The Application of Software “Rapid Processing of Perfusion and Diffusion” in Acute Ischemic Stroke
Abstract
1. Introduction
2. Development and Application of RAPID Software
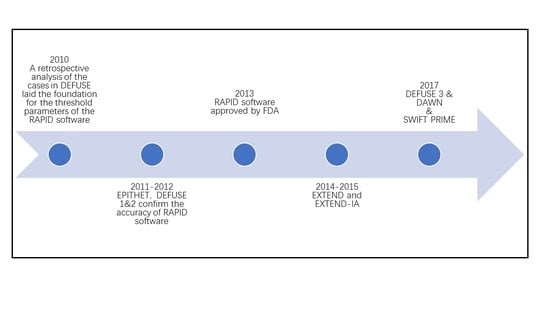
2.1. Background and Development of RAPID Software
2.2. Application of RAPID Software
2.3. Advantages and Limitations of RAPID Software
3. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
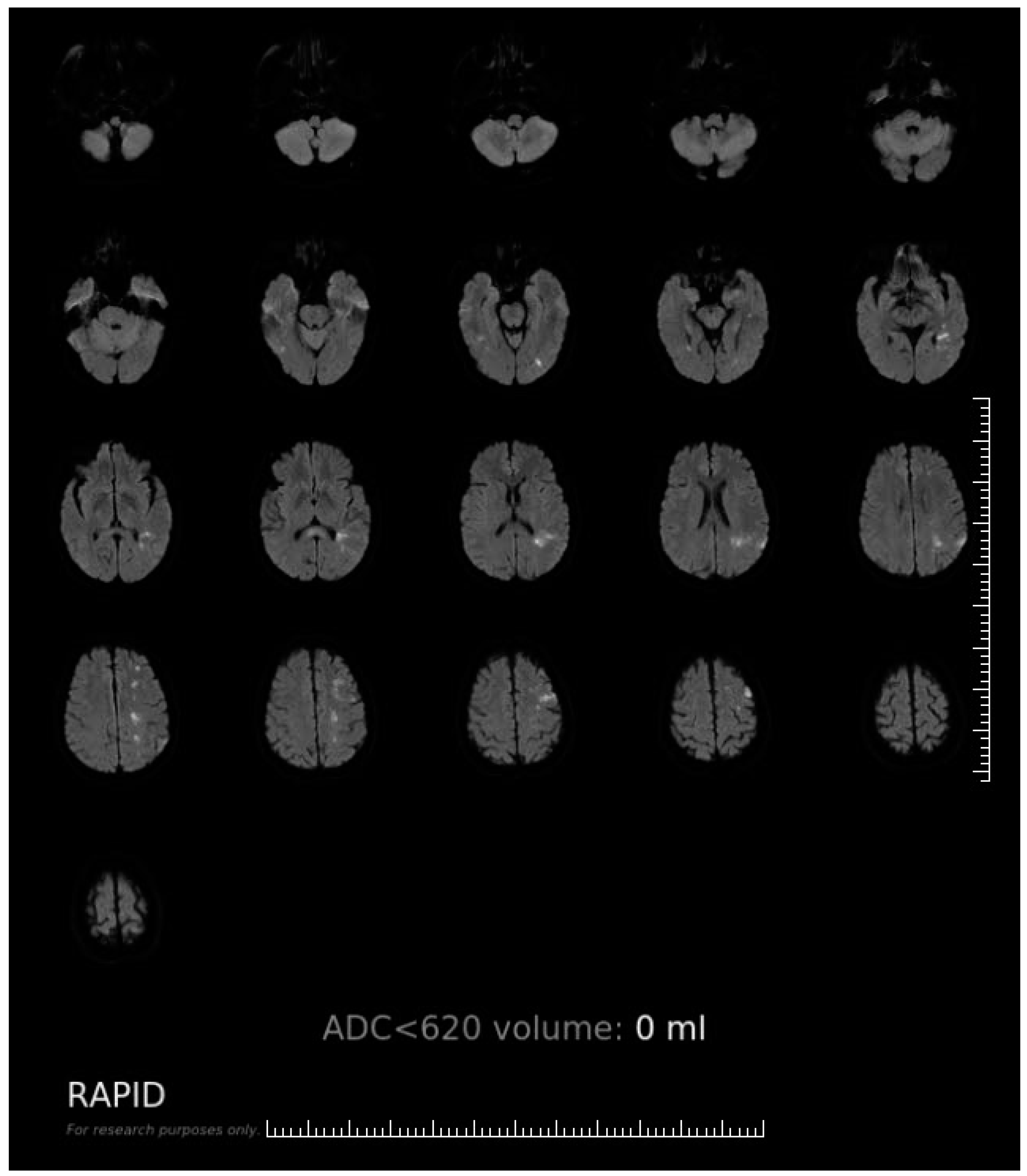
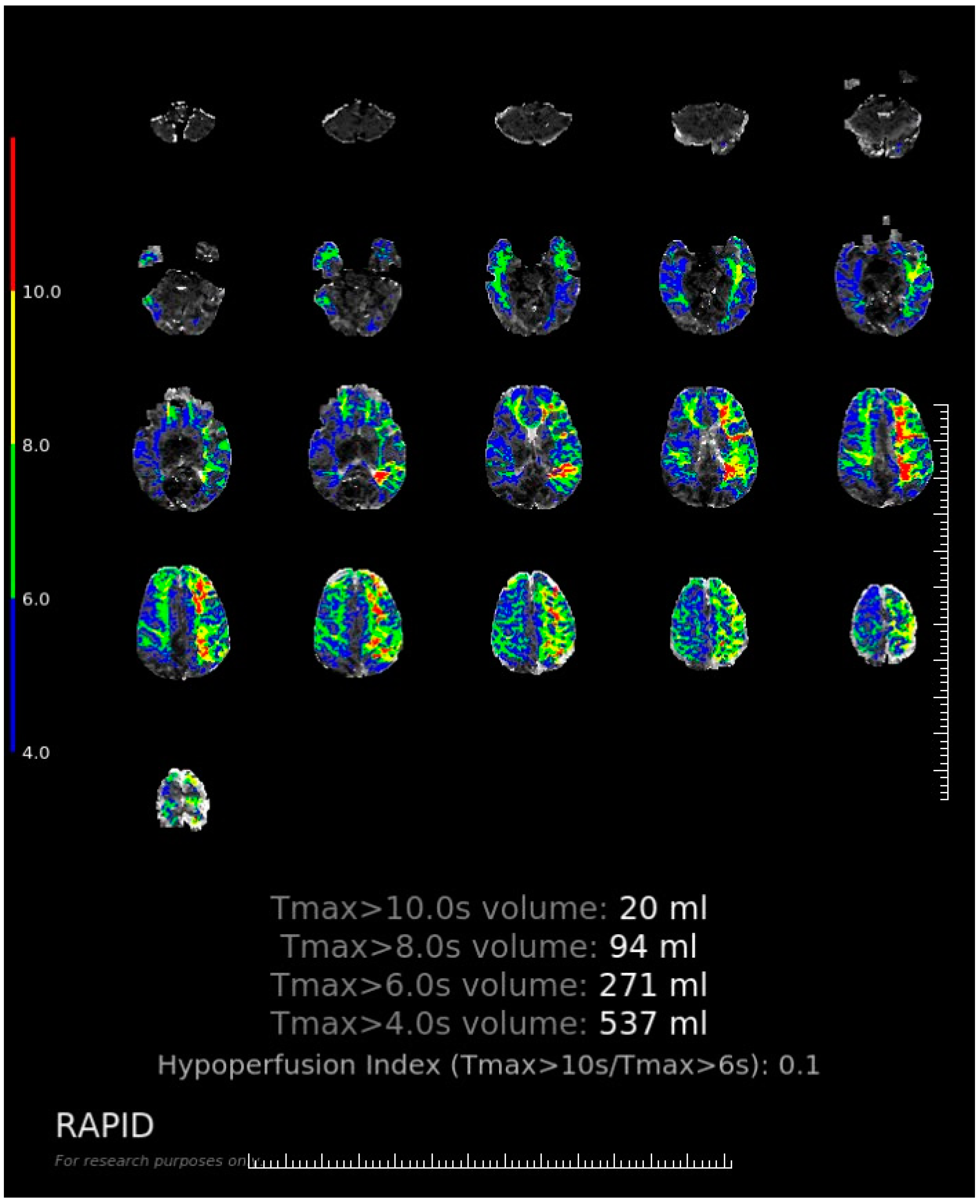
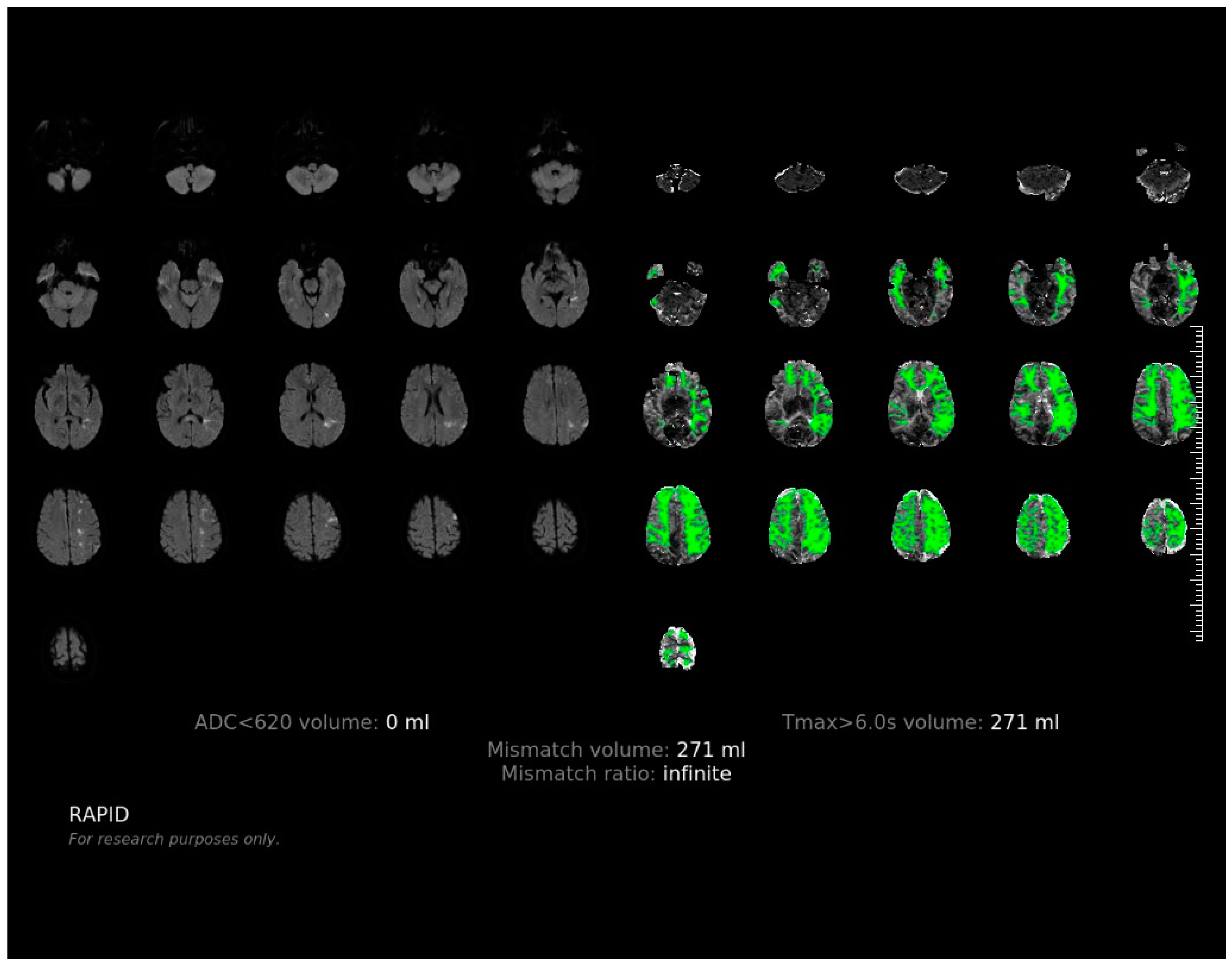
Appendix A. The Software Interface and Evaluating Procedure
References
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480,687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Baron, J.C.; Bousser, M.G.; Rey, A.; Guillard, A.; Comar, D.; Castaigne, P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke 1981, 12, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Thijs, V.N.; Wechsler, L.; Bs, S.K.; Schlaug, G.; Skalabrin, E.; Bammer, R.; Kakuda, W.; Lansberg, M.G.; Shuaib, A.; et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006, 60, 508–517. [Google Scholar] [CrossRef]
- Østergaard, L.; Weisskoff, R.M.; Chesler, D.A.; Gyldensted, C.; Rosen, B.R. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn. Reson. Med. 1996, 36, 715–725. [Google Scholar] [CrossRef]
- Østergaard, L.; Sorensen, A.G.; Kwong, K.K.; Weisskoff, R.M.; Gyldensted, C.; Rosen, B.R. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn. Reson. Med. 1996, 36, 726–736. [Google Scholar] [CrossRef]
- Straka, M.; Albers, G.W.; Bammer, R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J. Magn. Reson. Imaging 2010, 32, 1024–1037. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Lee, J.; Christensen, S.; Straka, M.; De Silva, D.A.; Mlynash, M.; Campbell, B.C.; Bammer, R.; Olivot, J.M.; Desmond, P.; et al. RAPID automated patient selection for reperfusion therapy: A pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 2011, 42, 1608–1614. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Straka, M.; Kemp, S.; Mlynash, M.; Wechsler, L.R.; Jovin, T.G.; Wilder, M.J.; Lutsep, H.L.; Czartoski, T.J.; A Bernstein, R.; et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012, 11, 860–867. [Google Scholar] [CrossRef]
- Campbell, B.C.; Yassi, N.; Ma, H.; Sharma, G.; Salinas, S.; Churilov, L.; Meretoja, A.; Parsons, M.W.; Desmond, P.M.; Lansberg, M.; et al. Imaging Selection in Ischemic Stroke: Feasibility of Automated CT-Perfusion Analysis. Int. J. Stroke 2015, 10, 51–54. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Mitchell, P.J.; Yan, B.; Parsons, M.W.; Christensen, S.; Churilov, L.; Dowling, R.; Dewey, H.; Brooks, M.; Miteff, F.; et al. A Multicenter, Randomized, Controlled Study to Investigate Extending the Time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial Therapy (EXTEND-IA). Int. J. Stroke 2014, 9, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Lansberg, M.G.; Kemp, S.; Tsai, J.P.; Lavori, P.; Christensen, S.; Mlynash, M.; Kim, S.; Hamilton, S.; Yeatts, S.D.; et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int. J. Stroke 2017, 12, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Saver, J.L.; Ribo, M.; Pereira, V.; Furlan, A.; Bonafe, A.; Baxter, B.; Gupta, R.; Lopes, D.; Jansen, O.; et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int. J. Stroke 2017, 12, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Levy, E.I.; Saver, J.L.; Siddiqui, A.H.; Goyal, M.; Bonafé, A.; Cognard, C.; Jahan, R.; Albers, G.W. Predictive Value of RAPID Assessed Perfusion Thresholds on Final Infarct Volume in SWIFT PRIME (Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke 2017, 48, 932–938. [Google Scholar] [CrossRef]
- Lin, L.; Bivard, A.; Levi, C.R.; Parsons, M.W. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: Back-to-back quantitative analysis. Stroke 2014, 45, 1727–1732. [Google Scholar] [CrossRef]
