Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis
Abstract
1. Introduction
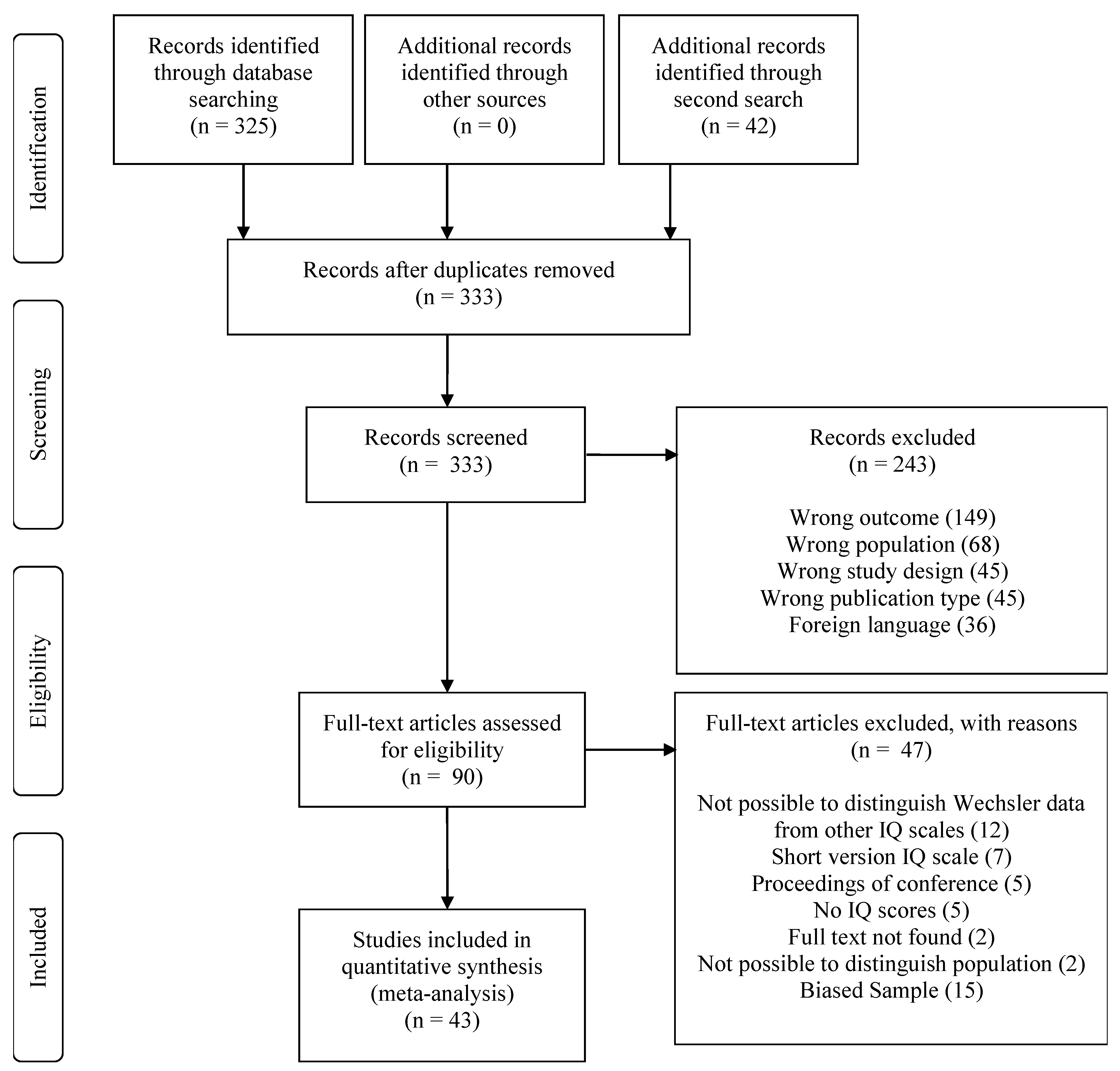
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Data Abstraction
2.4. Quality Assessment of Studies
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Quality Assessment of Studies
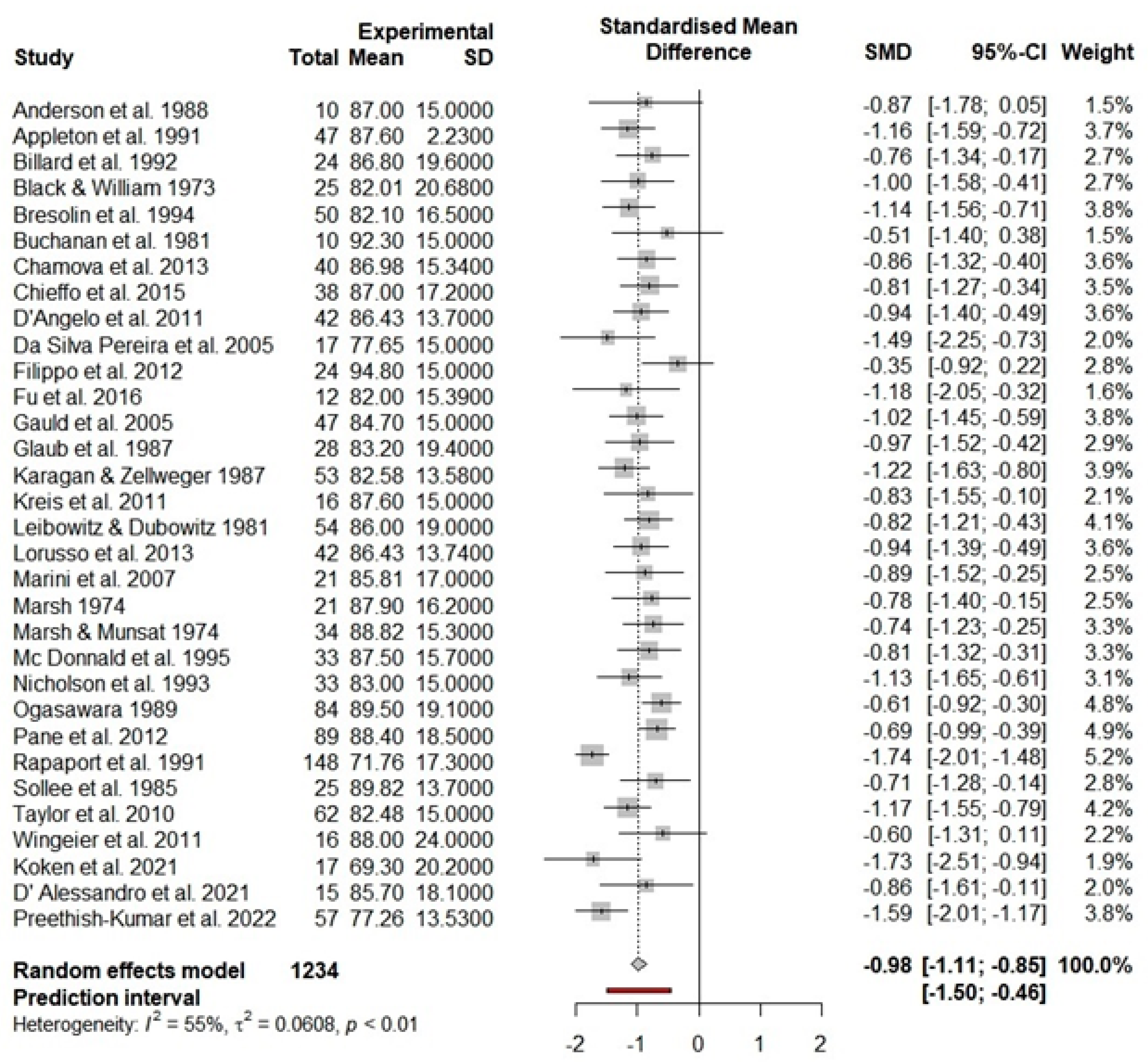
3.3. Main Effects for FSIQ
3.4. Tentative Post Hoc Analysis for VIQ and PIQ
3.5. Moderator Analyses
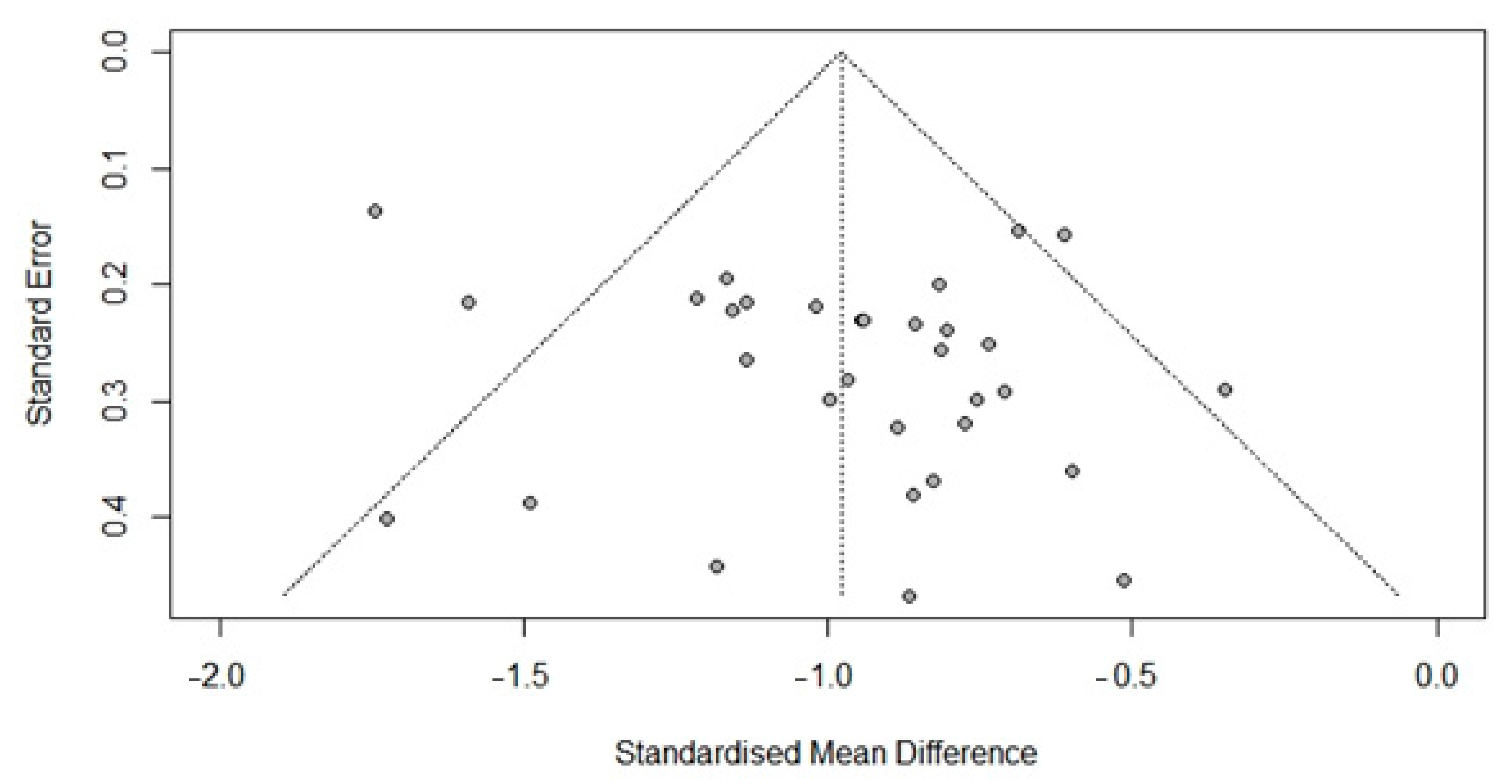
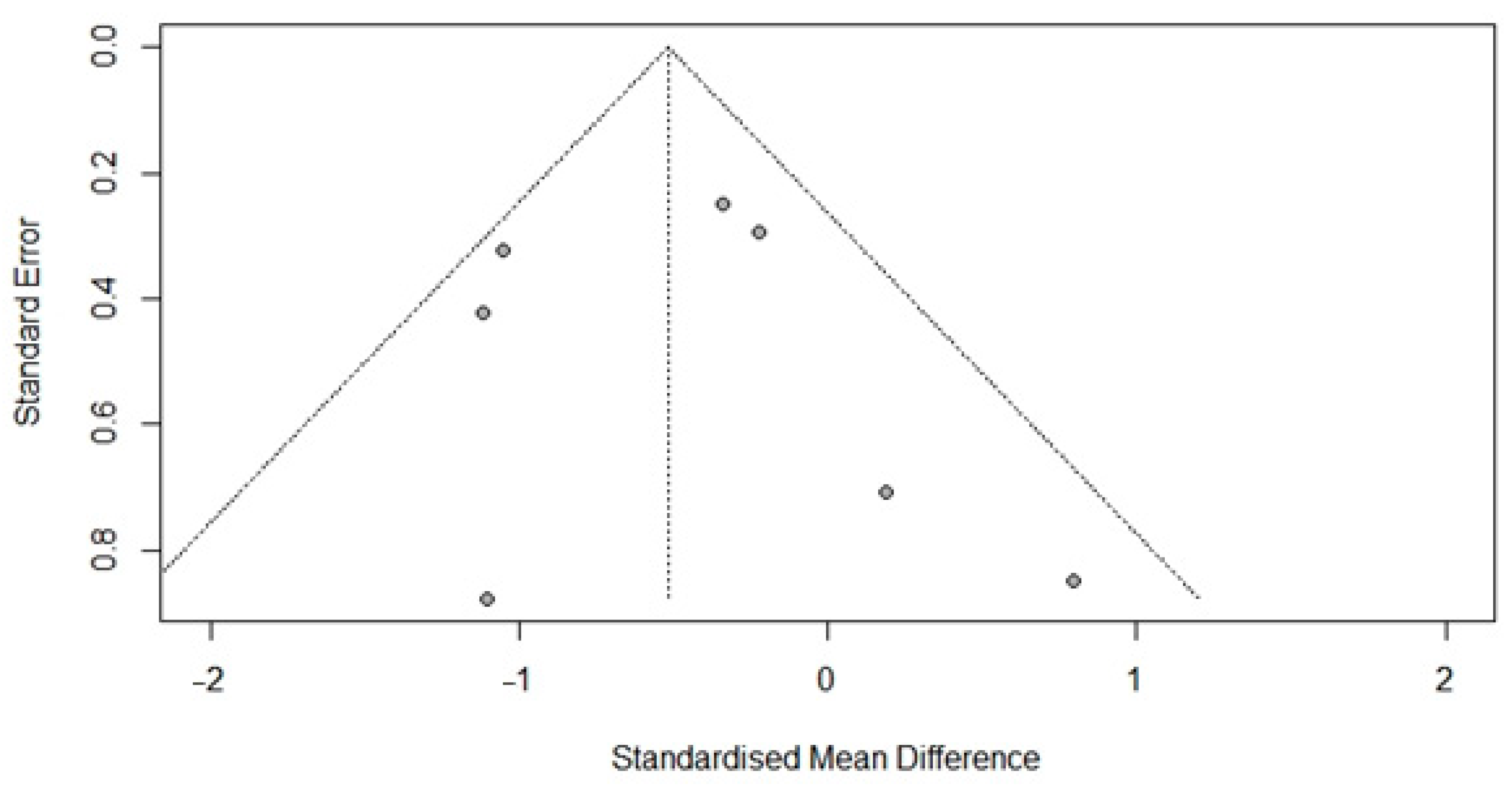
3.6. Publication Bias
3.7. Missing Values Analysis
3.8. Comparison with Data from Cotton et al. (2001)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Authors | Year | 1. Were the Criteria for Inclusion in the Sample Clearly Defined? | 2. Were the Study Subjects and the Setting Described in Detail? | 3. Was the Exposure Measured in a Valid and Reliable Way? | 4. Were Objective, Standard Criteria Used for Measurement of the Condition? | 5. Were Confounding Factors Identified? | 6. Were Strategies to Deal with Confounding Factors Stated? | 7. Were the Outcomes Measured in a Valid and Reliable Way? | 8. Was Appropriate Statistical Analysis Used? | Total | Level of Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al. | 1988 | N | Y | Y | Y | Y | Y | UC | Y | 13 | strong |
| Appleton et al. | 1991 | UC | Y | Y | Y | N | N | UC | Y | 10 | weak |
| Billard et al. | 1992 | UC | Y | Y | Y | Y | Y | UC | Y | 14 | strong |
| Black & William | 1973 | UC | Y | Y | Y | UC | N | UC | Y | 11 | weak |
| Bresolin et al. | 1994 | UC | UC | Y | Y | Y | UC | UC | Y | 12 | moderate |
| Bunchanan et al. | 1981 | UC | N | Y | UC | N | N | UC | Y | 7 | weak |
| Chamova et al. | 2013 | N | UC | Y | Y | N | N | Y | Y | 9 | weak |
| Chieffo et al. | 2015 | UC | UC | Y | Y | Y | N | Y | Y | 12 | moderate |
| D’Angelo et al. | 2011 | Y | Y | Y | Y | Y | Y | UC | Y | 15 | strong |
| Da Silva Pereira et al. | 2005 | N | N | Y | Y | N | N | Y | N | 6 | weak |
| Filippo et al. | 2012 | Y | Y | Y | Y | Y | Y | Y | N | 14 | strong |
| Fu et al. | 2016 | Y | UC | Y | Y | Y | Y | Y | Y | 15 | strong |
| Glaub et al. | 2005 | UC | UC | Y | UC | N | N | UC | Y | 8 | weak |
| Glaub et al. | 1987 | N | UC | Y | UC | Y | Y | UC | Y | 11 | weak |
| Karagan & Zellweger | 1987 | Y | Y | Y | Y | Y | Y | UC | Y | 15 | strong |
| Kreis et al. | 2011 | N | Y | Y | UC | N | Y | UC | Y | 10 | weak |
| Leibowitz &Dubowitz | 1981 | N | Y | Y | Y | Y | Y | Y | Y | 14 | strong |
| Lorusso et al. | 2013 | Y | Y | Y | Y | Y | Y | Y | Y | 16 | strong |
| Marini et al. | 2007 | N | Y | Y | Y | Y | Y | UC | Y | 13 | strong |
| Marsh | 1974 | N | Y | Y | Y | N | N | UC | UC | 8 | weak |
| Marsh & Munsat | 1974 | N | Y | Y | Y | N | N | UC | Y | 9 | weak |
| Mc Donnald et al. | 1995 | N | Y | Y | Y | UC | Y | UC | Y | 12 | moderate |
| Nicholson et al. | 1993 | N | N | Y | UC | N | N | UC | Y | 6 | weak |
| Ogasawara | 1989 | N | N | Y | Y | UC | Y | UC | UC | 9 | weak |
| Pane et al. | 2012 | Y | N | Y | Y | UC | N | UC | Y | 10 | weak |
