Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Management of Low-Grade Gliomas and Radiation Necrosis: A Single-Institution Case Series
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Lesion Volume Estimation
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Indications for LITT
3.3. Molecular Markers
3.4. Lesion Volume and Treatment Parameters
3.5. Clinical Outcomes
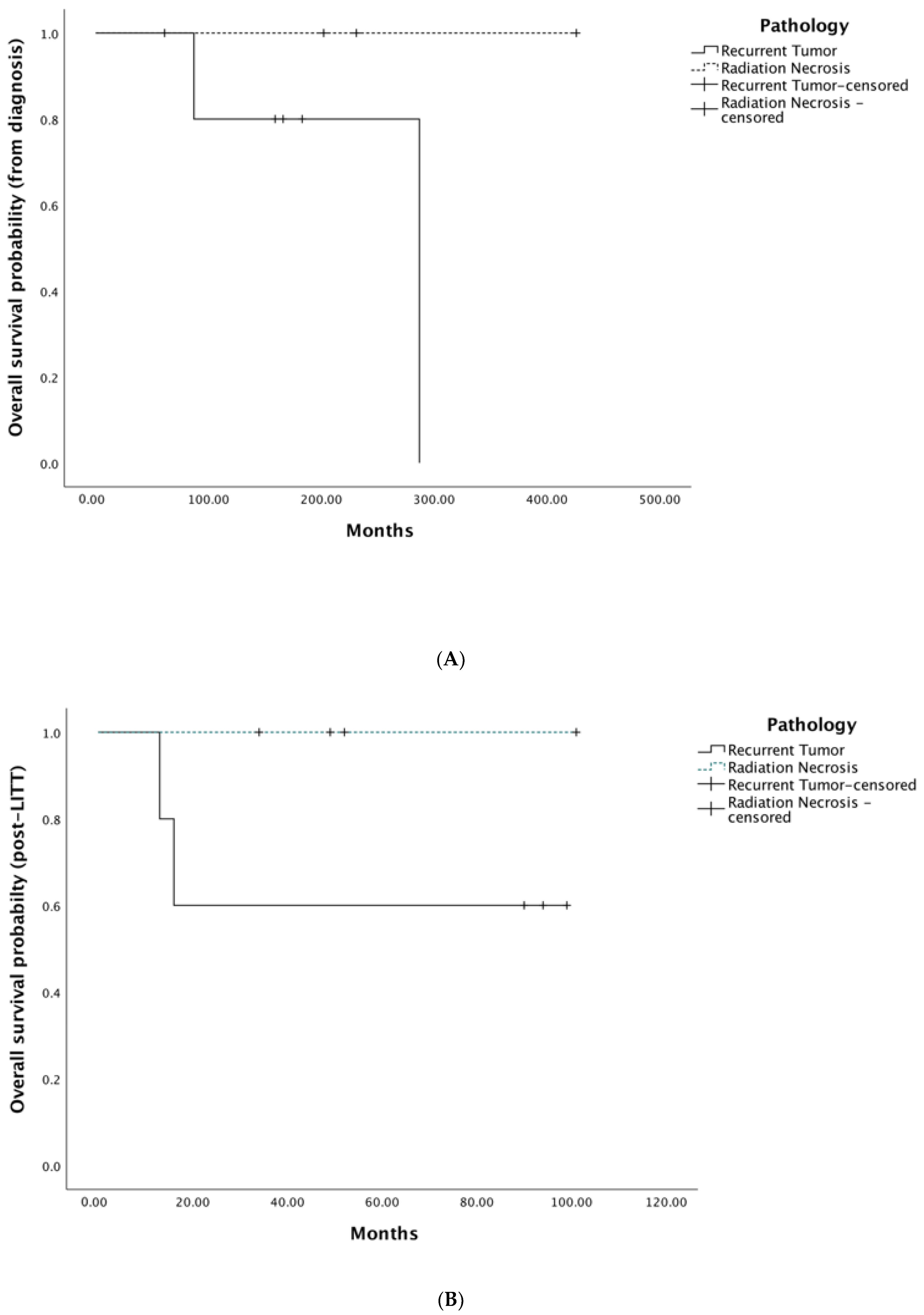
3.6. Survival Outcomes
4. Discussion
4.1. Safety and Efficacy of LITT
4.2. LITT Treatment Planning
4.3. LITT Survival Outcomes
4.4. LITT Survival Outcomes in Context
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Morshed, R.A.; Young, J.S.; Hervey-Jumper, S.L.; Berger, M.S. The management of low-grade gliomas in adults. J. Neurosurg. Sci. 2019, 63, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2021, 102, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Youssef, G.; Miller, J.J. Lower Grade Gliomas. Curr. Neurol. Neurosci. Rep. 2020, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; de Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivan, M.E.; Mohammadi, A.M.; De Deugd, N.; Reyes, J.; Rodriguez, G.; Shah, A.; Barnett, G.H.; Komotar, R.J. Laser Ablation of Newly Diagnosed Malignant Gliomas: A Meta-Analysis. Neurosurgery 2016, 79 (Suppl. S1), S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Jethwa, P.R.; Barrese, J.C.; Gowda, A.; Shetty, A.; Danish, S.F. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: Initial experience. Neurosurgery 2012, 71, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Hargreaves, E.L.; Khan, A.J.; Haffty, B.G.; Danish, S.F. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 2014, 74, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Wu, C.; Tracy, J.; Lorenzo, M.; Evans, J.; Nei, M.; Skidmore, C.; Mintzer, S.; Sharan, A.D.; Sperling, M.R. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016, 57, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Guichard, J.P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; George, B. Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg. Med. 2011, 43, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.E.; Ahluwalia, M.S.; Valerio-Pascua, J.; Manjila, S.; Torchia, M.G.; Jones, S.E.; Sunshine, J.L.; Phillips, M.; Griswold, M.A.; Clampitt, M.; et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J. Neurosurg. 2013, 118, 1202–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrows, A.M.; Marsh, W.R.; Worrell, G.; Woodrum, D.A.; Pollock, B.E.; Gorny, K.R.; Felmlee, J.P.; Watson, R.E.; Kaufmann, T.J.; Goerss, S.; et al. Magnetic resonance imaging-guided laser interstitial thermal therapy for previously treated hypothalamic hamartomas. Neurosurg. Focus 2016, 41, E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, R.; Hebb, A.; Barber, J.; Rostomily, R.; Silbergeld, D. Outcomes in Reoperated Low-Grade Gliomas. Neurosurgery 2015, 77, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, S.E.; Ahmadi, R.; Schulz-Ertner, D.; Thilmann, C.; Debus, J. Recurrent low-grade gliomas: The role of fractionated stereotactic re-irradiation. J. Neuro-Oncol. 