Association of Elevated Serum Uric Acid with Nerve Conduction Function and Peripheral Neuropathy Stratified by Gender and Age in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Materials and Methods
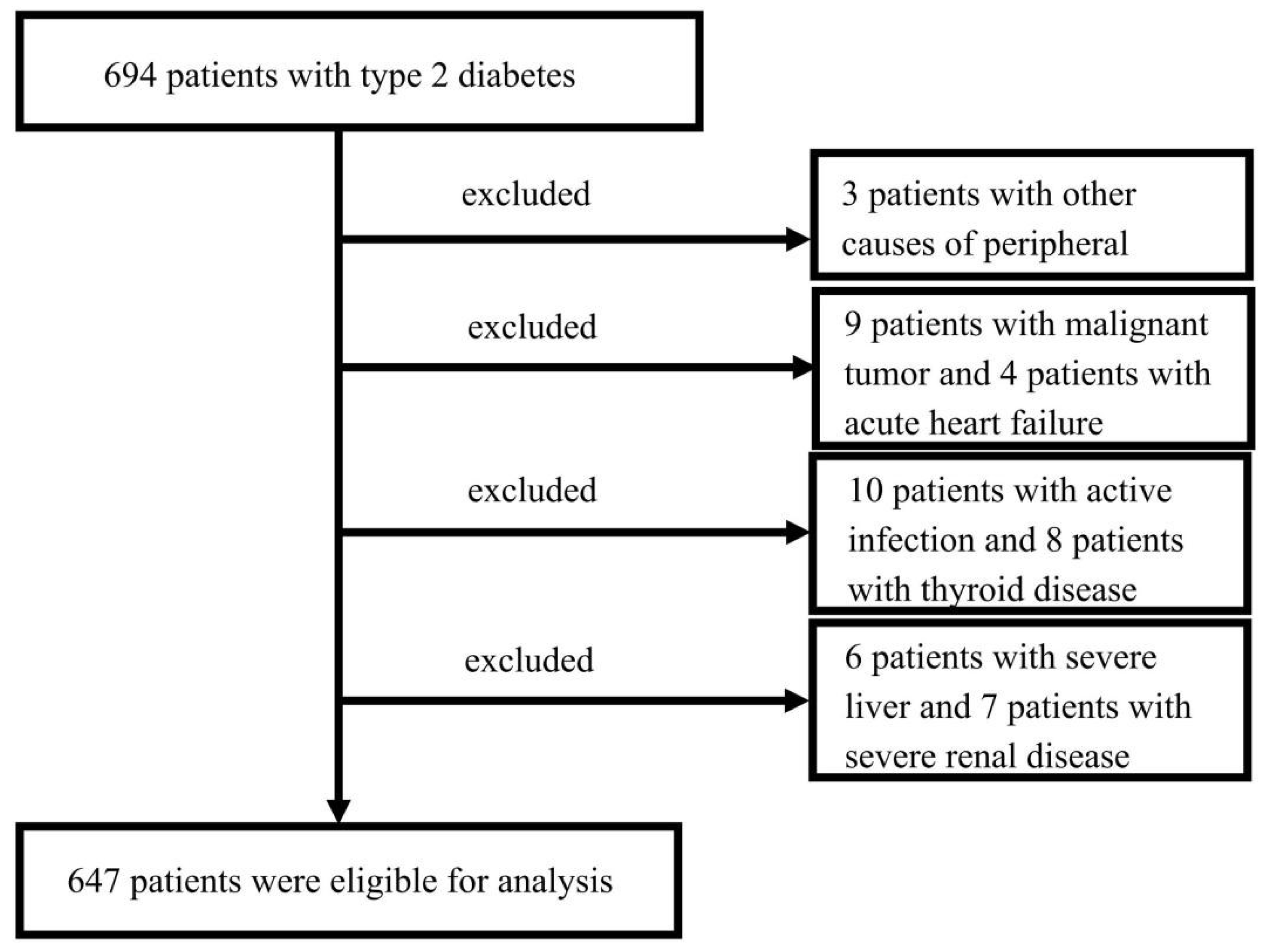
2.1. Study Population
2.2. Peripheral Neuropathy Assessment
2.3. Clinical Feature Collection and Laboratory Examination
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
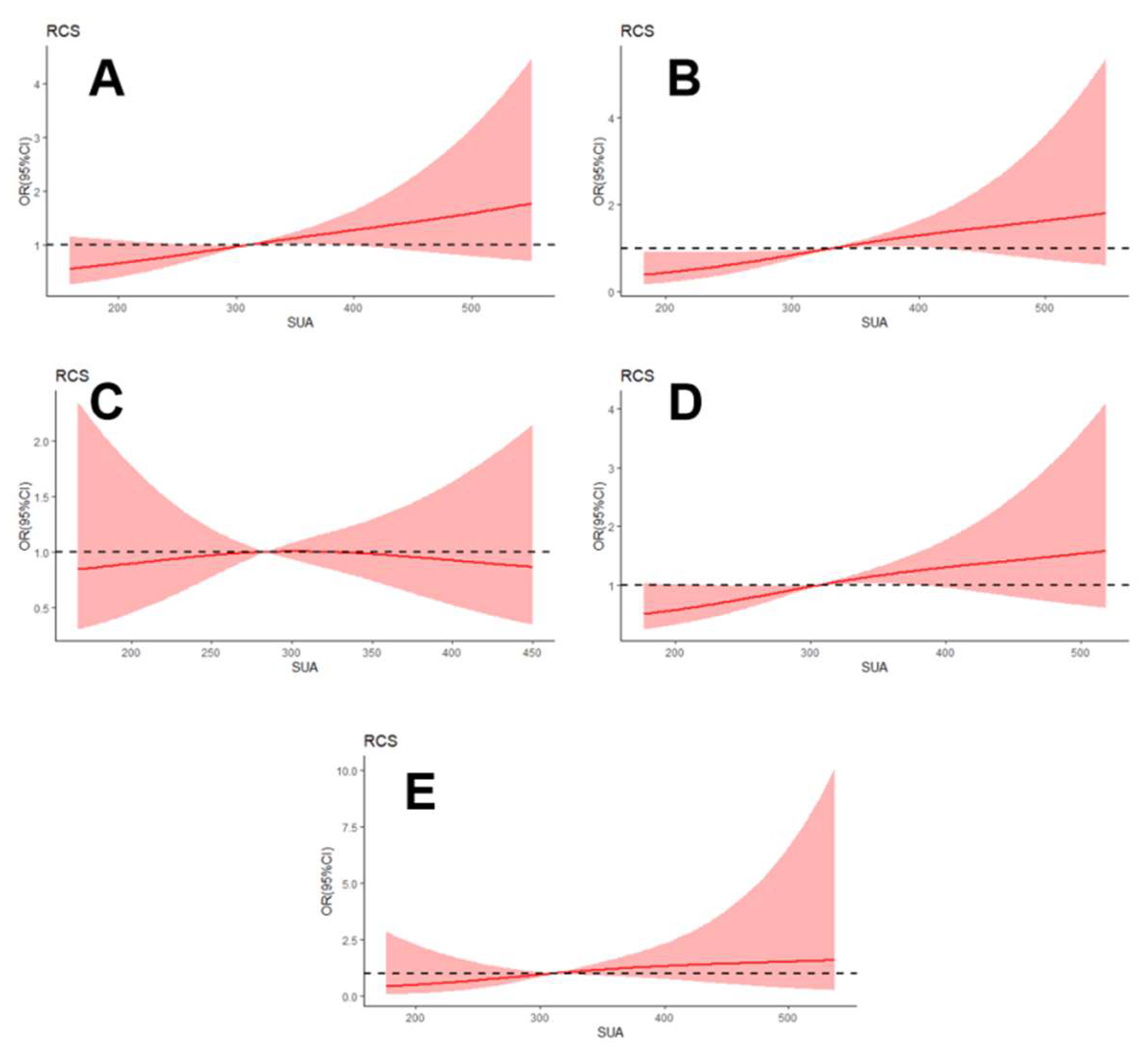
3.2. Association between SUA Level and the Presence of DPN
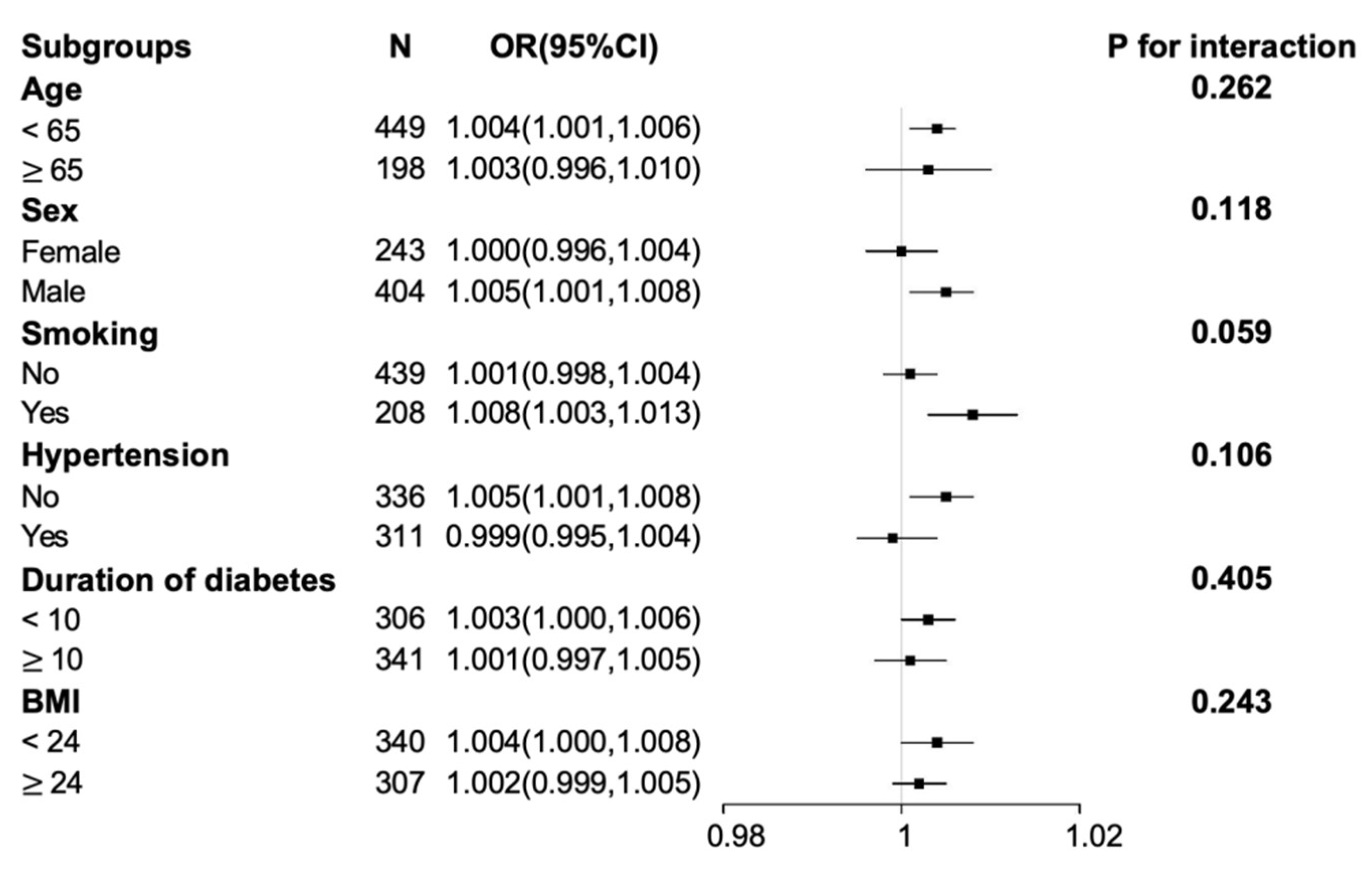
3.3. Subgroup Analysis for the Association between SUA Level and the Presence of DPN
3.4. Relationship between SUA Level and NCSs Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Hu, J.; Wen, J.; Zhang, Z.; Zhou, L.; Li, Y.; Hu, R. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes—ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS ONE 2013, 8, e61053. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Yamagishi, S.; Wada, R. Pathology and pathogenetic mechanisms of diabetic neuropathy: Correlation with clinical signs and symptoms. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. S1), S184–S189. [Google Scholar] [CrossRef] [PubMed]
- Waldfogel, J.M.; Nesbit, S.A.; Dy, S.M.; Sharma, R.; Zhang, A.; Wilson, L.M.; Bennett, W.L.; Yeh, H.-C.; Chelladurai, Y.; Feldman, D.; et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017, 88, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Ribu, L.; Rustoen, T.; Birkeland, K.; Hanestad, B.R.; Paul, S.M.; Miaskowski, C. The prevalence and occurrence of diabetic foot ulcer pain and its impact on health-related quality of life. J. Pain 2006, 7, 290–299. [Google Scholar] [CrossRef]
- Jiang, A.J.; Gu, H.; Feng, Z.R.; Ding, Y.; Xu, X.-H.; Yin, G.-P.; Zhang, W.-L.; Shen, Z.-Y.; Li, Q. Heart rate-corrected QT interval: A novel diagnostic biomarker for diabetic peripheral neuropathy. J. Diabetes Investig. 2021, 13, 850–857. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, H.; Zhu, X.; Mao, F.; Zhang, S.; Shi, H.; Li, Y.; Lu, B. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2017, 130, 90–97. [Google Scholar] [CrossRef]
- Niu, Y.; Li, J.; Peng, R.; Zhao, X.; Wu, J.; Tang, Q. Low vitamin D is associated with diabetes peripheral neuropathy in older but not in young and middle-aged patients. Diabetes/Metab. Res. Rev. 2019, 35, e3162. [Google Scholar] [CrossRef]
