1. Introduction
Herpes zoster (HZ), caused by reactivation of the latent varicella-zoster virus (VZV) in the spinal cord or cranial sensory ganglia, is typically characterized by a unilateral acute painful rash of the affected skin, which is self-limiting and generally disappears within a few weeks [
1]. Postherpetic neuralgia (PHN) is the most common clinical complication of HZ and presents with severe neuropathic pain, usually persisting for more than 1 month after the rash heals, which is in line with Chinese expert consensus [
2]. A study showed that the prevalence rates of HZ and PHN are 7.7% and 2.3%, respectively [
2]. Among HZ patients, 29.8% developed PHN, and the prevalence of both HZ and PHN tends to increase with age. These patients suffer from chronic pain, which seriously affects their quality of life and imposes a burden on society [
3].
The pathogenesis of PHN is not yet fully understood. Currently, PHN is thought to be caused by abnormally high excitability of pain-related neurons in the spinal cord and above the spinal cord or by enhanced synaptic transmission, thereby amplifying the central sensitization mechanism of pain signal transmission, leading to neuropathic pain for an extended period in patients with HZ and PHN [
1]. Moreover, dorsal root ganglia atrophy seen in PHN may arise due to direct infection of the spinal cord or transsynaptic degeneration [
4]. Studies have shown that this kind of neuropathic pain remodels brain structure and function [
5], and more studies have explored altered brain function in patients with HZ and PHN [
6,
7,
8]; however, research on the brain structure of patients with PHN is relatively limited. Of note, Chen et al. found decreased fractional anisotropy (FA) and axial diffusivity (AD) of the white matter in the insula, anterior central gyrus, cerebellum, occipital lobe, and other regions in PHN patients [
9]. In a diffusion kurtosis imaging (DKI) study, Zhang et al. found that DKI parameters of the left middle frontal gyrus and occipital lobe, bilateral insula and superior temporal gyrus, and right anterior cerebellar lobe were significantly reduced in PHN patients compared with those in healthy controls [
10]. In addition, a longitudinal study of PHN patients before and after treatment showed that the AD of the right thalamus in PHN patients decreased significantly after treatment, and the FA of the right superior temporal gyrus increased greatly [
11]. These results suggest that the white matter microstructure of PHN patients is influenced by long-term pain. However, whether similar changes occur in HZ patients and how these changes manifest require further study.
Diffusion tensor imaging (DTI), a noninvasive method of central nervous system imaging, can fully characterize diffusion anisotropy by estimating the diffusion characteristics of water molecules in brain tissues; this characterization indicates the connectivity of white matter tracts and provides more detailed information about the tissue microstructure. Various methods have been developed to analyze DTI data, including voxel-based analysis (VBA) and tract-based spatial statistics (TBSS), which both utilize voxel-based analysis as the registration algorithm cannot accurately pinpoint the actual position of the fiber tract in individual brains [
12,
13]. However, tractography, which reconstructs white matter tracts through algorithms that estimate the orientation distribution function, is widely considered the most accurate method for identifying white matter fiber fascicles in the living human brain [
14,
15]. Notably, automatic fiber quantification (AFQ) is a method based on tractography that can quickly and reliably identify 20 white matter tracts in the brain; this method can be used to further analyze the diffusion characteristics of anatomically equivalent positions along the fiber track. Since neuropathic pain can manifest at specific positions along the different fiber tracts [
16], AFQ provides an opportunity to investigate the local specificity of white matter fiber changes in HZ and PHN patients. Therefore, the purpose of this study was to explore alterations in the white matter microstructure in HZ and PHN patients by AFQ and to investigate whether these diffusion indices are significantly correlated with clinical data.
3. Results
3.1. Group Differences in Demographic and Clinical Characteristics
No significant differences in age (
p = 0.617), sex (
p = 0.867) were found among the three groups. VAS scores (
p = 0.674) and HAMD scores (
p = 0.084) were not significantly different between the HZ and PHN groups, while there were significant differences in the duration of disease (
p = 0.021), SCL-90-R scores (
p = 0.006) and HAMA scores (
p = 0.044) (
Table 1).