- Albers, G.W.; Goyal, M.; Jahan, R.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann. Neurol. 2016, 79, 76–89. [Google Scholar] [CrossRef]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Kemp, S.; Lansberg, M.; Wechsler, L.; Bammer, R.; Marks, M.P.; Albers, G.W. Optimal Tmax Threshold for Predicting Penumbral Tissue in Acute Stroke. Stroke 2009, 40, 469–475. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Saver, J.; Starkman, S.; Duckwiler, G.; Jahan, R.; Vespa, P.; Villablanca, J.P.; Liebeskind, D.S.; Gobin, Y.P.; Vinuela, F.; et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann. Neurol. 2002, 52, 698–703. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Hunter, G.J.; He, J.; Hamberg, L.M.; Sorensen, A.G.; Schwamm, L.H.; Koroshetz, W.J.; Gonzalez, R.G. Predicting Cerebral Ischemic Infarct Volume with Diffusion and Perfusion MR Imaging. Am. J. Neuroradiol. 2002, 23, 1785–1794. [Google Scholar]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Cereda, C.W.; Christensen, S.; Campbell, B.C.V.; Mishra, N.; Mlynash, M.; Levi, C.; Straka, M.; Wintermark, M.; Bammer, R.; Albers, G.W.; et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J. Cereb. Blood Flow Metab. 2016, 36, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Campbell, B.C.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Campbell, B.; Straka, M.; Mlynash, M.; Olivot, J.-M.; Bammer, R.; Kemp, S.M.; Albers, G.W.; Lansberg, M. Apparent Diffusion Coefficient Threshold for Delineation of Ischemic Core. Int. J. Stroke 2015, 10, 348–353. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Dowling, R.J.; Yan, B.