| Rapaport et al. | 1991 | UC | N | Y | Y | N | N | UC | Y | 8 | weak |
| Sollee et al. | 1985 | UC | Y | Y | UC | Y | Y | UC | Y | 13 | strong |
| Taylor et al. | 2010 | N | Y | Y | Y | N | N | Y | Y | 10 | weak |
| Wingeier et al. | 2011 | Y | Y | Y | Y | UC | N | Y | Y | 13 | strong |
| Koken et al. | 2021 | Y | Y | Y | Y | UC | N | Y | Y | 13 | strong |
| D’Alessandro et al. | 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 16 | strong |
| Preethish-Kumar et al. | 2022 | Y | Y | Y | Y | Y | Y | UC | Y | 15 | strong |
| Authors | Year | 1. Were the Criteria for Inclusion in the Sample Clearly Defined? | 2. Were the Study Subjects and the Setting Described in Detail? | 3. Was the Exposure Measured in a Valid and Reliable Way? | 4. Were Objective, Standard Criteria Used for Measurement of the Condition? | 5. Were Confounding Factors Identified? | 6. Were Strategies to Deal with Confounding Factors Stated? | 7. Were the Outcomes Measured in a Valid and Reliable Way? | 8. Was Appropriate Statistical Analysis Used? | Total | Level of Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chamova et al. | 2013 | N | UC | Y | Y | N | N | Y | Y | 9 | weak |
| Melo et al. | 1995 | N | Y | Y | Y | Y | Y | Y | Y | 14 | strong |
| Mori-Yoshimura et al. | 2019 | UC | Y | Y | UC | Y | Y | Y | Y | 14 | strong |
| Young et al. | 2008 | Y | Y | Y | Y | Y | Y | Y | Y | 16 | strong |
| Koken et al. | 2021 | Y | Y | Y | Y | UC | N | Y | Y | 13 | strong |
| Hellebrekers et al. | 2020 | NA | Y | Y | Y | Y | NA | Y | Y | 12 | strong |
| D’alessandro et al. | 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 16 | strong |
References
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef]
- Waldrop, M.; Flanigan, K.M. Update in Duchenne and Becker muscular dystrophy. Curr. Opin. Neurol. 2019, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Thangarajh, M. The Dystrophinopathies. CONTINUUM Lifelong Learn. Neurol. 2019, 25, 1619–1639. [Google Scholar] [CrossRef]
- Anderson, J.L.; Head, S.; Rae, C.; Morley, J.W. Brain function in Duchenne muscular dystrophy. Brain 2002, 125, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Doorenweerd, N.; Hooijmans, M.; Schubert, S.A.; Webb, A.G.; Straathof, C.S.; van Zwet, E.W.; van Buchem, M.A.; Verschuuren, J.J.; Hendriksen, J.G.; Niks, E.H.; et al. Proton Magnetic Resonance Spectroscopy Indicates Preserved Cerebral Biochemical Composition in Duchenne Muscular Dystrophy Patients. J. Neuromuscul. Dis. 2017, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.G.; Hoogland, G.; Schipper, S.; Hendriksen, J.G.; Vles, J.S.; Aalbers, M.W. A possible role of dystrophin in neuronal excitability: A review of the current literature. Neurosci. Biobehav. Rev. 2015, 51, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, J.G.; Thangarajh, M.; Kan, H.E.; Muntoni, F.; Aoki, Y.; Collin, P.; Colvin, M.; Doorenweerd, N.; Ferlini, A.; Goyenvalle, A.; et al. 249th ENMC International Workshop: The role of brain dystrophin in muscular dystrophy: Implications for clinical care and translational research, Hoofddorp, The Netherlands, November 29th–December 1st 2019. Neuromuscul. Disord. 2020, 30, 782–794. [Google Scholar] [CrossRef]
