Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson’s Disease: A Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Assessment of Quality of Literature
2.4. Data Selection
2.5. Statistical Analysis
3. Results
3.1. Search Results and Characteristics of Included Articles
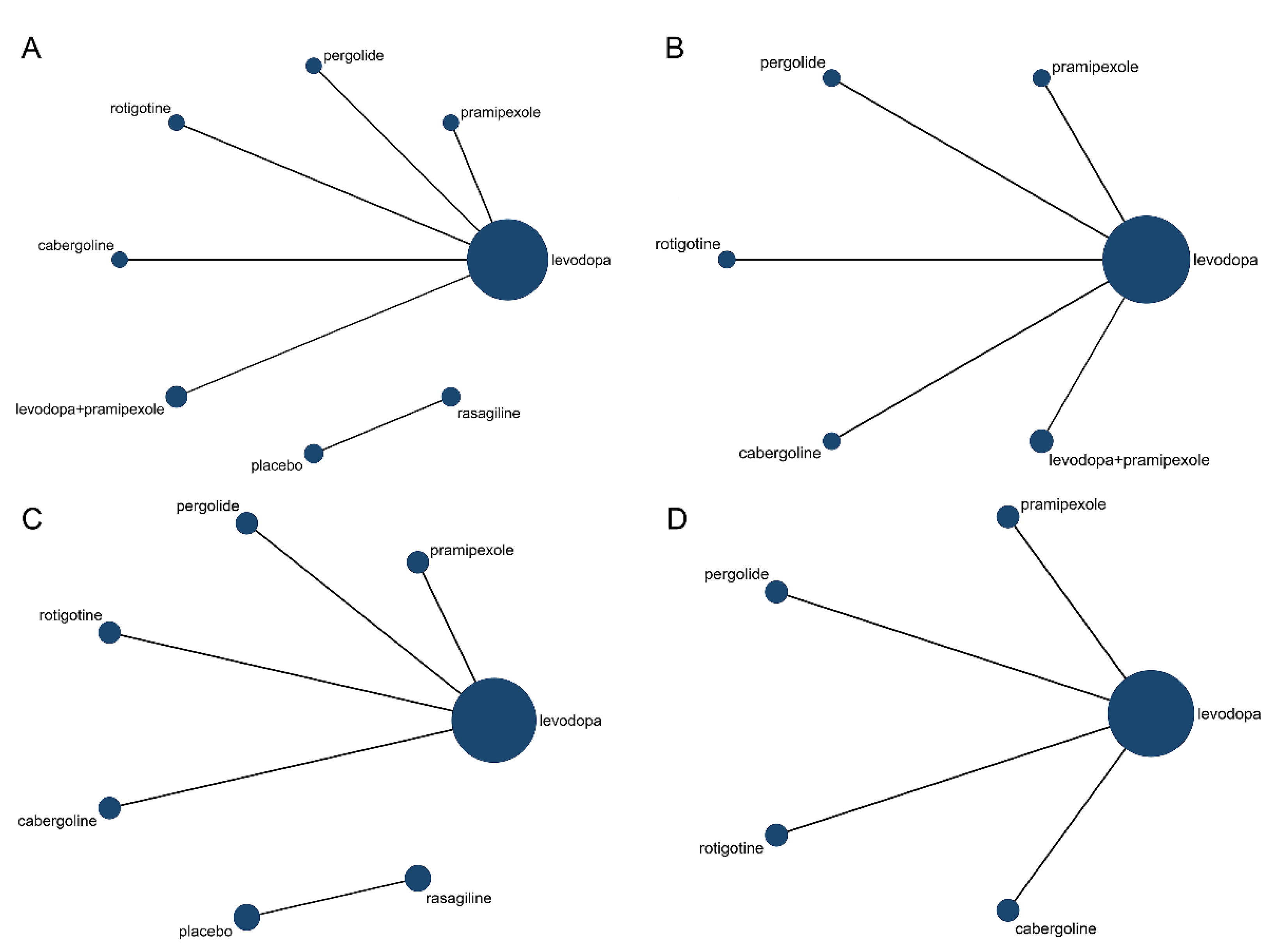
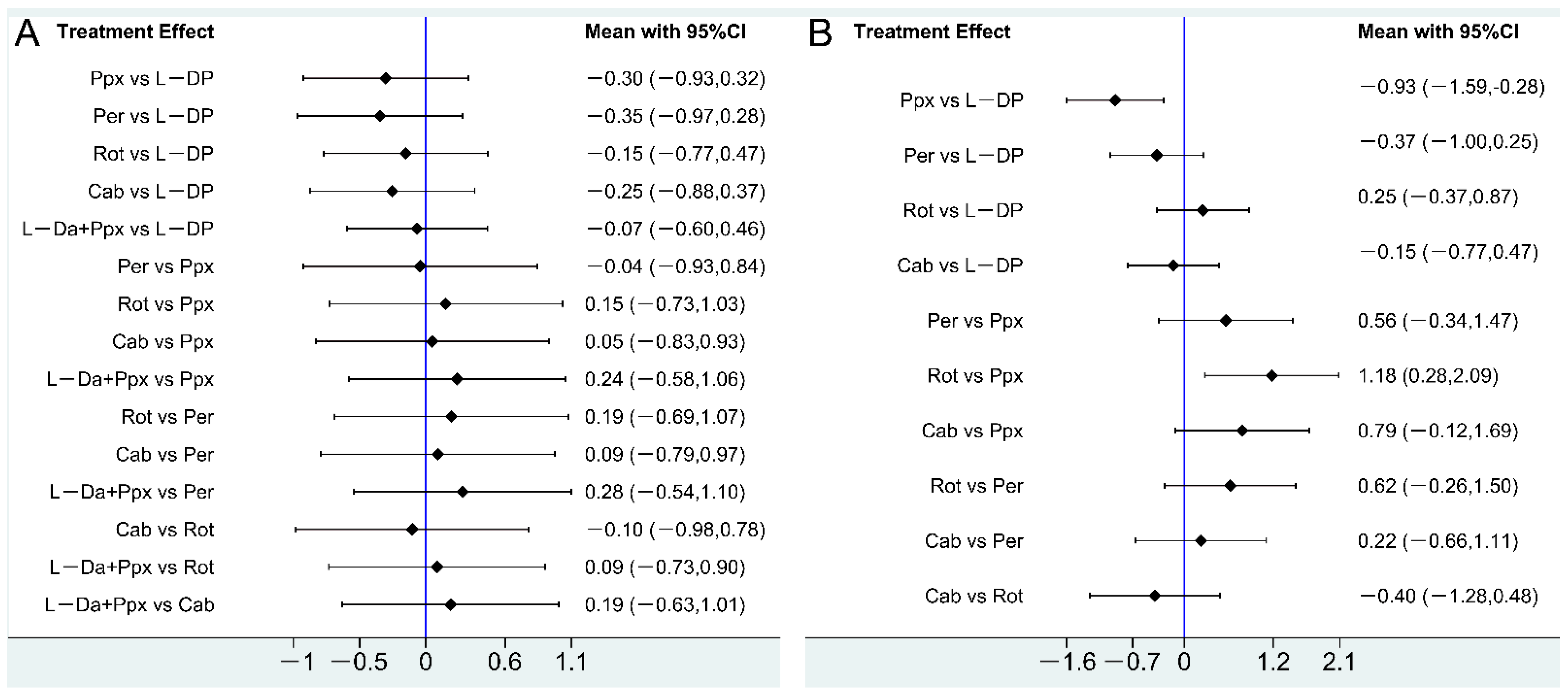
3.2. Effect of Drug Therapy on Letter Fluency in PD Patients
3.3. Effect of Drug Therapy on Semantic Fluency in PD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K.M.; Caplan, D.N. Communication Impairment in Parkinson’s Disease: Impact of Motor and Cognitive Symptoms on Speech and Language. Brain Lang. 2018, 185, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Allcock, L.; Jones, D.; Noble, E.; Hildreth, A.J.; Burn, D.J. Prevalence and Pattern of Perceived Intelligibility Changes in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Noble, E.; Jones, D.; Burn, D. Life with Communication Changes in Parkinson’s Disease. Age Ageing 2006, 35, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Miller, N. Communication Changes in Parkinson’s Disease. Pract. Neurol. 2017, 17, 266–274. [Google Scholar] [CrossRef]
- Dadgar, H.; Khatoonabadi, A.R. Verbal Fluency Performance in Patients with Non- Demented Parkinson’s Disease. Iran. J. Psychiatry 2013, 8, 55–58. [Google Scholar] [PubMed] [PubMed Central]
- Villalobos, D.; Torres-Simón, L.; Pacios, J.; Paúl, N.; del Río, D. A Systematic Review of Normative Data for Verbal Fluency Test in Different Languages. Neuropsychol. Rev. 2022. [Google Scholar] [CrossRef]