2005, 71, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, M.; Engenhart-Cabillic, R.; Debus, J.; Hess, T.; Schad, L.R.; Wannenmacher, M.F. Low-grade astrocytoma: Treatment with conventionally fractionated stereotactic radiation therapy. Radiology 1996, 201, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Plathow, C.; Schulz-Ertner, D.; Thilman, C.; Zuna, I.; Lichy, M.; Weber, M.-A.; Schlemmer, H.-P.; Wannenmacher, M.; Debus, J. Fractionated stereotactic radiotherapy in low-grade astrocytomas: Long-term outcome and prognostic factors. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Barresi, V.; Castellano, A.; Tabouret, E.; Pasqualetti, F.; Salvalaggio, A.; Cerretti, G.; Caccese, M.; Padovan, M.; Zagonel, V.; et al. Clinical Management of Diffuse Low-Grade Gliomas. Cancers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Paulino, A.C.; Mazloom, A.; Terashima, K.; Su, J.; Adesina, A.M.; Okcu, M.F.; Teh, B.S.; Chintagumpala, M. Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 2013, 119, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.A.; Lumenta, C.B. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: Clinical expierence. Minim. Invasive Neurosurg. 2002, 45, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, K.C.; Khanna, P.C.; Elster, J.D.; Paul, M.R.; Levy, M.L.; Crawford, J.R.; Gonda, D.D. Clinical and Neuroimaging Features of Magnetic Resonance-Guided Stereotactic Laser Ablation for Newly Diagnosed and Recurrent Pediatric Brain Tumors: A Single Institutional Series. World Neurosurg. 2021, 150, e378–e387. [Google Scholar] [CrossRef]
- Ahluwalia, M.; Barnett, G.H.; Deng, D.; Tatter, S.B.; Laxton, A.W.; Mohammadi, A.M.; Leuthardt, E.; Chamoun, R.; Judy, K.; Asher, A.; et al. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J. Neurosurg. 2018, 130, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.S.; Deng, D.; Vera, A.; Chiang, V.L. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J. Neurooncol. 2019, 142, 309–317. [Google Scholar] [CrossRef]
- Bastos, D.C.A.; Weinberg, J.; Kumar, V.A.; Fuentes, D.T.; Stafford, J.; Li, J.; Rao, G.; Prabhu, S.S. Laser Interstitial Thermal Therapy in the treatment of brain metastases and radiation necrosis. Cancer Lett. 2020, 489, 9–18. [Google Scholar] [CrossRef]
- Sharma, M.; Balasubramanian, S.; Silva, D.; Barnett, G.H.; Mohammadi, A.M. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert Rev. Neurother. 2016, 16, 223–232. [Google Scholar] [CrossRef]
- Chen, C.; Lee, I.; Tatsui, C.; Elder, T.; Sloan, A.E. Laser interstitial thermotherapy (LITT) for the treatment of tumors of the brain and spine: A brief review. J. Neurooncol. 2021, 151, 429–442. [Google Scholar] [CrossRef]