- Snow, W.M.; Anderson, J.E.; Jakobson, L.S. Neuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: A review. Neurosci. Biobehav. Rev. 2013, 37, 743–752. [Google Scholar] [CrossRef]
- Cyrulnik, S.E.; Hinton, V.J. Duchenne muscular dystrophy: A cerebellar disorder? Neurosci. Biobehav. Rev. 2008, 32, 486–496. [Google Scholar] [CrossRef]
- Kreis, R.; Wingeier, K.; Vermathen, P.; Giger, E.; Joncourt, F.; Zwygart, K.; Kaufmann, F.; Boesch, C.; Steinlin, M. Brain metabolite composition in relation to cognitive function and dystrophin mutations in boys with Duchenne muscular dystrophy. NMR Biomed. 2011, 24, 253–262. [Google Scholar] [CrossRef]
- Young, H.K.; Barton, B.A.; Waisbren, S.; Dale, L.P.; Ryan, M.M.; Webster, R.I.; North, K.N. Cognitive and Psychological Profile of Males With Becker Muscular Dystrophy. J. Child Neurol. 2008, 23, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Banihani, R.; Smile, S.; Yoon, G.; Dupuis, A.; Mosleh, M.; Snider, A.; McAdam, L. Cognitive and neurobehavioral profile in boys with duchenne muscular dystrophy. J. Child Neurol. 2015, 30, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chamova, T.; Guergueltcheva, V.; Raycheva, M.; Todorov, T.; Genova, J.; Bichev, S.; Bojinova, V.; Mitev, V.; Tournev, I.; Todorova, A. Association between loss of Dp140 and cognitive impairment in Duchenne and Becker dystrophies. Balk. J. Med. Genet. 2013, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Daoud, F.; Angeard, N.; Demerre, B.; Martie, I.; Benyaou, R.; Leturcq, F.; Cossée, M.; Deburgrave, N.; Saillour, Y.; Tuffery, S.; et al. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum. Mol. Genet. 2009, 18, 3779–3794. [Google Scholar] [CrossRef] [PubMed]
- Darmahkasih, A.J.; Rybalsky, I.; Tian, C.; Shellenbarger, K.C.; Horn, P.S.; Ma, J.T.L.; Wong, B.L. Neurodevelopmental, behavioral, and emotional symptoms common in Duchenne muscular dystrophy. Muscle Nerve 2020, 61, 466–474. [Google Scholar] [CrossRef]
- Bardoni, A.; Felisari, G.; Sironi, M.; Comi, G.; Lai, M.; Robotti, M.; Bresolin, N. Loss of Dp140 regulatory sequences is associated with cognitive impairment in dystrophinopathies. Neuromuscul. Disord. 2000, 10, 194–199. [Google Scholar] [CrossRef]
- Hendriksen, J.G.; Vles, J.S. Are Males With Duchenne Muscular Dystrophy at Risk for Reading Disabilities? Pediatr. Neurol. 2006, 34, 296–300. [Google Scholar] [CrossRef]
- Ricotti, V.; Mandy, W.P.L.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef]
- Taylor, P.J.; Betts, G.A.; Maroulis, S.; Gilissen, C.; Pedersen, R.L.; Mowat, D.R.; Johnston, H.M.; Buckley, M.F. Dystrophin gene mutation location and the risk of cognitive impairment in duchenne muscular dystrophy. PLoS ONE 2010, 5, e8803. [Google Scholar] [CrossRef]
- Felisari, G.; Boneschi, F.M.; Bardoni, A.; Sironi, M.; Comi, G.; Robotti, M.; Turconi, A.C.; Lai, M.; Corrao, G.; Bresolin, N. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000, 55, 559–564. [Google Scholar] [CrossRef]
