Health Clin. 2014, 4, 170–176. [Google Scholar] [CrossRef]
- Liu, J.P.; Chang, L.R.; Song, Y.Z.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Malinow, R. New developments on the role of NMDA receptors in Alzheimer’s disease. Curr. Opin. Neurobiol. 2012, 22, 559–563. [Google Scholar] [CrossRef]
- Di Carlo, A.; Lamassa, M.; Consoli, D.; Valentia, V.; Inzitari, D. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010, 75, 670–671. [Google Scholar] [CrossRef]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef]
- Jean, J.C.; Moos, W.H.; Johnson, G. Cholinomimetics and Alzheimer’s disease. Bioorganic Med. Chem. Lett. 1992, 2, 777–780. [Google Scholar] [CrossRef]
- Geerts, H.; Spiros, A.; Roberts, P. Assessing the synergy between cholinomimetics and memantine as augmentation therapy in cognitive impairment in schizophrenia. A virtual human patient trial using quantitative systems pharmacology. Front. Pharmacol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Nikiforuk, A.; Potasiewicz, A.; Kos, T.; Popik, P. The combination of memantine and galantamine improves cognition in rats: The synergistic role of the α7 nicotinic acetylcholine and NMDA receptors. Behav. Brain Res. 2016, 313, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.D.; Spiros, A.; Geerts, H. Simulations of symptomatic treatments for Alzheimer’s disease: Computational analysis of pathology and mechanisms of drug action. Alzheimers Res. Ther. 2012, 4, 50. [Google Scholar] [CrossRef]
- Mueller, C.; Soysal, P.; Rongve, A.; Isik, A.T.; Thompson, T.; Maggi, S.; Smith, L.; Basso, C.; Stewart, R.; Ballard, C.; et al. Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res. Rev. 2019, 50, 72–80. [Google Scholar] [CrossRef]
- Bleckwenn, M.; Kleineidam, L.; Wagner, M.; Jessen, F.; Weyerer, S.; Werle, J.; Wiese, B.; Luhmann, D.; Posselt, T.; Konig, H.H.; et al. Impact of coronary heart disease on cognitive decline in Alzheimer’s disease: A prospective longitudinal cohort study in primary care. Br. J. Gen. Pract. 2017, 67, E111–E117. [Google Scholar] [CrossRef]
- Rountree, S.D.; Chan, W.; Pavlik, V.N.; Darby, E.J.; Doody, R.S. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimers Res. Ther. 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Fazal, K.; Perera, G.; Khondoker, M.; Howard, R.; Stewart, R. Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer’s disease. Bjpsych. Open 2017, 3, 158–164. [Google Scholar] [CrossRef]
- Pimouguet, C.; Delva, F.; Le Goff, M.; Stern, Y.; Pasquier, F.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Helmer, C. Survival and early recourse to care for dementia: A population based study. Alzheimers Dement. 2015, 11, 385–393. [Google Scholar] [CrossRef]
- Gasper, M.C.; Ott, B.R.; Lapane, K.L. Is donepezil therapy associated with reduced mortality in nursing home residents with dementia? Am. J. Geriatr. Pharmacother. 2002, 3, 1–7. [Google Scholar] [CrossRef]
- Rajamaki, B.; Hartikainen, S.; Tolppanen, A.M. The effect of comorbidities on survival in persons with Alzheimer’s disease: A matched cohort study. BMC Geriatr. 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, D.; Vermeiren, Y.; Dekker, A.D.; Naude, P.J.W.; De Deyn, P.P. Neuropsychiatric disturbances in Alzheimer’s disease: What have we learned from neuropathological studies? Curr. Alzheimer Res. 2016, 13, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Simic, G.; Leko, M.B.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.L.; Gruenewald, D.A. Pharmacologic management of agitation in patients with dementia. Curr. Geriatr. Rep. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Cruz, M.R.S.; Hidalgo, P.C.; Lee, M.S.; Thomas, C.W.; Holroyd, S. Buspirone for the treatment of dementia with behavioral disturbance. Int. Psychogeriatr. 2017, 29, 859–862. [Google Scholar] [CrossRef]