3.2. Group Differences in Mean Diffusion Properties
The numbers of subjects in whom the 20 fiber tracts were successfully reconstructed in three dimensions by AFQ among the three groups are displayed in column “n1:n2:n3”, which represents the numbers of subjects from the HZ, PHN, and HC groups, respectively (
Table 2,
Table 3,
Table 4 and
Table 5). The mean diffusion measurements (FA, MD, AD, and RD per tract) were compared among groups at the whole-brain level.
Regarding FA, the FA of the left CGC in the HZ and PHN groups was significantly lower than that in the HC group, the FA of the right CGC in the PHN group was significantly lower than that in the HC group, and the FA of the left SLF in the HZ group was higher than that in the HC group.
Regarding MD, the MD of the left CST, left CGC, and left SLF in the HZ and PHN groups were significantly higher than those in the HC group. The MD of the right CGC and left SLF in the PHN group were significantly higher than those in the HC group.
Regarding RD, the RD of the bilateral CGC in the HZ and PHN groups was significantly higher than that in the HC group, and the RD of the right ILF in the PHN group was significantly higher than that in the HC group.
Regarding AD, the AD of the left SLF and left AF in the HZ and PHN groups were significantly higher than that in the HC group, and the AD of the left CST in the PHN group was significantly higher than that in the HC group.
3.3. Group Differences in the Regional Distribution of Diffusion Properties
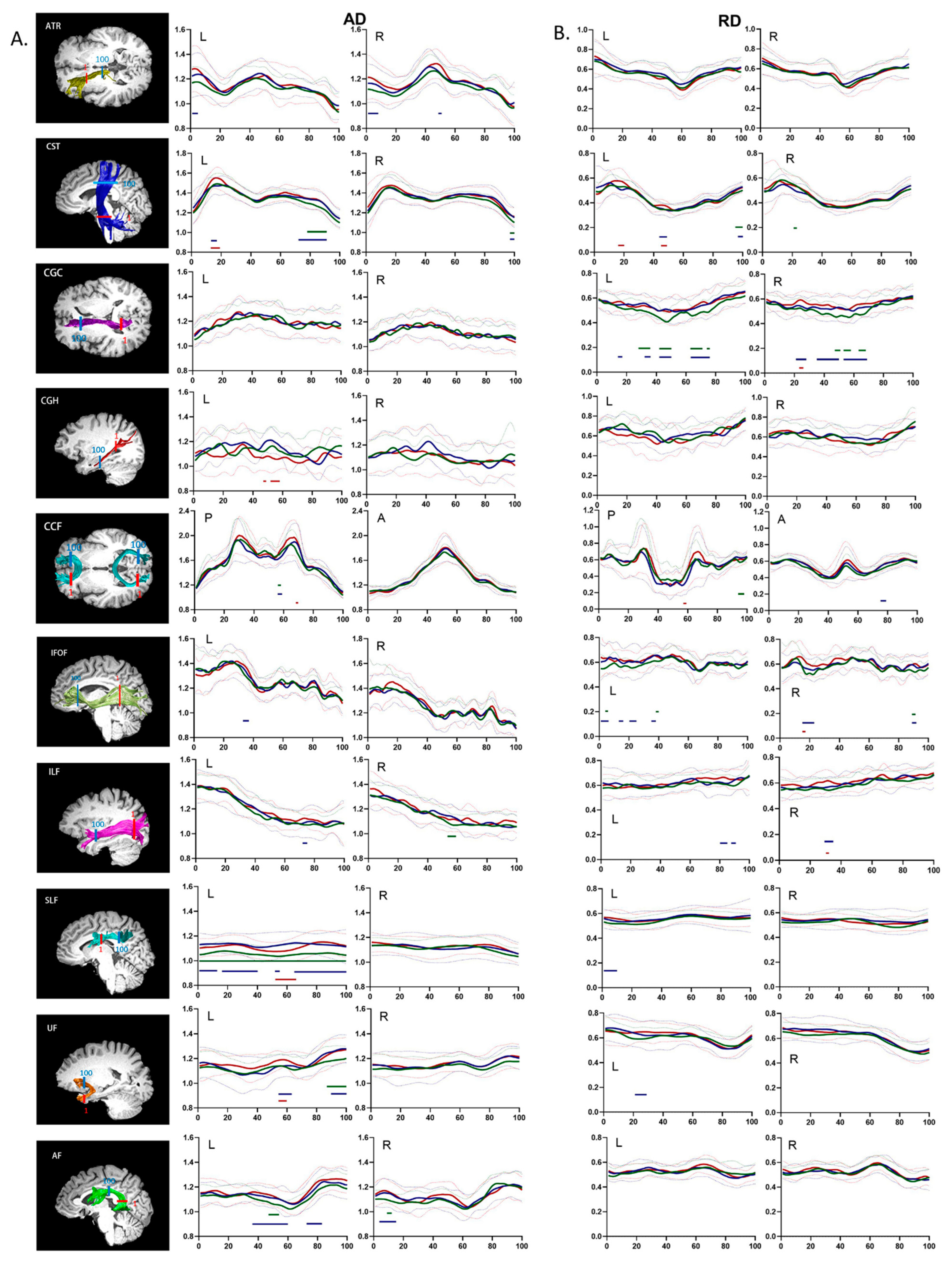
Unlike conventional fiber-tract imaging, AFQ analysis can quantify diffusion measurements along the white matter fiber tract to provide detailed diffusion characteristics. Dotted comparisons of the diffusion characteristics among the HZ, PHN, and HC groups are shown in
Figure 1 (FA and MD) and
Figure 2 (AD and RD). Multiple comparisons among all 100 points per fiber tract were corrected by FDR-adjusted
p values < 0.05 to reduce Type I errors.