- Moizard, M.P.; Billard, C.; Toutain, A.; Berret, F.; Marmin, N.; Moraine, C. Are Dp71 and Dp140 brain dystrophin isoforms related to cognitive impairment in Duchenne muscular dystrophy? Am. J. Med. Genet. 1998, 80, 32–41. [Google Scholar] [CrossRef]
- D’Angelo, M.G.; Lorusso, M.L.; Civati, F.; Comi, G.P.; Magri, F.; Del Bo, R.; Guglieri, M.; Molteni, M.; Turconi, A.C.; Bresolin, N. Neurocognitive profiles in duchenne muscular dystrophy and gene mutation site. Pediatr. Neurol. 2011, 45, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.J.; Vles, J.S.H.; Klinkenberg, S.; Hendriksen, J.G.M. The Neurocognitive and Behavioral Profiles of 3 Brothers With Becker Muscular Dystrophy. Child Neurol. Open 2020, 7, 2329048X2095721. [Google Scholar] [CrossRef] [PubMed]
- reethish-Kumar, V.; Shah, A.; Polavarapu, K.; Kumar, M.; Safai, A.; Vengalil, S.; Nashi, S.; Deepha, S.; Govindaraj, P.; Afsar, M.; et al. Disrupted structural connectome and neurocognitive functions in Duchenne muscular dystrophy: Classifying and subtyping based on Dp140 dystrophin isoform. J. Neurol. 2022, 269, 2113–2125. [Google Scholar] [CrossRef]
- Cotton, S.; Voudouris, N.J.; Greenwood, K.M. Intelligence and Duchenne muscular dystrophy: Full-scale, verbal, and performance intelligence quotients. Dev. Med. Child Neurol. 2001, 43, 497–501. [Google Scholar] [CrossRef]
- Sternberg, R.J. Intelligence. State of art. Dialogues Clin. Neurosci. 2012, 14, 19–27. [Google Scholar] [CrossRef]
- Bagdatlioglu, E.; Porcari, P.; Greally, E.; Blamire, A.M.; Straub, V.W. Cognitive impairment appears progressive in the mdx mouse. Neuromuscul. Disord. 2020, 30, 368–388. [Google Scholar] [CrossRef]
- Doorenweerd, N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy-a narrative review. Neuromuscul. Disord. 2020, 30, 437–442. [Google Scholar] [CrossRef]
- Perumal, A.R.; Rajeswaran, J.; Nalini, A. Neuropsychological Profile of Duchenne Muscular Dystrophy. Appl. Neuropsychol. Child 2015, 4, 49–57. [Google Scholar] [CrossRef]
- Ferrero, A.; Rossi, M. Cognitive profile and neuropsychiatric disorders in Becker muscular dystrophy: A systematic review of literature. Neurosci. Biobehav. Rev. 2022, 137, 104648. [Google Scholar] [CrossRef]
- Sattler, J.M. Assessment of Children; Cognitive Foundations: San Diego, CA, USA, 2008. [Google Scholar]
- Weiss, L.G.; Prifitera, A.; Holdnack, J.A.; Saklofske, D.H. WISC-IV Advanced Clinical Interpretation; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar] [CrossRef]
- Watkins, M.W.; Smith, L.G. Long-term stability of the wechsler intelligence scale for children-fourth edition. Psychol. Assess. 2013, 25, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Weerkamp, P.; Chieffo, D.; Collin, P.; Moriconi, F.; Papageorgiou, A.; Vainieri, I.; Miranda, R.; Hankinson, C.; Vogel, A.; Poncet, S.; et al. Psychological test usage in Duchenne muscular dystrophy: An EU Delphi based multi-centre study. (Unpublished manuscript).