- Agha, R.A.; Sohrabi, C.; Mathew, G.; Franchi, T.; Kerwan, A.; O’Neill, N. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) Guidelines. Int. J. Surg. 2020, 84, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ascher, P.W.; Justich, E.; Schrottner, O. A new surgical but less invasive treatment of central brain tumours Preliminary report. Acta Neurochir. Suppl. 1991, 52, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Merienne, L.; Fallet-Bianco, C.; Beuvon, F.; Devaux, B.; Leriche, B.; Cioloca, C. Stereotaxic laser interstitial thermotherapy. A new alternative in the therapeutic management of some brain tumors. Neurochirurgie 1992, 38, 238–244. [Google Scholar] [PubMed]
- Kahn, T.; Bettag, M.; Ulrich, F.; Schwarzmaier, H.J.; Schober, R.; Furst, G.; Modder, U. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J. Comput. Assist. Tomogr. 1994, 18, 519–532. [Google Scholar] [CrossRef]
- Kahn, T.; Harth, T.; Bettag, M.; Ulrich, B.S.F.; Schwarzmaier, H.J.; Modder, U. Preliminary experience with the application of Gadolinium-DTPA before MR imaging-guided laser-induced interstitial thermotherapy of brain tumors. J. Magn. Reson. Imaging 1997, 7, 226–229. [Google Scholar] [CrossRef]
- Schwabe, B.; Kahn, T.; Harth, T.; Ulrich, F.; Schwarzmaier, H.J. Laser-induced thermal lesions in the human brain: Short- and long-term appearance on MRI. J. Comput. Assist. Tomogr. 1997, 21, 818–825. [Google Scholar] [CrossRef]
- Von Tempelhoff, W.; Toktamis, S.; Schwarzmeier, H.-J.; Eickmeyer, F.; Niehoff, H.; Ulrich, F. LITT (Laser Induced Interstitial Thermotherapy) of Benign and Malignant Gliomas in the OPEN MR (0.5 Tesla, GE Signa SP). Med. Laser Appl. 2002, 17, 170–178. [Google Scholar] [CrossRef]
- Patel, P.; Patel, N.V.; Danish, S.F. Intracranial MR-guided laser-induced thermal therapy: Single-center experience with the Visualase thermal therapy system. J. Neurosurg. 2016, 125, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.A.; Salehi, A.; Limbrick, D.D., Jr.; Smyth, M.D. Applications of a robotic stereotactic arm for pediatric epilepsy and neurooncology surgery. J. Neurosurg. Pediatr. 2017, 20, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Karsy, M.; Patel, D.M.; Bollo, R.J. Trapped ventricle after laser ablation of a subependymal giant cell astrocytoma complicated by intraventricular gadolinium extravasation: Case report. J. Neurosurg. Pediatr. 2018, 21, 523–527. [Google Scholar] [CrossRef]
- Buckley, R.T.; Wang, A.C.; Miller, J.W.; Novotny, E.J.; Ojemann, J.G. Stereotactic laser ablation for hypothalamic and deep intraventricular lesions. Neurosurg. Focus 2016, 41, E10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadey, D.Y.; Kamath, A.A.; Smyth, M.D.; Chicoine, M.R.; Leuthardt, E.C.; Kim, A.H. Utilizing personalized stereotactic frames for laser interstitial thermal ablation of posterior fossa and mesiotemporal brain lesions: A single-institution series. Neurosurg. Focus 2016, 41, E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennert, R.C.; Khan, U.; Tatter, S.B.; Field, M.; Toyota, B.; Fecci, P.E.; Judy, K.; Mohammadi, A.M.; Landazuri, P.; Sloan, A.; et al. Patterns of Clinical Use of Stereotactic Laser Ablation: Analysis of a Multicenter Prospective Registry. World Neurosurg. 2018, 116, e566–e570. [Google Scholar] [CrossRef] [PubMed]