The pointwise comparison of FA revealed that HZ patients showed extensive FA decreases in the inferior portion of the right CST, the anterior and medial portions of bilateral CGC, the posterior portion of the left IFOF, and the posterior portion of the left SLF compared to HCs. Compared with HCs, PHN patients had decreased FA in the middle portion of the left CST, the anterior and middle portions of the left CGC, the anterior, middle, and posterior portions of the right CGC, the posterior portion of the left IFOF, the anterior and posterior portions of the right IFOF, and the middle portion of the right ILF. Compared with the HZ group, PHN patients had decreased FA in the posterior portion of the bilateral ATR, the medial portion of the left CST, the posterior portion of the right CGC, the anterior portion of the left SLF, and the posterior portion of the right SLF.
The pointwise comparison of MD showed that compared to HCs, HZ patients had substantial increases in MD in the superior portion of the bilateral CST; the anterior, middle, and posterior portions of the left CGC; the middle portion of the right CGC; the left portion of the anterior and posterior CCF; the anterior portion of the right IFOF; the anterior, middle, and posterior portions of the left SLF; and the middle of the portion of the left AF. Additionally, compared to HCs, PHN patients had increased MD in the following regions: the superior, middle, and inferior portions of the left CST; the superior portion of the right CST; the anterior, middle, and posterior portions of the bilateral CGC; the left portion of the anterior CCF; the middle portion of the left IFOF; the anterior and posterior portions of the right IFOF; the anterior portion of the left ILF; the middle portion of the right ILF; the anterior, middle, and posterior portions of left SLF; the posterior portion of the right SLF; the frontal and temporal lobe portions of the left UF; the middle and frontal lobe portions of the left AF; and the temporal portion of the right AF. Compared to the HZ group, PHN patients showed increased MD in the middle and inferior portions of the left CST, the posterior portion of the right CGC, the left portion of the posterior CCF, the posterior portion of the right IFOF, the anterior portion of the left ILF, and the frontal lobe portion of the left AF.