- Herrera, E.; Cuetos, F.; Ribacoba, R. Verbal Fluency in Parkinson’s Disease Patients on/off Dopamine Medication. Neuropsychologia 2012, 50, 3636–3640. [Google Scholar] [CrossRef]
- Piatt, A.L.; Fields, J.A.; Paolo, A.M.; Koller, W.C.; Tröster, A.I. Lexical, Semantic, and Action Verbal Fluency in Parkinson’s Disease with and without Dementia. J. Clin. Exp. Neuropsychol. 1999, 21, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Brucki, S.M.D.; Rocha, M.S.G. Category Fluency Test: Effects of Age, Gender and Education on Total Scores, Clustering and Switching in Brazilian Portuguese-Speaking Subjects. Braz. J. Med. Biol. Res. 2004, 37, 1771–1777. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. Verbal Fluency Deficits in Parkinson’s Disease: A Meta-Analysis. J. Int. Neuropsychol. Soc. 2004, 10, 608–622. [Google Scholar] [CrossRef]
- Lange, K.W.; Robbins, T.W.; Marsden, C.D.; James, M.; Owen, A.M.; Paul, G.M. L-Dopa Withdrawal in Parkinson’s Disease Selectively Impairs Cognitive Performance in Tests Sensitive to Frontal Lobe Dysfunction. Psychopharmacology 1992, 107, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Obeso, I.; Casabona, E.; Bringas, M.L.; Álvarez, L.; Jahanshahi, M. Semantic and Phonemic Verbal Fluency in Parkinson’s Disease: Influence of Clinical and Demographic Variables. Behav. Neurol. 2012, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Bayles, K.A.; Cruz, R.F.; Tomoeda, C.K.; Wood, J.A.; McGeagh, A.; Montgomery, E.B. Comparing the Difficulty of Letter, Semantic, and Name Fluency Tasks for Normal Elderly and Patients with Parkinson’s Disease. Neuropsychology 1997, 11, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Gotham, A.M.; Brown, R.G.; Marsden, C.D. ‘Frontal’ cognitive function in patients with parkinson’s disease ‘on’ and ‘off’ levodopa. Brain 1988, 111, 299–321. [Google Scholar] [CrossRef]