- Zhu, F.F.; Yang, L.Z. The Association Between the Levels of Thyroid Hormones and Peripheral Nerve Conduction in Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 493–504. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Lu, J.; Zhang, Y.; Shen, Y.; Wang, C.; Jia, W. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine 2015, 50, 397–404. [Google Scholar] [CrossRef]
- Xiong, Y.; Wangsheng, F.; Wang, S.; Zhou, W.; Huang, X.; Bao, H.; Cheng, X. Positive association between body fat percentage and hyperuricemia in patients with hypertension: The China H-type hypertension registry study. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, T.; Xuan, X.; Hu, H.; Xiao, X.; Li, J. Serum albumin predicts hyperuricemia in patients with idiopathic membranous nephropathy. Clin. Nephrol. 2021, 96, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, A.; Zuo, Y.; Chen, S.; Zhang, L.; Zhao, Y.; Liu, L.; Wu, S.; Luo, Y.; Gao, J. Time course of serum uric acid accumulation and the risk of diabetes mellitus. Nutr. Diabetes 2022, 12, 1. [Google Scholar] [CrossRef]
- Wang, T.; Bi, Y.; Xu, M.; Huang, Y.; Xu, Y.; Li, X.; Wang, W.; Ning, G. Serum uric acid associates with the incidence of type 2 diabetes in a prospective cohort of middle-aged and elderly Chinese. Endocrine 2011, 40, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Meng, X.F.; He, F.F.; Chen, S.; Su, H.; Xiong, J.; Gao, P.; Tian, X.-J.; Liu, J.-S.; Zhu, Z.-H.; et al. High serum uric acid and increased risk of type 2 diabetes: A systemic review and meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e56864. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xu, L.; Zhao, D.; Luo, Z.; Pan, S. Correlation between serum uric acid and diabetic peripheral neuropathy in T2DM patients. J. Neurol. Sci. 2018, 385, 78–82. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.; Hou, X.; Xu, D.; Che, K.; Li, C.; Yan, S.; Wang, Y.; Wang, B. Serum Uric Acid Levels and Diabetic Peripheral Neuropathy in Type 2 Diabetes: A Systematic Review and Meta-analysis. Mol. Neurobiol. 2016, 53, 1045–1051. [Google Scholar] [CrossRef]
- Jiang, T.N.; Li, Y.F.; Zhang, Q.; Wang, L.Y.; Zhao, C.-L.; Liu, L.-G. Association between serum uric acid and large-nerve fiber dysfunction in type 2 diabetes: A cross-sectional study. Chin. Med. J. 2019, 132, 1015–1022. [Google Scholar] [CrossRef]
- Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5. [CrossRef] [Green Version]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Qian, Y.; Zeng, Y.; Lin, Q.; Huang, H.; Zhang, W.; Yu, H.; Deng, B. Association of platelet count and plateletcrit with nerve conduction function and peripheral neuropathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2021, 12, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, F.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. Improvement in Neuropathy Outcomes with Normalizing HbA1c in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, F.; Zhu, X.; Liu, S.; Qiao, X.; Zheng, H.; Lu, B.; Li, Y. Age as an Independent Risk Factor for Diabetic Peripheral Neuropathy in Chinese Patients with Type 2 Diabetes. Aging Dis. 2019, 10, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Hotta, N.; Akanuma, Y.; Kawamori, R.; Matsuoka, K.; Oka, Y.; Shichiri, M.; Toyota, T.; Nalashima, M.; Yoshimura, I.; Sakamoto, N.; et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: The 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care 2006, 29, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Kohara, N.; Kimura, J.; Kaji, R.; Goto, Y.; Ishii, J.; Takiguchi, M.; Nakai, M. F-wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: Multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia 2000, 43, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Dyck, P.J.; Carter, R.E.; Litchy, W.J. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 2011, 44, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Li, Q.; Min, R.; Deng, Y.; Xu, Y.; Gao, L. The association between serum uric acid and diabetic complications in patients with type 2 diabetes mellitus by gender: A cross-sectional study. PeerJ 2021, 9, e10691. [Google Scholar] [CrossRef]
- Abraham, A.; Breiner, A.; Barnett, C.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Uric acid levels correlate with the severity of diabetic sensorimotor polyneuropathy. J. Neurol. Sci. 2017, 379, 94–98. [Google Scholar] [CrossRef]
- Perkins, B.A.; Lovblom, L.E.; Lewis, E.J.H.; Bril, V.; Ferdousi, M.; Orszag, A.; Edwards, K.; Pritchard, N.; Russel, A.; Dehghani, C.; et al. Corneal Confocal Microscopy Predicts the Development of Diabetic Neuropathy: A Longitudinal Diagnostic Multinational Consortium Study. Diabetes Care 2021, 44, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Sumino, H.; Ichikawa, S.; Kanda, T.; Nakamura, T.; Sakamaki, T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet 1999, 354, 650. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Choi, H.K. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 2008, 10, R116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyck, P.J.; O’Brien, P.C.; Litchy, W.J.; Harper, C.M.; Klein, C.J.; Dyck, P.J. Monotonicity of nerve tests in diabetes: Subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care 2005, 28, 2192–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuelwafaa, N.; Ahmed, H.; Omer, I.; Abdullah, M.; Ahmed, A.; Musa, A. Electrophysiological Characterization of Neuropathy Complicating Type 1 Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 2435261. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, A.; Cherubini, A.; Volpato, S.; Sparvieri, E.; Lauretani, A.; Franceschi, C.; Senin, U.L.; Abate, G.; Paganelli, R.; Martin, A.; et al. Markers of inflammation, vitamin E and peripheral nervous system function: The InCHIANTI study. Neurobiol. Aging 2006, 27, 1280–1288. [Google Scholar] [CrossRef] [Green Version]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Mittal, R.; Kumar, A.; Singh, D.P.; Bishnoi, M.; Nag, T.C. Ameliorative potential of rutin in combination with nimesulide in STZ model of diabetic neuropathy: Targeting Nrf2/HO-1/NF-kB and COX signalling pathway. Inflammopharmacology 2018, 26, 755–768. [Google Scholar] [CrossRef]
- Hong, Q.; Wang, L.; Huang, Z.; Feng, Z.; Cui, S.; Fu, B.; Cai, G.; Chen, X.; Wu, D. High Concentrations of Uric Acid and Angiotensin II Act Additively to Produce Endothelial Injury. Mediat. Inflamm. 2020, 2020, 8387654. [Google Scholar] [CrossRef]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef]
- Rock, K.L.; Kataoka, H.; Lai, J.J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013, 9, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens 2015, 33, 1729–1741, discussion 1741. [Google Scholar] [CrossRef]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Martinon, F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol. Rev. 2010, 233, 218–232. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, X.; Kong, J. Recent Progress on Uric Acid Detection: A Review. Crit. Rev. Anal. Chem. 2020, 50, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.H. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes. Metab. 2022, 24, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 647) | Non-DPN (n = 176) | DPN (n = 471) | p Value |
|---|---|---|---|---|
| Age (years) | 57.14 ± 13.09 | 51.26 ± 13.51 | 59.34 ± 12.23 | 0.000 |
| Male, n. (%) | 404 (62.44%) | 103 (58.52%) | 301 (63.91%) | 0.208 |