- Wechsler, D. Wechsler Intelligence Scale for Children-Fifth Edition-Nederlandstalige Bewerking. Technische Handleiding; Pearson: London, UK, 2018. [Google Scholar]
- Wechsler, D. WAIS-IV-NL: Wechsler Adult Intelligence Scale—Nederlandstalige Bewerking; Pearson: London, UK, 2012. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary; Pearson: London, UK, 2020. [Google Scholar]
- Mori-Yoshimura, M.; Mizuno, Y.; Yoshida, S.; Ishihara, N.; Minami, N.; Morimoto, E.; Maruo, K.; Nonaka, I.; Komaki, H.; Nishino, I.; et al. Psychiatric and neurodevelopmental aspects of Becker muscular dystrophy. Neuromuscul. Disord. 2019, 9, 930–939. [Google Scholar] [CrossRef]
- Köken, Ö.Y.; Kucur, Ö.; Taşkiran, C.; Öztoprak, Ü.; Sel, Ç.G.; Aksoy, E.; Aksoy, A.; Yoldaş, T.; Yüksel, D. Clinical features and quality of life in duchenne and becker muscular dystrophy patients from a tertiary center in Turkey. Guncel Pediatri 2021, 19, 15–22. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.J.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Inst. Rev. Man. 2017, 5, 219. [Google Scholar]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. Rstudio Team; PBC: Boston, MA, USA, 2020; Available online: http//www.rstudio.com (accessed on 18 October 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. -Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- Gonthier, C. Cross-cultural differences in visuo-spatial processing and the culture-fairness of visuo-spatial intelligence tests: An integrative review and a model for matrices tasks. Cogn. Res. Princ. Implic. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Cockcroft, K.; Alloway, T.; Copello, E.; Milligan, R. A cross-cultural comparison between South African and British students on the wechsler adult intelligence scales third edition (WAIS-III). Front. Psychol. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Thangarajh, M.; Elfring, G.L.; Trifillis, P. Longitudinal evaluation of working memory in duchenne muscular dystrophy. J. Clin. Med. 2020, 9, 2940. [Google Scholar] [CrossRef] [PubMed]
- Battini, R.; Lenzi, S.; Lucibello, S.; Chieffo, D.; Moriconi, F.; Cristofani, P.; Bulgheroni, S.; Cumbo, F.; Pane, M.; Baranello, G.; et al. Longitudinal data of neuropsychological profile in a cohort of Duchenne muscular dystrophy boys without cognitive impairment. Neuromuscul. Disord. 2021, 31, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sharawat, I.K. Mental health and behavioral function in children with neuromuscular disorders. Eur. J. Paediatr. Neurol. 2021, 30, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, G.; Hendriksen, R.G.F.; Slegers, R.J.; Hendriks, M.P.H.; Schijns, O.E.M.G.; Aalbers, M.W.; Vles, J.S.H. The expression of the distal dystrophin isoforms Dp140 and Dp71 in the human epileptic hippocampus in relation to cognitive functioning. Hippocampus 2019, 29, 102–110. [Google Scholar] [CrossRef]
- Lionarons, J.M.; Hellebrekers, D.M.; Klinkenberg, S.; Faber, C.G.; Vles, J.S.; Hendriksen, J.G. Methylphenidate use in males with Duchenne muscular dystrophy and a comorbid attention-deficit hyperactivity disorder. Eur. J. Paediatr. Neurol. 2019, 23, 152–157. [Google Scholar] [CrossRef]
- Bushby, K.M.D.; Appleton, R.; Anderson, L.V.B.; Welch, J.L.; Kelly, P.; Gardner-Medwin, D. Deletion status and intellectual impairment in duchenne muscular dystrophy. Dev. Med. Child Neurol. 2008, 37, 260–269. [Google Scholar] [CrossRef]
- Rapaport, D.; Passos-Bueno, M.R.; Takata, R.I.; Campiotto, S.; Eggers, S.; Vainzof, M.; Makover, A.; Nudel, U.; Yaffe, D.; Zatz, M. A deletion including the brain promoter of the Duchenne muscular dystrophy gene is not associated with mental retardation. Neuromuscul. Disord. 1992, 2, 117–120. [Google Scholar] [CrossRef]
- Moizard, M.-P.; Toutain, A.; Fournier, D.; Berret, F.; Raynaud, M.; Billard, C.; Andres, C.; Moraine, C. Severe cognitive impairment in DMD: Obvious clinical indication for Dp71 isoform point mutation screening. Eur. J. Hum. Genet. 2000, 8, 552–556. [Google Scholar] [CrossRef]
- Florek, M.; Karolak, S. Intelligence level of patients with the duchenne type of progressive muscular dystrophy (PMD-D). Eur. J. Pediatr. 1977, 126, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Desguerre, I.; Christov, C.; Mayer, M.; Zeller, R.; Becane, H.-M.; Bastuji-Garin, S.; Leturcq, F.; Chiron, C.; Chelly, J.; Gherardi, R.K. Clinical heterogeneity of Duchenne muscular dystrophy (DMD): Definition of sub-phenotypes and predictive criteria by long-term follow-up. PLoS ONE 2009, 4, e4347. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Tunnecliffe, M.; Psych, M.A.; Douglas, P.S.; Dip, G. IQ, prognosis and duchenne muscular dystrophy. Brain Dev. 1985, 7, 7–9. [Google Scholar] [CrossRef]
- Savage, R.D.; Adams, M. Cognitive functioning and neurological deficit: Duchenne muscular dystrophy and cerebral palsy. Aust. Psychol. 1979, 14, 59–75. [Google Scholar] [CrossRef]
- Ueda, Y.; Suwazono, S.; Maedo, S.; Higuchi, I. Profile of cognitive function in adults with duchenne muscular dystrophy. Brain Dev. 2017, 39, 225–230. [Google Scholar] [CrossRef]
- HHausmanowa-Petrusewicz, I.; Zaremba, J.; Fidziańska, A.; Zimowski, J.; Bisko, M.; Badurska, B.; Fidziańska, E.; Lusakowska, A.; Borkowska, J. Interrelationship between gene, its product and phenotype in Duchenne and Becker muscular dystrophy. Acta Neurobiol. Exp. (Wars) 1993, 53, 297–303. [Google Scholar]
- Parisi, L.; Di Filippo, T.; Glorioso, P.; La Grutta, S.; Epifanio, M.S.; Roccella, M. Autism spectrum disorders in children affected by Duchenne muscular dystrophy. Minerva Pediatr. 2018, 70, 233–239. [Google Scholar] [CrossRef]