The pointwise comparison of AD demonstrated that compared to HCs, HZ patients showed substantial increases in AD in the superior portion of the bilateral CST, the middle portion of the right ILF, the entire left SLF, the frontal lobe portion of the left UF, the middle portion of the left AF, and the temporal lobe portion of the right AF. Compared with HCs, PHN patients showed substantially increased AD in the anterior portion of the left ATR, the anterior and middle portions of the right ATR, the superior and inferior portions of the left CST, the superior portion of the right CST, the middle portion of the left IFOF, the anterior portion of the left ILF, the anterior, middle, and posterior portions of the left SLF, the middle and frontal lobe portions of the left UF, the middle and frontal lobe portions of the left AF, and the temporal lobe portion of the right AF. Compared to the HZ group, PHN patients showed substantially increased AD in the inferior portion of the left CST, the middle portion of the left CGH, the middle portion of the left SLF, and the middle portion of the left UF.
Pointwise comparison of RD revealed that compared to HCs, HZ patients showed substantially increased RD in the superior portion of the left CST; the inferior portion of the right CST; the anterior, middle, and posterior portions of the left CGC; anterior and middle portions of right CGC; the left portion of the posterior CCF; the middle and posterior portions of the left IFOF; and the anterior portion of the right IFOF. Compared with HCs, PHN patients showed substantially increased RD in the anterior and middle portions of the left CST; the anterior, middle, and posterior portions of the bilateral CGC; the left portion of the anterior CCF; the middle and posterior portions of the left IFOF; the anterior and posterior portions of the right IFOF; the anterior portion of the left ILF; the middle and posterior portions of the right ILF; the anterior portion of the left SLF; and the temporal lobe portion of the left UF. Compared to the HZ group, PHN patients showed substantially increased RD in the middle and inferior portions of the left CST, the posterior portion of the right CGC, the left portion of the posterior CCF, the posterior portion of the right IFOF, and the posterior portion of the right ILF.
3.4. Correlation Analysis
In the analysis of the correlations between clinical characteristics and average diffusion measurements (FA, MD, AD, and RD) of the significantly altered fiber tracts in the PHN and HZ groups, it was found that the FA of the left cingulum cingulate gyrus in the PHN group was negatively correlated with HAMA scores, HAMD scores, and the duration of disease; the RD of the left cingulum cingulate was positively correlated with HAMA scores and the duration of disease. There were no significant correlations among the variables in the HZ group (
Figure 3).
4. Discussion
AFQ can more accurately locate tract segments that differ among groups by tracking 20 fiber tracts in the whole brain and segmenting them at equal intervals of 100 points, thereby enabling assessment of the integrity of white matter fiber tracts in participants in the HC, HZ, and PHN groups. This method revealed a wide range of changes in the diffusion properties of fiber tracts among the three groups. Specifically, the differences between the HZ and PHN groups mainly manifested as decreased FA of the bilateral SLF, increased MD of the left CST, increased AD of the left CGH and left SLF and increased RD of the left CST. Compared with HCs, the FA of the bilateral CGC and left SLF in the HZ group were decreased to different extents, while increases in the MD, RD, and AD were mainly observed in the left CST, left CGC, and left SLF. The abovementioned properties were also increased in some segments of the right CGC, left IFOF, and left UF. In PHN patients, more extensive changes in the diffusion properties of the fiber tracts were observed. A decrease in the FA was observed in the bilateral CGC and left IFOF. Increases in the MD, RD, and AD were observed in many fiber tracts, such as the left CGC, left SLF, left UF, and left AF. As HZ progressed to PHN, the abnormalities in some white matter fiber tracts expanded. For example, the left CST showed increased MD in the superior portion in HZ patients. However, in PHN patients, increased MD was also observed in the middle and inferior portions of the left CST. The areas with altered tract diffusion properties (FA, MD, AD, and RD) of the right CGC expanded (
Table 6 and
Table 7). This expansion suggests that the persistent pain experienced by PHN patients might be related to the aggravation of injury to these specific fiber tracts.