| Smoking, n. (%) | 208 (32.15%) | 45 (25.57%) | 163 (34.61%) | 0.028 |
| Hypertension, n. (%) | 311 (48.07%) | 57 (32.39%) | 254 (53.93%) | 0.000 |
| Hyperlipidemia, n. (%) | 213 (32.92%) | 63 (35.80%) | 150 (31.85%) | 0.342 |
| Duration of diabetes (years) | 10 (4–14) | 5 (1–10) | 10 (5–17) | 0.000 |
| SUA (µmol/L) | 324.76 ± 96.81 | 309.16 ± 87.04 | 330.58 ± 99.67 | 0.012 |
| BMI (kg/m2) | 24.15 ± 3.38 | 24.51 ± 3.44 | 24.01 ± 3.35 | 0.101 |
| HbA1c (%) | 9.46 ± 2.34 | 9.49 ± 2.46 | 9.46 ± 2.29 | 0.887 |
| TC (mmol/L) | 4.75 ± 1.25 | 4.93 ± 1.22 | 4.68 ± 1.26 | 0.025 |
| TG (mmol/L) | 1.89 ± 1.46 | 1.94 ± 1.48 | 1.86 ± 1.46 | 0.539 |
| HDL-C (mmol/L) | 1.02 ± 0.29 | 0.99 ± 0.29 | 1.03 ± 0.29 | 0.161 |
| LDL-C (mmol/L) | 2.58 ± 0.92 | 2.73 ± 0.89 | 2.52 ± 0.92 | 0.008 |
| TSH (mIU/L) | 1.31 (0.86–1.87) | 1.31 (0.91–2.20) | 1.31 (0.84–1.81) | 0.543 |
| FT4 (pmol/L) | 11.21 ± 2.30 | 11.20 ± 2.11 | 11.22 ± 2.37 | 0.939 |
| FT3 (pmol/L) | 4.77 ± 1.56 | 4.93 ± 0.72 | 4.71 ± 1.78 | 0.107 |
| Characteristics | Serum Uric Acid (SUA) Levels | p Value | |
|---|---|---|---|
| ≤297.5 µmol/L (n = 284) | >297.5 µmol/L n = 363 | ||
| Age (years) | 57.13 ± 12.29 | 57.15 ± 13.69 | 0.981 |
| Male, n. (%) | 149 (52.46%) | 255 (70.25%) | 0.000 |
| Smoking, n. (%) | 83 (29.23%) | 125 (34.44%) | 0.159 |
| Hypertension, n. (%) | 126 (44.37%) | 185 (50.96%) | 0.096 |
| Hyperlipidemia, n. (%) | 94 (33.10%) | 119 (32.78%) | 0.932 |
| Duration of diabetes (years) | 10 (4–14) | 10 (4–15) | 0.812 |
| BMI (kg/m2) | 23.55 ± 3.02 | 24.61 ± 3.57 | 0.000 |
| HbA1c (%) | 9.56 ± 2.26 | 9.39 ± 2.40 | 0.349 |
| TC (mmol/L) | 4.69 ± 1.21 | 4.80 ± 1.29 | 0.283 |
| TG (mmol/L) | 1.30 (0.92–1.99) | 1.69 (1.18–2.35) | 0.000 |
| HDL-C (mmol/L) | 1.07 ± 0.32 | 0.99 ± 0.26 | 0.001 |
| LDL-C (mmol/L) | 2.56 ± 0.92 | 2.59 ± 0.91 | 0.624 |
| TSH (mIU/L) | 1.18 (0.84–1.81) | 1.34 (0.89–1.92) | 0.082 |
| FT4 (pmol/L) | 11.43 ± 2.63 | 11.04 ± 1.98 | 0.039 |
| FT3 (pmol/L) | 4.85 ± 2.21 | 4.72 ± 0.73 | 0.305 |
| DPN, n. (%) | 191 (67.25%) | 280 (77.13%) | 0.005 |
| Multivariate Adjusted Model (Model 1) | Multivariate Adjusted Model (Model 2) | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| All patients | SUA (>297.5 µmol/L vs. ≤297.5 µmol/L) | 1.689 (1.126–2.535) | 0.011 | 1.892 (1.225–2.922) | 0.004 |
| SUA as a continuous variable | 1.003 (1.000–1.005) | 0.026 | 1.003 (1.001–1.005) | 0.017 | |
| SUA Q1 (<257 µmol/L) | Ref. | Ref. | |||
| SUA Q2 (257–380 µmol/L) | 1.162 (0.724–1.865) | 0.535 | 1.173 (0.712–1.935) | 0.531 | |