- Dadey, D.Y.; Kamath, A.A.; Leuthardt, E.C.; Smyth, M.D. Laser interstitial thermal therapy for subependymal giant cell astrocytoma: Technical case report. Neurosurg. Focus 2016, 41, E9. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.V.; Jethwa, P.R.; Barrese, J.C.; Hargreaves, E.L.; Danish, S.F. Volumetric trends associated with MRI-guided laser-induced thermal therapy (LITT) for intracranial tumors. Lasers Surg. Med. 2013, 45, 362–369. [Google Scholar] [CrossRef]
- Hafez, D.M.; Liekweg, C.; Leuthardt, E.C. Staged Laser Interstitial Thermal Therapy (LITT) Treatments to Left Insular Low-Grade Glioma. Neurosurgery 2020, 86, E337–E342. [Google Scholar] [CrossRef] [Green Version]
- Pruitt, R.; Gamble, A.; Black, K.; Schulder, M.; Mehta, A.D. Complication avoidance in laser interstitial thermal therapy: Lessons learned. J. Neurosurg. 2017, 126, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, K.P.; Palejwala, A.H.; Milton, C.K.; Lu, V.M.; Glenn, C.A.; Sughrue, M.E.; Conner, A.K. Laser Interstitial Thermal Therapy Case Series: Choosing the Correct Number of Fibers Depending on Lesion Size. Oper. Neurosurg. 2020, 20, 18–23. [Google Scholar] [CrossRef]
- Brandmeir, N.J.; McInerney, J.; Zacharia, B.E. The use of custom 3D printed stereotactic frames for laser interstitial thermal ablation: Technical note. Neurosurg. Focus 2016, 41, E3. [Google Scholar] [CrossRef] [Green Version]
- Del Bene, M.; Carone, G.; Porto, E.; Barbotti, A.; Messina, G.; Tringali, G.; Rossi, D.; Lanteri, P.; Togni, R.; Demichelis, G.; et al. Neurophysiology-Guided Laser Interstitial Thermal Therapy: A Synergistic Approach for Motor Function Preservation. Technical Note. World Neurosurg. 2022, 168, 165–172. [Google Scholar] [CrossRef]
- Luedke, M.W.; Pietak, M.R.; Serafini, S.; Haglund, M.M.; Sinha, S.R. Intraoperative ECoG During MRI-Guided Laser-Interstitial Thermal Therapy for Intractable Epilepsy. J. Clin. Neurophysiol. 2016, 33, e28–e30. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Newcomb, N.; Hofer, S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med. Inform. Decis. Mak. 2013, 13, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawan, P.Y.; Islam, A.A.; July, J.; Patellongi, I.; Nasrum, M.; Aninditha, T. Karnofsky Performance Scale and Neurological Assessment of Neuro-Oncology Scale as Early Predictor in Glioma. Asian Pac. J. Cancer Prev. 2020, 21, 3387–3392. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Hawasli, A.H.; Rodriguez, A.; Schroeder, J.L.; Laxton, A.W.; Elson, P.; Tatter, S.B.; Barnett, G.H.; Leuthardt, E.C. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: A multicenter study. Cancer Med. 2014, 3, 971–979. [Google Scholar] [CrossRef]