- Matison, R.; Mayeux, R.; Rosen, J.; Fahn, S. “Tip-of-the-Tongue” Phenomenon in Parkinson Disease. Neurology 1982, 32, 567. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. Evolution of Cognitive Dysfunction in an Incident Parkinson’s Disease Cohort. Brain 2007, 130, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Sawcer, S.J.; et al. The Distinct Cognitive Syndromes of Parkinson’s Disease: 5 Year Follow-up of the CamPaIGN Cohort. Brain 2009, 132, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Brusa, L.; Tiraboschi, P.; Koch, G.; Peppe, A.; Pierantozzi, M.; Ruggieri, S.; Stanzione, P. Pergolide Effect on Cognitive Functions in Early-Mild Parkinson’s Disease. J. Neural Transm. 2005, 112, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Brusa, L.; Bassi, A.; Stefani, A.; Pierantozzi, M.; Peppe, A.; Caramia, M.D.; Boffa, L.; Ruggieri, S.; Stanzione, P. Pramipexole in Comparison to L-Dopa: A Neuropsychological Study. J. Neural Transm. 2003, 110, 373–380. [Google Scholar] [CrossRef]
- Brusa, L.; Pavino, V.; Massimetti, M.C.; Bove, R.; Iani, C.; Stanzione, P. The Effect of Dopamine Agonists on Cognitive Functions in Non-Demented Early-Mild Parkinson’s Disease Patients. Funct. Neurol. 2013, 28, 13–17. [Google Scholar]
- Deck, B.L.; Rick, J.; Xie, S.X.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; Chahine, L.M.; Dahodwala, N.; Trojanowski, J.Q.; Weintraub, D. Statins and Cognition in Parkinson’s Disease. JPD 2017, 7, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Hanagasi, H.A.; Gurvit, H.; Unsalan, P.; Horozoglu, H.; Tuncer, N.; Feyzioglu, A.; Gunal, D.I.; Yener, G.G.; Cakmur, R.; Sahin, H.A.; et