| SUA Q3 (≥380 µmol/L) | 1.675 (0.934–3.003) | 0.083 | 1.796 (0.961–3.356) | 0.067 | |
| Male subgroup | SUA (>297.5 µmol/L vs. ≤297.5 µmol/L) | 1.968 (1.161–3.338) | 0.012 | 2.507 (1.405–4.473) | 0.002 |
| SUA as a continuous variable | 1.004 (1.001–1.007) | 0.012 | 1.005 (1.001,1.008) | 0.004 | |
| SUA Q1 (<257 µmol/L) | Ref. | Ref. | |||
| SUA Q2 (257–380 µmol/L) | 1.461 (0.758–2.816) | 0.257 | 1.510 (0.761–2.994) | 0.238 | |
| SUA Q3 (≥380 µmol/L) | 2.028 (0.986–4.171) | 0.055 | 2.510 (1.149–5.482) | 0.021 | |
| Female subgroup | SUA (>297.5 µmol/L vs. ≤297.5 µmol/L) | 1.411 (0.741–2.685) | 0.295 | 1.263 (0.623–2.559) | 0.517 |
| SUA as a continuous variable | 1.001 (0.997–1.005) | 0.691 | 1.000 (0.996–1.004) | 0.953 | |
| SUA Q1 (<257 µmol/L) | Ref. | Ref. | |||
| SUA Q2 (257–380 µmol/L) | 0.957 (0.480–1.910) | 0.901 | 0.880 (0.406–1.908) | 0.746 | |
| SUA Q3 (≥380 µmol/L) | 1.339 (0.453–3.960) | 0.598 | 0.959 (0.300–3.065) | 0.944 | |
| Younger subgroup | SUA (>297.5 µmol/L vs. ≤297.5 µmol/L) | 1.787 (1.142–2.796) | 0.011 | 2.070 (1.278–3.352) | 0.003 |
| SUA as a continuous variable | 1.003 (1.000–1.006) | 0.029 | 1.004 (1.001–1.006) | 0.013 | |
| SUA Q1 (<257 µmol/L) | Ref. | Ref. | |||
| SUA Q2 (257–380 µmol/L) | 1.131 (0.671–1.907) | 0.644 | 1.284 (0.739–2.231) | 0.375 | |
| SUA Q3 (≥380 µmol/L) | 1.665 (0.880–3.151) | 0.117 | 1.897 (0.959–3.755) | 0.066 | |
| Older subgroup | SUA (>297.5 µmol/L vs. ≤297.5 µmol/L) | 1.485 (0.509–4.335) | 0.470 | 1.895 (0.514–6.988) | 0.337 |
| SUA as a continuous variable | 1.003 (0.998–1.009) | 0.27 | 1.003 (0.996–1.010) | 0.336 | |
| SUA Q1 (<257 µmol/L) | Ref. | Ref. | |||
| SUA Q2 (257–380 µmol/L) | 2.171 (0.607–7.761) | 0.233 | 1.561 (0.302–8.067) | 0.595 | |
| SUA Q3 (≥380 µmol/L) | 2.581 (0.477–13.963) | 0.271 | 2.061 (0.274–15.484) | 0.482 | |
| SUA Level | p Value | ||
|---|---|---|---|
| ≤297.5 µmol/L (n = 284) | >297.5 µmol/L (n = 363) | ||
| Motor amplitude (mV) | |||
| Ulnar | 12.52 ± 2.62 | 12.15 ± 3.08 | 0.103 |
| Median | 12.39 ± 3.44 | 12.25 ± 3.15 | 0.612 |
| Tibial | 13.22 ± 5.81 | 12.91 ± 6.44 | 0.531 |
| Common peroneal | 6.07 ± 3.34 | 6.38 ± 3.76 | 0.276 |
| Motor CV (m/s) | |||
| Ulnar | 51.41 ± 5.89 | 50.28 ± 6.50 | 0.022 |
| Median | 52.36 ± 5.56 | 51.58 ± 5.08 | 0.062 |
| Tibial | 44.33 ± 4.97 | 43.38 ± 5.56 | 0.024 |
| Common peroneal | 43.28 ± 4.98 | 42.70 ± 5.24 | 0.155 |
| Sensory amplitude (uV) | |||