- Tovar-Spinoza, Z.; Choi, H. Magnetic resonance-guided laser interstitial thermal therapy: Report of a series of pediatric brain tumors. J. Neurosurg. Pediatr. 2016, 17, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Voynov, G.; Kaufman, S.; Hong, T.; Pinkerton, A.; Simon, R.; Dowsett, R. Treatment of Recurrent Malignant Gliomas with Stereotactic Intensity Modulated Radiation Therapy. Am. J. Clin. Oncol. 2002, 25, 606–611. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for pilocytic astrocytomas part 1: Outcomes in adult patients. J. Neurooncol. 2009, 95, 211–218. [Google Scholar] [CrossRef]
- Kano, H.; Niranjan, A.; Kondziolka, D.; Flickinger, J.C.; Pollack, I.F.; Jakacki, R.I.; Lunsford, L.D. Stereotactic radiosurgery for pilocytic astrocytomas part 2: Outcomes in pediatric patients. J. Neurooncol. 2009, 95, 219–229. [Google Scholar] [CrossRef]
- Oberheim Bush, N.A.; Chang, S. Treatment Strategies for Low-Grade Glioma in Adults. J. Oncol. Pract. 2016, 12, 1235–1241. [Google Scholar] [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgard, G.; Solheim, O. Comparison of a strategy favoring early surgical resection vs. a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Role of surgical resection in low- and high-grade gliomas. Curr. Treat. Options Neurol. 2014, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Bansal, A.G.; Young, E.B.; Batchala, P.P.; Patrie, J.T.; Lopes, M.B.; Jain, R.; Fadul, C.E.; Schiff, D. Extent of Surgical Resection in Lower-Grade Gliomas: Differential Impact Based on Molecular Subtype. Am. J. Neuroradiol. 2019, 40, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Mei, Q.; Li, H.; Ke, C.; Yu, J.; Chen, J. A survival analysis of surgically treated incidental low-grade glioma patients. Sci. Rep. 2021, 11, 8522. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Ng, S.; Young, J.S.; Tomasino, B.; Polano, M.; Ben-Israel, D.; Kelly, J.J.P.; Skrap, M.; Duffau, H.; Berger, M.S. The benefit of early surgery on overall survival in incidental low grade glioma patients: A multicenter study. Neuro-Oncol. 2021, 24, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Shaw, E.; Arusell, R.; Scheithauer, B.; O’Fallon, J.; O’Neill, B.; Dinapoli, R.; Nelson, D.; Earle, J.; Jones, C.; Cascino, T.; et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2002, 20, 2267–2276. [Google Scholar] [CrossRef]
- Karim, A.B.; Maat, B.; Hatlevoll, R.; Menten, J.; Rutten, E.H.; Thomas, D.G.; Mascarenhas, F.; Horiot, J.C.; Parvinen, L.M.; van Reijn, M.; et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 549–556. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Afra, D.; de Witte, O.; Hassel, M.B.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Shaw, E.G.; Wang, M.; Coons, S.W.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; Mehta, M.P. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: Initial results of RTOG 9802. J. Clin. Oncol. 2012, 30, 3065–3070. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Vidiri, A.; Galie, E.; Carosi, M.; Telera, S.; Cianciulli, A.M.; Canalini, P.; Giannarelli, D.; Jandolo, B.; Carapella, C.M. Temozolomide chemotherapy for progressive low-grade glioma: Clinical benefits and radiological response. Ann. Oncol. 