- Sato, Y.; Yamauchi, A.; Urano, M.; Kondo, E.; Saito, K. Corticosteroid therapy for duchenne muscular dystrophy: Improvement of psychomotor function. Pediatr. Neurol. 2014, 50, 31–37. [Google Scholar] [CrossRef]
- Della Coletta, M.V.; Scola, R.H.; Wiemes, G.R.M.; Fonseca, C.N.; Mäder, M.J.; Freund, A.A.; Werneck, L.C. Event-related potentials (P300) and neuropsychological assessment in boys exhibiting duchenne muscular dystrophy. Arq. Neuropsiquiatr. 2007, 65, 59–62. [Google Scholar] [CrossRef]
- Colombo, P.; Nobile, M.; Tesei, A.; Civati, F.; Gandossini, S.; Mani, E.; Molteni, M.; Bresolin, N.; D’Angelo, M.G. Assessing mental health in boys with Duchenne muscular dystrophy: Emotional, behavioural and neurodevelopmental profile in an Italian clinical sample. Eur. J. Paediatr. Neurol. 2017, 21, 639–647. [Google Scholar] [CrossRef]
- Lincoln, N.B.; Staples, D.J. Psychological aspects of some chronic progressive neuromuscular disorders. 1. Cognitive abilities. J. Chronic Dis. 1977, 30, 207–215. [Google Scholar] [CrossRef]
- Donders, J.; Taneja, C. Neurobehavioral characteristics of children with duchenne muscular dystrophy. Child Neuropsychol. 2009, 15, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Thompson, C.; Dunn, J.; Barnes, P.; Styles, P.; Kemp, G.; Rae, C.; Radda, G.; Pike, M.; Scott, R. Brain abnormalities in Duchenne muscular dystrophy: Phosphorus-31 magnetic resonance spectroscopy and neuropsychological study. Lancet 1995, 345, 1260–1264. [Google Scholar] [CrossRef]
- Bardoni, A.; Sironi, M.; Felisari, G.; Comi, G.P.; Bresolin, N. Absence of brain Dp140 isoform and cognitive impairment in Becker muscular dystrophy. Lancet 1999, 353, 897–898. [Google Scholar] [CrossRef]
- Magri, F.; Govoni, A.; D’Angelo, M.G.; Del Bo, R.; Ghezzi, S.; Sandra, G.; Turconi, A.C.; Sciacco, M.; Ciscato, P.; Bordoni, A.; et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J. Neurol. 2011, 258, 1610–1623. [Google Scholar] [CrossRef]
- Rabbi-Bortolini, E.; Zatz, M.; Opitz, J.M.; Reynolds, J.F. Investigation on genetic heterogeneity in Duchenne muscular dystrophy. Am. J. Med. Genet. 1986, 24, 111–117. [Google Scholar] [CrossRef]
- Rasic, M.V.; Vojinovic, D.; Pešović, J.; Mijalkovic, G.; Lukic, V.; Mladenovic, J.; Kosac, A.; Novakovic, I.; Maksimovic, N.; Romac, S.; et al. Intellectual ability in the duchenne muscular dystrophy and dystrophin gene mutation location. Balk. J. Med. Genet. 2014, 17, 25–36. [Google Scholar] [CrossRef]
- Dorman, C.; Hurley, A.D.; Avignon, J.D. Language and Learning Disorders of Older Boys with Duchenne Muscular Dystrophy. Dev. Med. Child Neurol. 2008, 30, 316–327. [Google Scholar] [CrossRef]
- Karagan, N.J.; Richman, L.C.; Sorensen, J.P. Analysis of verbal disability in duchenne muscular dystrophy. J. Nerv. Ment. Dis. 1980, 168, 419–423. [Google Scholar] [CrossRef]
- Fabbro, F.; Felisari, G.; D’Angelo, M.G.; Turconi, A.C.; Marini, A.; Comi, G.P.; Bresolin, N. Language disturbances in a group of participants suffering from Duchenne Muscular Dystrophy: A pilot study. Percept. Mot. Ski. 2007, 104, 663–676. [Google Scholar] [CrossRef]
- Upadhyaya, M.; Smith, R.A.; Thomas, N.S.T.; Norman, A.M.; Harper, P.S. Intragenic deletions in 164 boys with Duchenne muscular dystrophy (DMD) studied with dystrophin cDNA. Clin. Genet. 1990, 37, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.; Doorenweerd, N.; Sweere, D.J.; van Kuijk, S.M.; Aartsma-Rus, A.M.; Klinkenberg, S.; Vles, J.S.; Hendriksen, J.G. Longitudinal follow-up of verbal span and processing speed in Duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2020, 25, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, H. Psychometric Studies. Dev. Med. Child Neurol. 1967, 9, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Mento, G.; Tarantino, V.; Bisiacchi, P.S. The neuropsychological profile of infantile Duchenne muscular dystrophy. Clin. Neuropsychol. 2011, 25, 1359–1377. [Google Scholar] [CrossRef]
- Astrea, G.; Pecini, C.; Gasperini, F.; Brisca, G.; Scutifero, M.; Bruno, C.; Santorelli, F.M.; Cioni, G.; Politano, L.; Chilosi, A.M.; et al. Reading impairment in Duchenne muscular dystrophy: A pilot study to investigate similarities and differences with developmental dyslexia. Res. Dev. Disabil. 2015, 45–46, 168–177. [Google Scholar] [CrossRef]
- Chamova, T.K.; Guerguelcheva, V.; Raycheva, M.; Mihaylova, Z.; Tournev, I. Genotype-phenotype correlations of the cognitive disorders in Bulgarian patients with Duchenne muscular dystrophy. Eur. J. Neurol. 2010, 17, 472. [Google Scholar]
- Vojinovic, D.; Rasic, V.M. Cognitive impairment of males with Duchenne muscular dystrophy. J. Neurol. 2013, 260, S260. [Google Scholar]
- Battini, R.; Chieffo, D.; Bulgheroni, S.; Piccini, G.; Pecini, C.; Lucibello, S.; Lenzi, S.; Moriconi, F.; Pane, M.; Astrea, G.; et al. Cognitive profile in Duchenne muscular dystrophy boys without intellectual disability: The role of executive functions. Neuromuscul. Disord. 2018, 28, 122–128. [Google Scholar] [CrossRef]
- North, K.N.; Miller, G.; Iannaccone, S.T.; Clemens, P.R.; Chad, D.A.; Bella, I.; Smith, T.W.; Beggs, A.; Specht, L.A. Cognitive dysfunction as the major presenting feature of Becker’s muscular dystrophy. Neurology 1996, 46, 461–465. [Google Scholar] [CrossRef]
- Colombo, P.; Civati, F.; Mani, E.; Gandossini, S.; Brighina, E.; Comi, G.; Bresolin, N.; Turconi, A.; Molteni, M.; Nobile, M.; et al. G.P.174. Neuromuscul. Disord. 2014, 24, 858. [Google Scholar] [CrossRef]