- Moret, C. Combination/augmentation strategies for improving the treatment of depression. Neuropsychiatr. Dis. Treat. 2005, 1, 301–309. [Google Scholar]
- Herrmann, N.; Lanctot, K.L. Pharmacologic management of neuropsychiatric symptoms of Alzheimer disease. Can. J. Psychiatry-Rev. Can. de Psychiatr. 2007, 52, 630–646. [Google Scholar] [CrossRef]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥ 65 years. Alzheimers Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef]
- Potter, G.G.; Plassman, B.L.; Burke, J.R.; Kabeto, M.U.; Langa, K.M.; Llewellyn, D.J.; Rogers, M.A.M.; Steffens, D.C. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009, 5, 445–453. [Google Scholar] [CrossRef]
- Barnes, L.L.; Bennett, D.A. Alzheimer’s Disease In African Americans: Risk Factors and Challenges for the Future. Health Aff. 2014, 33, 580–586. [Google Scholar] [CrossRef]
- Barnes, L.L.; Wilson, R.S.; Li, Y.; Aggarwal, N.T.; Gilley, D.W.; McCann, J.J.; Evans, D.A. Racial differences in the progression of cognitive decline in Alzheimer disease. Am. J. Geriatr. Psychiatry 2005, 13, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Babulal, G.M.; Quiroz, Y.T.; Albensi, B.C.; Arenaza-Urquijo, E.; Astell, A.J.; Babiloni, C.; Bahar-Fuchs, A.; Bell, J.; Bowman, G.L.; Brickman, A.M.; et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement. 2019, 15, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Vishwas, S.; Awasthi, A.; Corrie, L.; Singh, S.K.; Gulati, M. Multiple target-based combination therapy of galantamine, memantine and lycopene for the possible treatment of Alzheimer’s disease. Med. Hypotheses 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T.; Tong, G.; Burke, A.D.; Tariot, P.N. Present Algorithms and Future Treatments for Alzheimer’s Disease. J. Alzheimers Dis. 2019, 67, 1157–1171. [Google Scholar] [CrossRef]
| Characteristic | Men | Women | |
|---|---|---|---|
| Number of Patients | 2949 | 6341 | p-Value |
| Age Group: No. (%) | |||
| 60–69 | 104 (1.6) | 32 (1.1) | 0.007 *a |
| 70–79 | 1076 (17.0) | 562 (19.1) | |
| ≥80 | 5161 (81.4) | 2355 (79.9) | |
| Mean ± SD | 85.59 ± 7.15 | 86.60 ± 7.51 | <0.001 *b |
| Race: No (%) | |||
| White | 5218 (82.3) | 2590 (87.8) | <0.001 *a |
| Black | 790 (12.5) | 246 (8.3) | |
| Other | 333 (5.3) | 113 (3.8) | |
| Hispanic Ethnicity: No. (%) | 148 (2.3) | 31 (1.1) | <0.001 *a |
| ETOH | 680 (10.9) | 526 (18.2) | <0.001 *a |
| Tobacco | 2019 (32.5) | 1879 (65.5) | <0.001 *a |
| Medications | |||
| Central acetylcholinesterase inhibitor | 3983 (62.8) | 1902 (64.5) | 0.117 |
| Donepezil | 3456 (54.5) | 1657 (56.2) | 0.128 |
| Galantamine | 77 (1.2) | 62 (2.1) | 0.001 *a |
| Rivastigmine | 782 (12.3) | 342 (11.6) | 0.312 |
| Second Generation Antipsychotic | 1017 (16.0) | 470 (15.9) | 0.902 |
| Aripiprazole | 175 (2.8) | 34 (1.2) | <0.001 * |
| Olanzapine | 267 (4.2) | 131 (4.4) | 0.608 |
| Risperidone | 681 (10.7) | 318 (10.8) | 0.950 |