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018, 378, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.A.; Snyder, K.V.; Mokin, M.; Waqas, M.; Podgorsak, A.R.; Allman, A.B.; Senko, J.; Bhurwani, M.M.S.; Hoi, Y.; Davies, J.M.; et al. Enhancing performance of a computed tomography perfusion software for improved prediction of final infarct volume in acute ischemic stroke patients. Neuroradiol. J. 2021, 34, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.A.; Snyder, K.V.; Mokin, M.; Waqas, M.; Zhang, X.; Podgorsak, A.R.; Allman, A.B.; Senko, J.; Bhurwani, M.M.S.; Hoi, Y.; et al. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: A comparison of Sphere, Vitrea, and RAPID. J. Neurointerv. Surg. 2021, 13, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, W.; Zhou, H.; Duan, W.; Li, S.; Huo, X.; Xu, W.; Huang, L.; Zheng, H.; Liu, J.; et al. Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc. Neurol. 2020, 5, 159–176. [Google Scholar] [CrossRef]
- Pérez-Pelegrí, M.; Biarnés, C.; Thió-Henestrosa, S.; Remollo, S.; Gimeno, A.; Cuba, V.; Teceño, M.; Martí-Navas, M.; Serena, J.; Pedraza, S.; et al. Higher agreement in endovascular treatment decision-making than in parametric quantifications among automated CT perfusion software packages in acute ischemic stroke. J. X-ray Sci. Technol. 2021, 29, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, W.-H.A.; Wang, Y.-X.J.; Wang, C.; Zhao, F.; Qi, W.; Chan, W.M.; Huang, Y.; Wai, M.S.; Dong, J.; et al. DL-3-n-Butylphthalide, an anti-oxidant agent, prevents neurological deficits and cerebral injury following stroke per functional analysis, magnetic resonance imaging and histological assessment. Curr. Neurovascular Res. 2012, 9, 167–175. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Zhang, L.; Ying, Z.; Sha, O.; Li, C.; Lü, L.; Chen, X.; Li, Z.; Niu, F.; et al. The novel targets of DL-3-n-butylphthalide predicted by similarity ensemble approach in combination with molecular docking study. Quant. Imaging Med. Surg. 2017, 7, 532–536. [Google Scholar] [CrossRef]