- Ogasawara, A. Downward shift in IQ in persons with Duchenne muscular dystrophy compared to those with spinal muscular atrophy. Am. J. Ment. Retard. 1989, 93, 544–547. [Google Scholar] [PubMed]
- Kozicka, A.; Prot, J.; Wasilewski, R. Mental retardation in patients with Duchenne progressive muscular dystrophy. J. Neurol. Sci. 1971, 14, 209–213. [Google Scholar] [CrossRef]
- Lorusso, M.L.; Civati, F.; Molteni, M.; Turconi, A.C.; Bresolin, N.; D’Angelo, M.G. Specific profiles of neurocognitive and reading functions in a sample of 42 Italian boys with Duchenne Muscular Dystrophy. Child Neuropsychol. 2013, 19, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.G.; Munsat, T.L. Evidence for early impairment of verbal intelligence in Duchenne muscular dystrophy. Arch. Dis. Child. 1974, 49, 118–122. [Google Scholar] [CrossRef][Green Version]
- Rapaport, D.; Passos-Bueno, M.R.; Brandão, L.; Love, D.; Vainzof, M.; Zatz, M. Apparent association of mental retardation and specific patterns of deletions screened with probes cf56a and cf23a in Duchenne Muscular Dystrophy. Am. J. Med. Genet. 1991, 39, 437–441. [Google Scholar] [CrossRef]
- Anderson, S.W.; Routh, D.K.; Ionasescu, V.V. Serial position memory of boys with duchenne muscular dystrophy. Dev. Med. Child Neurol. 2008, 30, 328–333. [Google Scholar] [CrossRef]
- Al-Qudah, A.A.; Kobayashi, J.; Chuang, S.; Dennis, M.; Ray, P. Etiology of intellectual impairment in Duchenne muscular dystrophy. Pediatr. Neurol. 1990, 6, 57–59. [Google Scholar] [CrossRef]
- Pane, M.; Lombardo, M.E.; Alfieri, P.; D’Amico, A.; Bianco, F.; Vasco, G.; Piccini, G.; Mallardi, M.; Romeo, D.; Ricotti, V.; et al. Attention deficit hyperactivity disorder and cognitive function in duchenne muscular dystrophy: Phenotype-genotype correlation. J. Pediatr. 2012, 161, 705–709.e1. [Google Scholar] [CrossRef]
- Marsh, G.G. Impaired visual-motor ability of children with Duchenne muscular dystrophy. Percept. Mot. Ski. 1972, 35, 504–506. [Google Scholar] [CrossRef]
- Leibowitz, D.; Dubowitz, V. Intellect and Behaviour in Duchenne Muscular Dystrophy. Dev. Med. Child Neurol. 2008, 23, 577–590. [Google Scholar] [CrossRef]
- Karagan, N.J.; Zellweger, H.U. Early Verbal Disability in Children with Duchenne Muscular Dystrophy. Dev. Med. Child Neurol. 2008, 20, 435–441. [Google Scholar] [CrossRef]
- Suzuki, Y.; Higuchi, S.; Aida, I.; Nakajima, T.; Nakada, T. Abnormal distribution of GABAA receptors in brain of duchenne muscular dystrophy patients. Muscle Nerve 2017, 55, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.V.; Johnson, M.A.; Bushby, K.M.; Gardner-Medwin, D.; Curtis, A.; Ginjaar, I.B.; Dunnen, J.T.D.; Welch, J.L.; Butler, T.J.; Bakker, E. Integrated study of 100 patients with Xp21 linked muscular dystrophy using clinical, genetic, immunochemical, and histopathological data. Part 2. Correlations within individual patients. J. Med. Genet. 1993, 30, 737–744. [Google Scholar] [CrossRef]
- Black, F.W. Intellectual Ability as Related to Age and Stage of Disease in Muscular Dystrophy: A Brief Note. J. Psychol. 1973, 84, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.C.D.S.; Kiyomoto, B.H.; Cardoso, R.; Oliveira, A.S.B. Duchenne muscular dystrophy: δ-dystroglycan immunoexpression in skeletal muscle and cognitive performance. Arq. Neuropsiquiatr. 2005, 63, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, N.; Castelli, E.; Comi, G.; Felisari, G.; Bardoni, A.; Perani, D.; Grassi, F.; Turconi, A.C.; Mazzucchelli, F.; Gallotti, D.; et al. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul. Disord. 1994, 4, 359–369. [Google Scholar] [CrossRef]
- Filippini, M.; Guerra, A.; Negosanti, A.; Santi, S.; Sarajlija, J.; Musti, M.A.; Gobbi, G.; Lassonde, M.; Pini, A. Mismatch Negativity Recording in Children with Duchenne Muscular Dystrophy: A Preliminary Study Integrating Neurophysiological and Neuropsychological Results. J. Child Neurol. 2016, 31, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Appleton, R.E.; Bushby, K.; Gardner-Medwin, D.; Welch, J.; Kelly, P.J. Head Circumference And Intellectual Performance Of Patients With Duchenne Muscular Dystrophy. Dev. Med. Child Neurol. 2008, 33, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, A. Similarity of IQs of siblings with Duchenne progressive muscular dystrophy. Am. J. Ment. Retard. 1989, 93, 548–550. [Google Scholar]
- D’Alessandro, R.; Ragusa, N.; Vacchetti, M.; Rolle, E.; Rossi, F.; Brusa, C.; Davico, C.; Vitiello, B.; Mongini, T.; Ricci, F.S. Assessing cognitive function in neuromuscular diseases: A pilot study in a sample of children and adolescents. J. Clin. Med. 2021, 10, 4777. [Google Scholar] [CrossRef]
- Sollee, N.D.; Latham, E.E.; Kindlon, D.J.; Bresnan, M.J. Neuropsychological impairment in Duchenne Muscular Dystrophy. J. Clin. Exp. Neuropsychol. 1985, 7, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Lorusso, M.L.; D’Angelo, M.G.; Civati, F.; Turconi, A.C.; Fabbro, F.; Bresolin, N. Evaluation of narrative abilities in patients suffering from Duchenne Muscular Dystrophy. Brain Lang. 2007, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Lauriano, V.; Gentil, V.; Eggers, S.; Del Bianco, S.S.; Gimenez, P.R.; Akiyama, J.; Okabaiashi, H.; Frota-Pessoa, O.; Passos-Bueno, M.R.; et al. Becker and limb-girdle muscular dystrophies: A psychiatric and intellectual level comparative study. Am. J. Med. Genet. Neuropsychiatr. Genet. 1995, 60, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wingeier, K.; Giger, E.; Strozzi, S.; Kreis, R.; Joncourt, F.; Conrad, B.; Gallati, S.; Steinlin, M. Neuropsychological impairments and the impact of dystrophin mutations on general cognitive functioning of patients with Duchenne muscular dystrophy. J. Clin. Neurosci. 2011, 18, 90–95. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, Y.; Zhang, C.; Sun, Y.; Zhang, S.; Mu, X.; Wang, H.; Xu, W.; Wu, S. Diffusion tensor imaging study in Duchenne muscular dystrophy. Ann. Transl. Med. 2016, 4, 109. [Google Scholar] [CrossRef]