White matter, which comprises of axons coated with myelin sheaths, allows conduction of signals between neurons and coordinates signals among brain regions during normal operations [
27]. The gray matter (comprised of neuron bodies) is thus the basis of white matter fibers. Recent studies have reported changes in cortical thickness and gray matter volume in patients with HZ and PHN compared with normal control groups [
8,
28]. The peripheral and central neural networks related to nociception processing pathways show extensive plasticity in chronic neuropathic pain. This plasticity manifests as changes in individual molecules, synapses, cellular function, at the structural level [
29]. Reconstruction of the white matter fiber tract by DTI, which examines the movement of water molecules through and along the fiber tract, can characterize the fiber-tract diffusion properties, thus describing abnormalities in the white matter microstructure, such as the morphology and density of axons, the degree of myelin coverage, and changes in water content [
30,
31]. For example, according to Beaulieu et al., the FA plays an important role in assessing axonal membrane structures, and a decrease in the FA may mean that the fiber tracts become looser [
32]. Increases in the MD may indicate the aggravation of demyelination or fiber tract edema. The AD measures the diffusion rate parallel to the fiber tract, which is related to axonal integrity [
33]. Furthermore, the RD can be used to measure the diffusion rate perpendicular to the fiber tract. Relatedly, Brusini et al. found that the RD can be used to indicate changes in myelin sheath content [
34]. Our findings reveal widespread degradation of white matter tracts in patients with HZ and PHN, and the inconsistent results observed in the measured diffusion indicators may indicate differences in the severity of disruption between these regions. Among the significantly changed fiber tracts (the CST_L, CGC, SLF_L, IFOF_L, and AF_L), there were widespread reductions in the FA and widespread elevations in the MD, AD, and RD. Moreover, against the background of FA and MD alterations, the changes in the AD of some fiber tracts were more obvious than those in the RD. For example, the MD of the middle portion of the left SLF exhibited significant alterations in the HZ and PHN subjects, and HZ patients appeared to have changes in the overall fiber tract (AD of points 1–100) and most of the segments changed (AD of points 1–13, 16–40, 52–55, and 65–100) in PHN patients, while the RD of HZ patients (no points) and PHN patients (points 1–10) did not obviously change. A similar phenomenon was observed in the left UF of the HZ group and the left UF and bilateral AF of the PHN group, suggesting that the degradation of these fiber tracts may be mainly caused by axonal degeneration. However, the bilateral CGC and left IFOF of the HZ group and the bilateral CGC and bilateral IFOF of the PHN group exhibited more pronounced RD alterations, suggesting greater degradation of the myelin sheath in these fiber tracts. Additionally, the regions of axon and myelin-sheath degradation were further increased in patients with PHN compared with those with HZ, again supporting the above view that the damage to the white matter microstructure in PHN patients is aggravated compared with that in patients with HZ. Previously, a DTI study of PHN revealed changes in the FA and AD [
9] and it was suggested that the changes in DTI parameters in PHN patients were due to the degradation of axons rather than axon demyelination or other reasons. Despite the consistent conclusions of our study, we believe that the main reason for fiber damage may vary among tracts and that the degradation of the myelin sheath also has a significant effect. Some studies of animal models of PHN have shown that when the HZ virus reactivates in the ganglia, it not only leads to the degradation of the axons responsible for pain transmission but also causes damage to satellite glial cells and Schwann cells, resulting in myelin sheath loss and other phenomena [
35,
36]. Additionally, there is a unique T-junction in dorsal root ganglia, which can enhance the signal from the periphery toward the central nervous system after injury to the peripheral axon, causing ectopic discharges and axonal damage in the central nervous system [
37]. In addition, long-term pain can affect the function and chemical profile of neurons by activating, regulating, and modifying primary sensory neurons and dorsal horn neurons, thereby affecting neuroplasticity and widely affecting brain structure [
38].