2003, 14, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Cartalat-Carel, S.; Meyronet, D.; Ricard, D.; Jouvet, A.; Pallud, J.; Mokhtari, K.; Guyotat, J.; Jouanneau, E.; Sunyach, M.P.; et al. Prolonged response without prolonged chemotherapy: A lesson from PCV chemotherapy in low-grade gliomas. Neuro-Oncol. 2010, 12, 1078–1082. [Google Scholar] [CrossRef] [PubMed]

| No. | Age | Sex | Original Diagnosis | Number of Lesions | Side and Location | Number of Previous Resections | Previous SRS | Previous iMRT | Previous Chemotherapy | Intraoperative Histology | IDH1 Status | Complications | Evidence of Radiographic Progression after LITT | PFS (Months) | Clinical Status at Time of Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | M | Ganglioglioma | 2 | Bilateral frontal/peri-ventricular | 3 | Yes | No | No | RN | n/a | None | No | 101 | alive |
| 2 | 51 | M | Oligodendroglioma, grade II | 2 | Bilateral frontal/periventricular | 1 | No | Yes | Yes | Recurrent oligodendroglioma, grade II | n/a | None | No | 99 | alive |
| 3 | 50 | M | n/a | 1 | Left thalamic | 0 | No | No | No | Oligodendroglioma, grade II | Mutation | Post-operative, permanent; thalamic pain syndrome | Yes | 76 | alive |
| 4 | 32 | F | n/a | 1 | Left thalamic | 0 | No | No | No | Astrocytoma, grade II | Mutation | Post-operative, permanent; CN III/IV palsies | No | 90 | alive |
| 5 | 61 | M | Oligoastrocytoma, grade II | 2 | Left frontal/peri-ventricular | 3 | Yes | Yes | Yes | RN | Mutation | None | No | 52 | alive |
| 6 | 65 | M | Astrocytoma, grade II | 1 | Left frontal | 2 | Yes | Yes | Yes | RN | Mutation | None | No | 49 | alive |
| 7 | 24 | M | Astrocytoma, grade II | 2 | Left frontal/parieto-occipital | 3 | No | Yes | Yes | Recurrent astrocytoma, grade II | Wildtype | None | Yes | 9 | deceased |
| 8 | 55 | M | Oligodendroglioma, grade II | 2 | Right frontal/temporal | 1 | Yes | Yes | Yes | Anaplastic oligodendroglioma, grade III | Wildtype | None | Yes | 2 | deceased |
| 9 | 72 | F | Oligodendroglioma, grade II | 1 | Right parieto-occipital | 1 | No | Yes | Yes | RN | Mutation | Post-operative, transient; acute subdural hematoma | No | 34 | alive |
| Characteristic | Patients (n = 9) or Lesions (n = 14) |
|---|---|
| Age, mean (SD), years | 50 (16) |
| Sex | |
| Male | 7 (78) |
| Female | 2 (22) |
| Cerebral location, lesions | |
| Frontal | 6 (43) |
| Periventricular | 3 (21) |
| Parieto-occipital | 2 (14) |
| Sub-cortical (thalamic) | 2 (14) |
| Temporal | 1 (7) |
| Cerebral hemisphere, lesions | |
| Left | 7 (50) |
| Right | 3 (21) |
| Bilateral | 4 (29) |
| Number of previous resections (SD) | 1.6 (1.2) |
| Time from original diagnosis to LITT, mean (SD), years | 11.6 (8.5) |
| Treatment of recurrent lesions | 13.2 (9.0) |
| Treatment of primary lesions | 5.8 (0.2) |
| History of radiation therapy | |