- Buchanan, D.C.; Roelofs, R.I.; White, D.K.; De Arango, G.S. Intellectual function in Duchenne muscular dystrophy: The influence of penicillamine. Arch. Phys. Med. Rehabil. 1981, 62, 623–625. [Google Scholar]
- Gauld, L.M.; Boynton, A.; Betts, G.A.; Johnston, H. Spirometry is affected by intelligence and behavior in Duchenne muscular dystrophy. Pediatr. Pulmonol. 2005, 40, 408–413. [Google Scholar] [CrossRef]
- McDonald, C.M.; Abresch, R.T.; Carter, G.T.; Fowler, W.M.; Johnson, E.R.; Kilmer, D.D.; Sigford, B.J. Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 1995, 74, S70–S92. [Google Scholar] [CrossRef]
- Glaub, T.; Mechler, F. Intellectual function in muscular dystrophies. Eur. Arch. Psychiatry Neurol. Sci. 1987, 236, 379–382. [Google Scholar] [CrossRef]
- Chieffo, D.; Brogna, C.; Berardinelli, A.; D’Angelo, G.; Mallardi, M.; D’Amico, A.; Alfieri, P.; Mercuri, E.; Pane, M. Early neurodevelopmental findings predict school age cognitive abilities in duchenne muscular dystrophy: A longitudinal study. PLoS ONE 2015, 10, e0133214. [Google Scholar] [CrossRef]
- Di Filippo, T.; Parisi, L.; Roccella, M. Psychological aspects in children affected by duchenne de boulogne muscular dystrophy. Ment. Illn. 2012, 4, 21–24. [Google Scholar] [CrossRef]
- Billard, C.; Gillet, P.; Signoret, J.; Uicaut, E.; Bertrand, P.; Fardeau, M.; Barthez-Carpentier, M.; Santini, J. Cognitive functions in duchenne muscular dystrophy: A reappraisal and comparison with spinal muscular atrophy. Neuromuscul. Disord. 1992, 2, 371–378. [Google Scholar] [CrossRef]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Canivez, G.L.; Watkins, M.W. Long-term stability of the Wechsler Intelligence Scale for Children—Third Edition. Psychol. Assess 1998, 10, 285. [Google Scholar] [CrossRef]
- Resing, W.C.M.; Bleichrodt, N.; Drenth, P.J.D.; Zaal, J.N. RAKIT-2: Revisie Amsterdamse Kinder Intelligentie Test; Pearson: London, UK, 2012. [Google Scholar]
- Watkins, M.W.; Glutting, J.J.; Youngstrom, E.A. Issues in subtest profile analysis. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; The Guilford Press: New York, NY, USA, 2005. [Google Scholar]
- Hinton, V.J.; DE Vivo, D.C.; Nereo, N.E.; Goldstein, E.; Stern, Y. Selective deficits in verbal working memory associated with a known genetic etiology: The neuropsychological profile of Duchenne muscular dystrophy. J. Int. Neuropsychol. Soc. 2001, 7, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Foo, R.Y.; Guppy, M.; Johnston, L.M. Intelligence assessments for children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2013, 55, 911–918. [Google Scholar] [CrossRef]
- Sherwell, S.; Reid, S.M.; Reddihough, D.S.; Wrennall, J.; Ong, B.; Stargatt, R. Measuring intellectual ability in children with cerebral palsy: Can we do better? Res. Dev. Disabil. 2014, 35, 2558–2567. [Google Scholar] [CrossRef]
- Piovesana, A.M.; Harrison, J.L.; Ducat, J.J. The Development of a Motor-Free Short-Form of the Wechsler Intelligence Scale for Children–Fifth Edition. Assessment 2019, 26, 1564–1572. [Google Scholar] [CrossRef]
- Coceski, M.; Hocking, D.R.; Abu-Rayya, H.M.; Sherwell, S.; Reid, S.M.; Reddihough, D.S.; Wrennall, J.; Stargatt, R. WISC-V motor-free cognitive profile and predictive factors in adolescents with cerebral palsy. Res. Dev. Disabil. 2021, 113, 103934. [Google Scholar] [CrossRef]

| 95% CI | Homogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| k | n | M | LL | UL | p | Q | I2 | |
| DMD FSIQ | 32 | 1234 | 84.76 | 82.81 | 86.70 | <0.001 | 69.09 | 55.10 |
| BMD FSIQ | 7 | 101 | 92.11 | 86.71 | 97.50 | 0.12 | 10.08 | 40.50 |
| DMD VIQ | 35 | 1107 | 84.21 | 83.44 | 85.95 | 0.03 | 51.27 | 33.70 |
| BMD VIQ | 6 | 91 | 90.81 | 82.45 | 99.70 | 0.02 | 13.34 | 62.50 |
| DMD PIQ | 32 | 1075 | 88.82 | 87.55 | 91.05 | <0.01 | 58.88 | 47.30 |
| BMD PIQ | 6 | 91 | 89.18 | 77.93 | 95.83 | 0.02 | 14.02 | 64.30 |
| Cotton * FSIQ | 32 | 1145 | 80.20 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerkamp, P.M.M.; Mol, E.M.; Sweere, D.J.J.; Schrans, D.G.M.; Vermeulen, R.J.; Klinkenberg, S.; Hurks, P.P.M.; Hendriksen, J.G.M. Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis. Brain Sci. 2022, 12, 1544. https://doi.org/10.3390/brainsci12111544
Weerkamp PMM, Mol EM, Sweere DJJ, Schrans DGM, Vermeulen RJ, Klinkenberg S, Hurks PPM, Hendriksen JGM. Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis. Brain Sciences. 2022; 12(11):1544. https://doi.org/10.3390/brainsci12111544
Chicago/Turabian StyleWeerkamp, Pien M. M., Eva M. Mol, Dirk J. J. Sweere, Debby G. M. Schrans, R. Jeroen Vermeulen, Sylvia Klinkenberg, Petra P. M. Hurks, and Jos G. M. Hendriksen. 2022. "Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis" Brain Sciences 12, no. 11: 1544. https://doi.org/10.3390/brainsci12111544
APA StyleWeerkamp, P. M. M., Mol, E. M., Sweere, D. J. J., Schrans, D. G. M., Vermeulen, R. J., Klinkenberg, S., Hurks, P. P. M., & Hendriksen, J. G. M. (2022). Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis. Brain Sciences, 12(11), 1544. https://doi.org/10.3390/brainsci12111544