| Selective Serotonin Receptor Inhibitor | 2260 (35.6) | 806 (27.3) | <0.001 *a |
| Citalopram | 819 (12.9) | 286 (9.7) | <0.001 *a |
| Escitalopram | 1489 (23.5) | 529 (17.9) | <0.001 *a |
| Paroxetine | 0 (0.0) | 0 (0.0) | |
| Memantine | 2783 (43.9) | 1385 (47.0) | 0.006 *a |
| Trazadone | 0 (0.0) | 0 (0.0) | |
| Trazadone | 501 (7.9) | 170 (5.8) | <0.001 *a |
| Valproate | 0 (0.0) | 0 (0.0) |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Characteristic | No CAI | CAI | No CAI | CAI | ||
| Number of Patients | 1047 | 1902 | p-Value | 2358 | 3982 | p-Value |
| Age Group: No. (%) | ||||||
| 60–69 | 2 (0.2) | 30 (1.6) | 0.002 *a | 25 (1.1) | 79 (2.0) | <0.001 *a |
| 70–79 | 198 (18.9) | 364 (19.1) | 359 (15.2) | 717 (18.0) | ||
| ≥ 80 | 847 (80.9) | 1508 (79.3) | 1974 (83.7) | 3187 (80.0) | ||
| Mean ± SD | 86.21 ± 7.24 | 85.26 ± 7.07 | 0.001 *b | 87.85 ± 7.88 | 85.86 ± 7.18 | <0.001 *b |
| Race: No (%) | ||||||
| White | 933 (89.1) | 1657 (87.1) | 0.136 | 1979 (83.9) | 3239 (81.3) | 0.001 *a |
| Black | 73 (7.0) | 173 (9.1) | 247 (10.5) | 543 (13.6) | ||
| Other | 41 (3.9) | 72 (3.8) | 132 (5.6) | 201 (5.0) | ||
| Hispanic Ethnicity: No. (%) | 12 (1.2) | 19 (1.0) | 0.716 | 48 (2.1) | 100 (2.5) | 0.237 |
| ETOH | 154 (15.1) | 372 (19.9) | 0.002 *a | 154 (6.7) | 526 (13.3) | <0.001 *a |
| Tobacco | 674 (67.0) | 1205 (64.7) | 0.213 | 744 (32.5) | 1275 (32.5) | 0.965 |
| Medications | ||||||
| Second Generation Antipsychotic | 140 (13.4) | 330 (17.4) | 0.005 *a | 386 (16.4) | 631 (15.8) | 0.580 |
| Aripiprazole | 8 (0.8) | 26 (1.4) | 0.142 | 70 (3.0) | 105 (2.6) | 0.435 |
| Olanzapine | 39 (3.7) | 92 (4.8) | 0.161 | 100 (4.2) | 167 (4.2) | 0.927 |
| Risperidone | 100 (9.6) | 218 (11.5) | 0.109 | 251 (10.6) | 430 (10.8) | 0.851 |
| Selective Serotonin Receptor Inhibitor | 193 (18.4) | 613 (32.3) | <0.001 *a | 753 (31.9) | 1507 (37.8) | <0.001 *a |
| Citalopram | 32 (3.1) | 254 (13.4) | <0.001 *a | 288 (12.2) | 531 (13.3) | 0.200 |
| Escitalopram | 161 (15.4) | 368 (19.3) | 0.007 *a | 487 (20.7) | 1002 (25.2) | <0.001 *a |
| Paroxetine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Memantine | 254 (24.3) | 1131 (59.5) | <0.001 *a | 590 (25.0) | 2193 (55.1) | <0.001 *a |
| Trazadone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Buspirone | 33 (3.2) | 137 (7.2) | <0.001 *a | 192 (8.1) | 309 (7.8) | 0.583 |
| Valproate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey-Taylor, M.J.; Poupore, N.; Theriot Roley, L.; Goodwin, R.L.; Mcphail, B.; Nathaniel, T.I. Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer’s Disease. Brain Sci. 2022, 12, 160. https://doi.org/10.3390/brainsci12020160
Bailey-Taylor MJ, Poupore N, Theriot Roley L, Goodwin RL, Mcphail B, Nathaniel TI. Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer’s Disease. Brain Sciences. 2022; 12(2):160. https://doi.org/10.3390/brainsci12020160
Chicago/Turabian StyleBailey-Taylor, Melissa J., Nicolas Poupore, Laurie Theriot Roley, Richard L. Goodwin, Brooks Mcphail, and Thomas I. Nathaniel. 2022. "Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer’s Disease" Brain Sciences 12, no. 2: 160. https://doi.org/10.3390/brainsci12020160
APA StyleBailey-Taylor, M. J., Poupore, N., Theriot Roley, L., Goodwin, R. L., Mcphail, B., & Nathaniel, T. I. (2022). Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer’s Disease. Brain Sciences, 12(2), 160. https://doi.org/10.3390/brainsci12020160