| Ulnar | 34.83 ± 19.86 | 31.76 ± 19.44 | 0.050 |
| Median | 34.82 ± 18.58 | 34.85 ± 19.89 | 0.985 |
| Superficial peroneal | 10.38 (5.50–14.02) | 10.36 (5.15–14.12) | 0.795 |
| Sensory CV (m/s) | |||
| Ulnar | 51.45 ± 6.24 | 51.38 ± 5.95 | 0.896 |
| Median | 51.00 ± 7.58 | 50.84 ± 7.59 | 0.780 |
| Superficial peroneal | 44.35 ± 5.44 | 44.08 ± 5.30 | 0.545 |
| F-wave minimum latency (ms) | 44.10 ± 4.81 | 45.21 ± 4.77 | 0.004 |
| MNAmp | 11.08 ± 2.78 | 10.98 ± 3.04 | 0.685 |
| MNCV | 47.87 ± 4.36 | 47.06 ± 4.42 | 0.022 |
| SNAmp | 27.20 (18.34–36.96) | 25.51 (19.29–34.91) | 0.648 |
| SNCV | 49.30 ± 5.00 | 49.74 ± 8.42 | 0.466 |
| Total Patients (n = 647) | Male Subgroup (n = 404) | Female Subgroup (n = 243) | Younger Subgroup (n = 449) | Older Subgroup (n = 198) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Motor amplitude (mV) | ||||||||||
| Ulnar | −0.023 | 0.553 | −0.036 | 0.470 | 0.045 | 0.484 | −0.043 | 0.361 | 0.026 | 0.714 |
| Median | −0.045 | 0.252 | −0.079 | 0.112 | −0.040 | 0.537 | −0.033 | 0.483 | −0.041 | 0.565 |
| Tibial | −0.060 | 0.128 | −0.101 | 0.042 | 0.031 | 0.629 | −0.042 | 0.379 | −0.079 | 0.270 |
| Common peroneal | −0.018 | 0.652 | −0.054 | 0.287 | 0.026 | 0.692 | −0.011 | 0.814 | 0.004 | 0.960 |
| Motor CV (m/s) | ||||||||||
| Ulnar | −0.097 | 0.013 | −0.062 | 0.215 | −0.022 | 0.733 | −0.128 | 0.007 | −0.037 | 0.601 |
| Median | −0.070 | 0.075 | −0.096 | 0.054 | 0.003 | 0.965 | −0.077 | 0.102 | −0.046 | 0.525 |
| Tibial | −0.104 | 0.008 | −0.152 | 0.002 | 0.010 | 0.883 | −0.107 | 0.023 | −0.087 | 0.229 |
| Common peroneal | −0.104 | 0.009 | −0.105 | 0.037 | 0.044 | 0.501 | −0.141 | 0.003 | −0.019 | 0.792 |
| Sensory amplitude (uV) | ||||||||||
| Ulnar | −0.101 | 0.011 | −0.081 | 0.107 | 0.044 | 0.497 | −0.130 | 0.006 | −0.027 | 0.705 |
| Median | −0.007 | 0.861 | −0.013 | 0.797 | 0.042 | 0.516 | −0.030 | 0.528 | 0.077 | 0.286 |
| Superficial peroneal | −0.056 | 0.182 | −0.036 | 0.512 | 0.042 | 0.530 | −0.042 | 0.402 | −0.007 | 0.934 |
| Sensory CV (m/s) | ||||||||||
| Ulnar | −0.057 | 0.153 | −0.026 | 0.610 | 0.023 | 0.722 | −0.075 | 0.112 | −0.010 | 0.886 |
| Median | −0.005 | 0.900 | −0.047 | 0.347 | −0.048 | 0.463 | −0.019 | 0.694 | 0.042 | 0.562 |
| Superficial peroneal | −0.065 | 0.124 | −0.122 | 0.024 | 0.082 | 0.219 | −0.104 | 0.037 | 0.016 | 0.840 |
| F-wave minimum latency (ms) | 0.138 | 0.000 | 0.171 | 0.001 | −0.086 | 0.187 | 0.121 | 0.010 | 0.041 | 0.577 |
| MNAmp | −0.051 | 0.203 | −0.096 | 0.057 | 0.026 | 0.688 | −0.038 | 0.421 | −0.047 | 0.520 |