Studies have shown that the cingulate fasciculus connects the frontal lobe, parietal lobe, and temporal lobes [
14]; this tract, along with the prefrontal lobe cortex, amygdala, anterior cingulate cortex, hippocampus, and insula, forms the limbic system. The cingulate fasciculus, the core link among these regions, plays an important role in executive function, emotions, pain perception, and so on [
39]; in addition, the cingulum hippocampus plays an important role in episodic memory [
40]. Our study revealed extensive DTI index abnormalities were found in the bilateral CGC, the CGH was more difficult to track and showed few changes, but comparison of the HZ and PHN groups revealed increased AD in some segments of the left CGH. This finding was consistent with the results of previous TBSS and DKI studies [
9,
10], suggesting that the abnormal processing of emotion and pain in patients with HZ and PHN might be related to changes in the microstructure of the bilateral CGC. In addition, the central anterior gyrus of the motor cortex and the somatomotor cortex, which are dominated by the CST, play an important role in the perception and regulation of pain [
41]. A recent study found that in PHN patients, the thickness of the motor cortex decreases, and the primary somatosensory cortex expands accordingly [
28]. Another study combining DTI and resting-state MRI revealed microstructural changes in brain regions, such as the anterior central gyrus, in patients with PHN [
42]. In addition, microstructural changes have been reported in different segments of liaison fibers, such as the SLF, ILF, UF, and IFOF. The temporal-parietal-occipital region, a complex brain region formed by these fiber tracts, participates in or coordinates many important brain functional activities, such as language, vision, working memory, and recognition [
43]. Many previous studies revealed changes in gray matter volume and cortical thickness in the temporal, parietal, and occipital lobes of the brain in PHN patients [
8,
28,
44]. This finding indicates that HZ and PHN patients demonstrate extensive changes in brain structure, which may be related to the inflammatory response and prolonged activation of glial cells, which contribute to the modification of the central nervous system [
38,
45].
In our study, the FA of the left CGC in PHN subjects was negatively correlated with HAMA scores, HAMD scores, and the duration of disease. Moreover, the RD was positively correlated with HAMA scores and the duration of disease, suggesting that the integrity of the white matter might be related to the generation of negative emotions and worsened with the continuation of pain. Many studies have shown that the cingulate fasciculus is key for emotional processing and critically contributes to patterned activities associated with pain perception [
46,
47,
48]. This phenomenon was more obvious in the PHN group because these patients endured long-term pain. Studies have shown that in chronic pain, such as that experienced by trigeminal neuralgia patients, negative emotions and anxiety disorders are associated with cingulate fasciculus activity because the cingulate fasciculus is one of the components of the emotional circuit, and it plays an important role in emotion regulation [
49]. Therefore, it is reasonable to speculate that negative emotions, anxiety, and depression in patients with HZ and PHN may further aggravate the damage to the white matter fiber tract.
However, this study has some limitations. First, as HCs were not assessed with HAMA and HAMD, there is insufficient evidence on whether HCs are completely free of anxiety and depression during MRI scanning, and the correlation between white matter integrity changes and anxiety and depression is not clear in HCs. We will collect the scale of each subject to improve our study in future studies. Second, since some fibers, such as the bilateral cingulate hippocampus, are adjacent to gray matter, the threshold setting for fiber tracking and the FA in this portion is low and may lead to partial fiber tracking failure. Third, although we performed a multipoint analysis with age as a covariate and correction for multiple comparisons, the effect of age on white matter hyperintensities should be taken into account. Fourth, the design of this study was a cross-sectional design. Therefore, we could not determine the causal dynamic mechanisms by which white matter alterations in HZ patients developed into those in PHN patients, and more longitudinal studies are required to further explore the underlying mechanisms.