- Chen, D.; Yin, Y.; Shi, J.; Yang, F.; Wang, K.; Zhao, F.; Li, W.; Li, B. DL-3-n-butylphthalide improves cerebral hypoperfusion in patients with large cerebral atherosclerotic stenosis: A single-center, randomized, double-blind, placebo-controlled study. BMC Neurol. 2020, 20, 212. [Google Scholar] [CrossRef] [PubMed]
| Time | Researcher | Study | When to Apply Rapid Software | Parameters Set by Rapid Software | Conclusions | ||
|---|---|---|---|---|---|---|---|
| Hypoperfusion | Ischemic Core | Mismatch | |||||
| 2006 [4] | Albers GW et al. | DEFUSE | MRI scan was obtained 3 to 6 h after treatment with intravenous tPA 3 to 6 h after symptom onset | PWI Tmax>2s | Determined by the semi-automated thresholding method (exceeding the DWI signal intensity of the contralateral hemisphere by more than three standard deviations) | Vh ≥ 10 mL Vh/Vi ≥ 1.2 | For tPA-treated patients with a mismatch, especially target mismatch, there was a strong and highly significant association between early reperfusion and favorable clinical outcomes |
| 2010 [7] | Straka M et al. | Review DEFUSE | Use RAPID software to analyze cases in DEFUSE | PWI Tmax>6s VP6 ≥ 3 mL | ADCth: 600 × 10−6 mm2/s Vi ≥ 1 mL | Vh−Vi ≥ 10 mL Vh/Vi ≥ 1.2 | Developed RAPID software, which can automatically segment and calculate lesion volume on PWI and DWI scans. The software has consistent results compared with a human reader |
| 2011 [8] | Lansberg MG et al. | Review EPITHET and DEFUSE | Use RAPID software to analyze cases in EPITHET and DEFUSE (two studies of intravenous tPA administered in the 3 to 6 h time window). | PWI Tmax>6s VP6 ≥ 10 mL VP8 ≤ 100 mL | 10 mL ≤ Vi ≤ 100 mL | VP6−Vi ≥ 10 mL VP6/Vi ≥ 1.2 | Comparing RAPID with processing methods used in previous stroke studies confirms that RAPID software can be used for patients with acute cerebral infarctions to rapidly select target patients |
