CNS Superficial Siderosis Mimicking a Motor Neuron Disease
Abstract
1. Introduction
2. Case Presentation
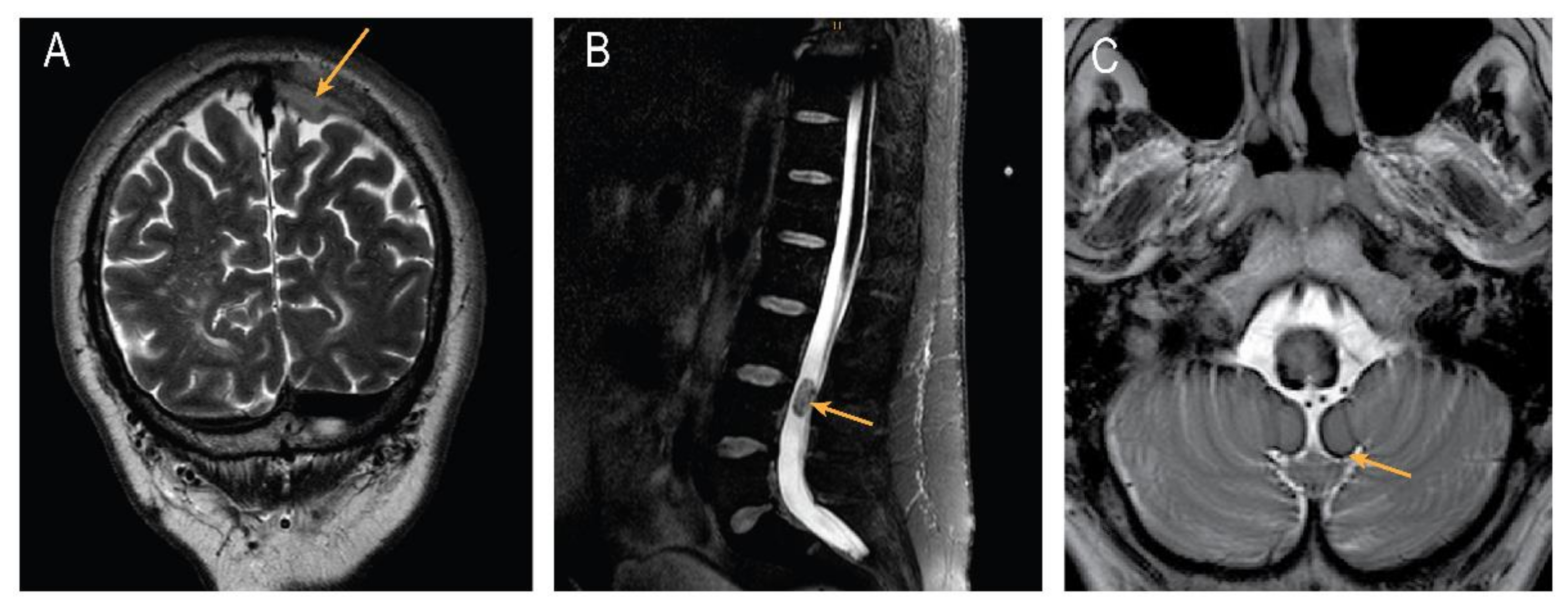
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearnley, J.M.; Stevens, J.M.; Rudge, P. Superficial Siderosis of the Central Nervous System. Brain 1995, 118, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Cohen-Gadol, A.A.; Wright, R.A.; Miller, G.M.; Piepgras, D.G.; Ahlskog, J.E. Superficial Siderosis. Neurology 2006, 66, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Vemuri, P.; Rabinstein, A.A.; Aakre, J.; Flemming, K.D.; Brown, R.D.; Kumar, N.; Kantarci, K.; Kremers, W.; Mielke, M.M.; et al. Prevalence and Natural History of Superficial Siderosis: A Population-Based Study. Stroke 2017, 48, 3210–3214. [Google Scholar] [CrossRef] [PubMed]
- Leussink, V.I.; Flachenecker, P.; Brechtelsbauer, D.; Bendszus, M.; Sliwka, U.; Gold, R.; Becker, G. Superficial Siderosis of the Central Nervous System: Pathogenetic Heterogeneity and Therapeutic Approaches. Acta Neurol Scand 2003, 107, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Beyond Superficial Siderosis: Introducing “Duropathies. ” Neurology 2012, 78, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Pradas, J.; Marín-lahoz, J.; De Luna, N.; Clarimón, J.; Turon-Sans, J.; Gelpí, E.; Díaz-Manera, J.; Illa, I.; Rojas-Garcia, R. Early Diagnosis of Amyotrophic Lateral Sclerosis Mimic Syndromes: Pros and Cons of Current Clinical Diagnostic Criteria. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Turner, B. Superficial Siderosis Associated with Anterior Horn Cell Dysfunction. J. Neurol. Neurosurg. Psychiatry 2002, 72, 275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Driver-Dunckley, E.D.; Hoxworth, J.M.; Patel, N.P.; Bosch, E.P.; Goodman, B.P. Superficial Siderosis Mimicking Amyotrophic Lateral Sclerosis. J. Clin. Neuromuscul. Dis. 2010, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Payer, M.; Sottas, C.; Bonvin, C. Superficial Siderosis of the Central Nervous System: Secondary Progression despite Successful Surgical Treatment, Mimicking Amyotrophic Lateral Sclerosis. Case Report and Review. Acta Neurochir. 