| IMRT | 6 (67) |
| SRS | 4 (44) |
| History of chemotherapy | 6 (67) |
| History of resection | 7 (78) |
| ≥3 previous treatments | 7 (78) |
| Variable | Patients (n = 4) |
|---|---|
| Enhancing T1-weighted lesion volume, cm3, mean (SD) | 4.1 (6.5) |
| Fluid-attenuated inversion recovery lesion volume, cm3, mean (SD) | 26.7 (27.9) |
| Total energy delivered, kJ, mean (SD) | 7.5 (9.5) |
| Number of pulses, mean (SD) | 306 (400) |
| Laser on time, min, mean (SD) | 9.8 (13.5) |
| Variable | Oligodendroglioma, WHO Grade II (n = 2) | Astrocytoma, WHO Grade II (n = 2) | Anaplastic Oligodendroglioma, WHO Grade III (n = 1) | Radiation Necrosis (n = 4) | Pooled (n = 9) |
|---|---|---|---|---|---|
| Progression-free survival from LITT, mo, median (IQR) | 88 (12) | 50 (41) | 2 (n/a) | 51 (19) | 52 (56) |
| Overall survival from LITT, mo, median (IQR) | 97 (3) | 110 (16) | 16 (n/a) | 501 (21) | 52 (60) |
| Overall survival from diagnosis, mo, median (IQR) | 175 (9) | 123 (36) | 287 (n/a) | 217 (120) | 183 (72) |
| Karnofsky Performance Score, mean (SD) | |||||
| Preoperative | 90 (0) | 85 (7) | 70 (n/a) | 75 (10) | 80 (15) |
| First clinical follow-up | 80 (14) | 75 (7) | 70 (n/a) | 75 (10) | 76 (9) |
| Last clinical follow-up | 80 (14) | 65 (21) | 50 (n/a) | 80 (8) | 73 (15) |
| Time to last clinical follow-up, mo, mean (SD) | 38 (36) | 38 (25) | 15 (n/a) | 37 (26) | 35 (23) |
| Clinical status at end of study period | |||||
| Deceased | 0 (0) | 1 (50) | 1 (100) | 0 (0) | 2 (22) |
| Alive | 2 (100) | 1 (50) | 0 (0) | 4 (100) | 7 (78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherschinski, L.; Jubran, J.H.; Shaftel, K.A.; Furey, C.G.; Farhadi, D.S.; Benner, D.; Hendricks, B.K.; Smith, K.A. Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Management of Low-Grade Gliomas and Radiation Necrosis: A Single-Institution Case Series. Brain Sci. 2022, 12, 1627. https://doi.org/10.3390/brainsci12121627
Scherschinski L, Jubran JH, Shaftel KA, Furey CG, Farhadi DS, Benner D, Hendricks BK, Smith KA. Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Management of Low-Grade Gliomas and Radiation Necrosis: A Single-Institution Case Series. Brain Sciences. 2022; 12(12):1627. https://doi.org/10.3390/brainsci12121627
Chicago/Turabian StyleScherschinski, Lea, Jubran H. Jubran, Kelly A. Shaftel, Charuta G. Furey, Dara S. Farhadi, Dimitri Benner, Benjamin K. Hendricks, and Kris A. Smith. 2022. "Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Management of Low-Grade Gliomas and Radiation Necrosis: A Single-Institution Case Series" Brain Sciences 12, no. 12: 1627. https://doi.org/10.3390/brainsci12121627
APA StyleScherschinski, L., Jubran, J. H., Shaftel, K. A., Furey, C. G., Farhadi, D. S., Benner, D., Hendricks, B. K., & Smith, K. A. (2022). Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Management of Low-Grade Gliomas and Radiation Necrosis: A Single-Institution Case Series. Brain Sciences, 12(12), 1627. https://doi.org/10.3390/brainsci12121627