al. The Effects of Rasagiline on Cognitive Deficits in Parkinson’s Disease Patients without Dementia: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study: Rasagiline and Cognitive Deficits in PD. Mov. Disord. 2011, 26, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Relja, M.; Klepac, N. A Dopamine Agonist, Pramipexole, and Cognitive Functions in Parkinson’s Disease. J. Neurol. Sci. 2006, 248, 251–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of Non-Invasive Brain Stimulation on Cognitive Functioning in Brain Disorders: A Meta-Analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Altmann, L.J.P.; Stegemöller, E.; Hazamy, A.A.; Wilson, J.P.; Bowers, D.; Okun, M.S.; Hass, C.J. Aerobic Exercise Improves Mood, Cognition, and Language Function in Parkinson’s Disease: Results of a Controlled Study. J. Int. Neuropsychol. Soc. 2016, 22, 878–889. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep Brain Stimulation for Parkinson Disease: An Expert Consensus and Review of Key Issues. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef]
- Højlund, A.; Petersen, M.V.; Sridharan, K.S.; Østergaard, K. Worsening of Verbal Fluency after Deep Brain Stimulation in Parkinson’s Disease: A Focused Review. Comput. Struct. Biotechnol. J. 2017, 15, 68–74. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane, 2021. Available online: http://www.training.cochrane.org/handbook (accessed on 10 December 2021).
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available online: http://www.ncbi.nlm.nih.gov/books/NBK35156 (accessed on 10 December 2021).