2010, 152, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Fogelson, J.L.; Morris, J.M.; Pichelmann, M.A. Superficial Siderosis Should Be Included in the Differential Diagnosis of Motor Neuron Disease. Neurologist 2012, 18, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Honjo, N.; Takata, T.; Touge, T.; Masaki, T. Flail Arm Syndrome Mimic Caused by Hemosiderin Deposition in the Anterior Horn. Acta Neurol. Belg. 2020, 120, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.A.; Li, X.; Schwartz, K.; Huang, H.; Mealy, M.A.; Levy, M. Two-Year Observational Study of Deferiprone in Superficial Siderosis. CNS Neurosci. Ther. 2018, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H.; Michael, S.C.; Li, D.; Chen, Z.; Cusack, M.J.; Gibson, W.M.; Petrocine, S.V.; Qian, J. The Pathology of Superficial Siderosis of the Central Nervous System. Acta Neuropathol. 2008, 116, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T. Microglia Take Centre Stage in Neurodegenerative Disease. Nat. Rev. Immunol. 2019, 19, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H.; Dickson, A.C. Tin-Protoporphyrin Prevents Experimental Superficial Siderosis in Rabbits. J. Neuropathol. Exp. Neurol. 2002, 61, 689–701. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Gomez, S.; Binder, J.; Schievelkamp, A.-H.; Heneka, M.T. CNS Superficial Siderosis Mimicking a Motor Neuron Disease. Brain Sci. 2022, 12, 1558. https://doi.org/10.3390/brainsci12111558
Castro-Gomez S, Binder J, Schievelkamp A-H, Heneka MT. CNS Superficial Siderosis Mimicking a Motor Neuron Disease. Brain Sciences. 2022; 12(11):1558. https://doi.org/10.3390/brainsci12111558
Chicago/Turabian StyleCastro-Gomez, Sergio, Julius Binder, Arndt-Hendrik Schievelkamp, and Michael Thomas Heneka. 2022. "CNS Superficial Siderosis Mimicking a Motor Neuron Disease" Brain Sciences 12, no. 11: 1558. https://doi.org/10.3390/brainsci12111558
APA StyleCastro-Gomez, S., Binder, J., Schievelkamp, A.-H., & Heneka, M. T. (2022). CNS Superficial Siderosis Mimicking a Motor Neuron Disease. Brain Sciences, 12(11), 1558. https://doi.org/10.3390/brainsci12111558