| MNCV | −0.113 | 0.004 | −0.126 | 0.012 | 0.014 | 0.826 | −0.135 | 0.004 | −0.059 | 0.423 |
| SNAmp | −0.037 | 0.384 | −0.020 | 0.714 | 0.098 | 0.145 | −0.072 | 0.151 | 0.066 | 0.405 |
| SNCV | −0.020 | 0.641 | −0.038 | 0.482 | 0.030 | 0.658 | −0.061 | 0.221 | 0.033 | 0.679 |
| Total Patients (n = 647) | Male Subgroup (n = 404) | Younger Subgroup (n = 449) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| MNCV | −0.006 [(−0.009)–(−0.002)] | 0.002 | −0.007 [(−0.012)–(−0.003)] | 0.001 | −0.007 [(−0.012)–(−0.002)] | 0.004 |
| F-wave minimum latency (ms) | 0.007 (0.003–0.011) | 0.000 | 0.01 (0.005–0.015) | 0.000 | 0.006 (0.001–0.011) | 0.028 |
| Ulnar Sensory amplitude (µV) | −0.007 [(−0.023)–(0.008)] | 0.353 | −0.013 [(−0.03)–(0.004)] | 0.136 | −0.014 [(−0.035)–(0.006)] | 0.179 |
| Motor CV (m/s) | ||||||
| Ulnar | −0.004 [(−0.010)–(0.001)] | 0.103 | −0.005 [(−0.012)–(0.002)] | 0.143 | −0.007 [(−0.014)–(−0.001)] | 0.034 |
| Median | −0.005 [(−0.010)–(0.000)] | 0.032 | −0.006 [(−0.012)–(−0.001)] | 0.024 | −0.008 [(−0.014)–(−0.001)] | 0.016 |
| Tibial | −0.008 [(−0.012)–(−0.003)] | 0.001 | −0.010 [(−0.015)–(−0.005)] | 0.000 | −0.007 [(−0.013)–(−0.002)] | 0.010 |
| Common peroneal | −0.006 [(−0.010)–(−0.002)] | 0.004 | −0.009 [(−0.014)–(−0.003)] | 0.001 | −0.008 [(−0.014)–(−0.002)] | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, L.; Lou, M. Association of Elevated Serum Uric Acid with Nerve Conduction Function and Peripheral Neuropathy Stratified by Gender and Age in Type 2 Diabetes Patients. Brain Sci. 2022, 12, 1704. https://doi.org/10.3390/brainsci12121704
Zhang W, Chen L, Lou M. Association of Elevated Serum Uric Acid with Nerve Conduction Function and Peripheral Neuropathy Stratified by Gender and Age in Type 2 Diabetes Patients. Brain Sciences. 2022; 12(12):1704. https://doi.org/10.3390/brainsci12121704
Chicago/Turabian StyleZhang, Wanli, Lingli Chen, and Min Lou. 2022. "Association of Elevated Serum Uric Acid with Nerve Conduction Function and Peripheral Neuropathy Stratified by Gender and Age in Type 2 Diabetes Patients" Brain Sciences 12, no. 12: 1704. https://doi.org/10.3390/brainsci12121704
APA StyleZhang, W., Chen, L., & Lou, M. (2022). Association of Elevated Serum Uric Acid with Nerve Conduction Function and Peripheral Neuropathy Stratified by Gender and Age in Type 2 Diabetes Patients. Brain Sciences, 12(12), 1704. https://doi.org/10.3390/brainsci12121704