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Perry, E.; Walker, M.; Grace, J.; Perry, R. Acetylcholine in Mind: A Neurotransmitter Correlate of Consciousness? Trends Neurosci. 1999, 22, 273–280. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, Y.; Manor, B.; Novak, P.; Milberg, W.; Zhang, J.; Fang, J.; Novak, V. Intranasal Insulin Enhanced Resting-State Functional Connectivity of Hippocampal Regions in Type 2 Diabetes. Diabetes 2015, 64, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Bak, T.H.; O’Donovan, D.G.; Xuereb, J.H.; Boniface, S.; Hodges, J.R. Selective Impairment of Verb Processing Associated with Pathological Changes in Brodmann Areas 44 and 45 in the Motor Neurone Disease–Dementia–Aphasia Syndrome. Brain 2001, 124, 103–120. [Google Scholar] [CrossRef]
- Damasio, A.R.; Tranel, D. Nouns and Verbs Are Retrieved with Differently Distributed Neural Systems. Proc. Natl. Acad. Sci. USA 1993, 90, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.A.; Moo, L.R.; Caramazza, A. Cortical Signatures of Noun and Verb Production. Proc. Natl. Acad. Sci. USA 2006, 103, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S.; Castner, S.A.; Svensson, T.H.; Siever, L.J.; Williams, G.V. Targeting the Dopamine D1 Receptor in Schizophrenia: Insights for Cognitive Dysfunction. Psychopharmacology 2004, 174, 3–16. [Google Scholar] [CrossRef]
- Xu, T.-X.; Sotnikova, T.D.; Liang, C.; Zhang, J.; Jung, J.U.; Spealman, R.D.; Gainetdinov, R.R.; Yao, W.-D. Hyperdopaminergic Tone Erodes Prefrontal Long-Term Potential via a D2 Receptor-Operated Protein Phosphatase Gate. J. Neurosci. 2009, 29, 14086–14099. [Google Scholar] [CrossRef]
- Leriche, L.; Bezard, E.; Gross, C.; Guillin, O.; Foll, B.; Diaz, J.; Sokoloff, P. The Dopamine D3 Receptor: A Therapeutic Target for the Treatment of Neuropsychiatric Disorders. CNSNDDT 2006, 5, 25–43. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Sibley, D.R. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, U.; Del Dotto, P.; Rascol, O. Role of Dopamine Receptor Agonists in the Treatment of Early Parkinson’s Disease. Parkinsonism Relat. Disord. 2009, 15, S44–S53. [Google Scholar] [CrossRef]
- Poletti, M.; Bonuccelli, U. Acute and Chronic Cognitive Effects of Levodopa and Dopamine Agonists on Patients with Parkinson’s Disease: A Review. Ther. Adv. Psychopharmacol. 2013, 3, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, B.; Pinborg, L.H.; Mortensen, E.L.; Friberg, L.; Baaré, W.F.C.; Gade, A.; Svarer, C.; Glenthøj, B.Y. Relationship of Frontal D2/3 Binding Potentials to Cognition: A Study of Antipsychotic-Naive Schizophrenia Patients. Int. J. Neuropsychopharmacol. 2013, 16, 23–36. [Google Scholar] [CrossRef][Green Version]
- Sharma, T.; Mockler, D. The Cognitive Efficacy of Atypical Antipsychotics in Schizophrenia. J. Clin. Psychopharmacol. 1998, 18, 12S–19S. [Google Scholar] [CrossRef]
- Cools, R.; Altamirano, L.; D’Esposito, M. Reversal Learning in Parkinson’s Disease Depends on Medication Status and Outcome Valence. Neuropsychologia 2006, 44, 1663–1673. [Google Scholar] [CrossRef]
- Kempler, D.; Teng, E.L.; Dick, M.; Taussig, I.M.; Davis, D.S. The Effects of Age, Education, and Ethnicity on Verbal Fluency. J. Int. Neuropsychol. Soc. 1998, 4, 531–538. [Google Scholar] [CrossRef]



| Study | Country | Study Design | Type of Interventions | Sample Size | Hoehn and Yahr Scale | Gender (Female/Male) | Age (Year) | Duration (Year) | Outcome Evaluation Index | Period of Treatment | Change of Scores after Drug Administration (Mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relja [23] 2006 | Croatia | RCT | levodopa | 25 | 2.3 ± 1 | 10/15 | 63.0 ± 12.7 | 5.4 ± 3.1 | Letter fluency | 6 months | −0.6 ± 7.95 |
| levodopa & pramipexole | 30 | 2.0 ± 0.8 | 13/17 | 61.7 ± 14.2 | 4.6 ± 4.8 | Letter fluency | 6 months | −1.2 ± 9.7 | |||
| Gotham [14] 1988 | UK | cross-sectional | levodopa | 15 | NA | NA | 64.4 ± 5.9 | 9.9 ± NA | Semantic fluency | On-Off | 1.72 ± 3.34 |
| Brusa [18] 2005 | Italy | cross-sectional | levodopa | 20 | ≤2.5 | 7/13 | 58 ± 7.83 | 2.6 ± 1.8 | Letter fluency Semantic fluency | 16 weeks | 6.25 ± 10.13 2.97 ± 5.6 |
| pergolide | 20 | ≤2.5 | 7/13 | 58 ± 7.83 | 2.6 ± 1.8 | Letter fluency Semantic fluency | 16 weeks | 2.63 ± 10.43 1.24 ± 3.22 | |||
| Brusa [19] 2003 | Italy | cross-sectional | levodopa | 20 | ≤2.5 | 7/13 | 57 ± 9.32 | 2.5 ± 1.3 | Letter fluency Semantic fluency | 4 months | 0.94 ± 9.22 2.33 ± 2.57 |
| pramipexole | 20 | ≤2.5 | 7/13 | 57 ± 9.32 | 2.5 ± 1.3 | Letter fluency Semantic fluency | 4 months | −1.93 ± 9.38 −0.73 ± 3.75 | |||
| Brusa [20] 2013 | Italy | RCT | levodopa | 20 | ≤2.5 | NA | 56 ± 5.63 | 2.3 ± 1.4 | Letter fluency Semantic fluency | 3 months | 6.71 ± 11.92 0.53 ± 5.85 |
| rotigotine | 20 | ≤2.5 | NA | 56 ± 5.63 | 2.3 ± 1.4 | Letter fluency Semantic fluency | 3 months | 5 ± 10.08 1.98 ± 5.51 | |||
| Brusa [20] 2013 | Italy | RCT | levodopa | 20 | ≤2.5 | NA | 57 ± 2.13 | 3.1 ± 0.5 | Letter fluency Semantic fluency | 3 months | 3.11 ± 9.69 0.79 ± 4.31 |
| cabergoline | 20 | ≤2.5 | NA | 57 ± 2.13 | 3.1 ± 0.5 | Letter fluency Semantic fluency | 3 months | 0.69 ± 9.01 0.22 ± 3.19 | |||
| Hanagasi [22] 2011 | Turkey | RCT | rasagiline | 23 | 2.00 ± 0.69 | 6/17 | 65.17 ± 9.5 | 4.09 ± 2.54 | Letter fluency Semantic fluency | 12 weeks | 3.14 ± 6.78 1.45 ± 4.44 |
| placebo | 25 | 1.64 ± 0.60 | 9/16 | 67.56 ± 10.13 | 3.96 ± 2.26 | Letter fluency Semantic fluency | 12 weeks | 0.52 ± 5.66 −0.72 ± 5.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, S.; Lai, H.; Mo, L.; Tan, C.; Liu, X.; Deng, F.; Chen, L. Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson’s Disease: A Network Meta-Analysis. Brain Sci. 2022, 12, 1496. https://doi.org/10.3390/brainsci12111496
Zhu Y, Li S, Lai H, Mo L, Tan C, Liu X, Deng F, Chen L. Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson’s Disease: A Network Meta-Analysis. Brain Sciences. 2022; 12(11):1496. https://doi.org/10.3390/brainsci12111496
Chicago/Turabian StyleZhu, Yuxia, Sichen Li, Hongyu Lai, Lijuan Mo, Changhong Tan, Xi Liu, Fen Deng, and Lifen Chen. 2022. "Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson’s Disease: A Network Meta-Analysis" Brain Sciences 12, no. 11: 1496. https://doi.org/10.3390/brainsci12111496
APA StyleZhu, Y., Li, S., Lai, H., Mo, L., Tan, C., Liu, X., Deng, F., & Chen, L. (2022). Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson’s Disease: A Network Meta-Analysis. Brain Sciences, 12(11), 1496. https://doi.org/10.3390/brainsci12111496