| 2012 [9] | Lansberg MG et al. | DEFUSE 2 | Baseline MRI scan was obtained before treatment of patients. Endovascular treatment within 12 h of onset of stroke, followed by analysis with RAPID software to establish whether the patient has a mismatch | PWI Tmax>6s VP10 ≤ 100 mL | ADCth: 600 × 10−6 mm2/s | Vh/Vi ≥ 1.8 Vh−Vi ≥ 15 mL Vi < 70 mL | For endovascular-treated patients with the target mismatches, there was a strong and highly significant association between early reperfusion and favorable clinical outcomes |
| 2014 [11] | Campbell BC et al. | EXTEND-IA | Baseline MRI or multimodal CT scan was obtained before or immediately after treatment of patients commencing intravenous tPA within 4.5 h of onset of anterior circulation ischemic stroke, followed by analysis with RAPID software to establish whether the patient has a mismatch | CTP Tmax>6s or PWI Tmax>6s | Diffusion lesion or rCBF decrease < 30% or ADCth: 620 × 10−6 mm2/s | Vh/Vi > 1.2 Vh−Vi > 10 mL Vi < 70 mL | For patients within a 4.5 h onset of anterior circulation ischemic stroke with the target mismatch, treatment with intra-arterial clot retrieval after intravenous tPA improved reperfusion and early nervous system compared with intravenous tPA alone |
| 2015 [10] | Campbell BC et al. | part of the EXTEND (tPA/placebo 4.5–9 h poststroke) and EXTEND-IA (tPA < 4.5 h ± thrombectomy) | Patients presenting < 9 h from stroke had CT, CTP, or CTA, followed by analysis with RAPID software to establish whether the patient has a mismatch | CTP Tmax>6s | rCBF < 30% | Vh/Vi > 1.2 Vh−Vi > 10 mL Vi < 70 mL | CTP and perfusion–diffusion MRI data were processed using RAPID software to generate a ‘mismatch’ classification that determined eligibility for trial therapies |
| 2017 [12] | Albers GW et al. | DEFUSE 3 | Baseline MRI or multimodal CT scans were obtained before treatment of patients. Endovascular treatment within 6–16 h of onset of ICA or MCA occlusion, followed by analysis with RAPID software to establish whether the patient has a mismatch | PWI Tmax>6s VP10 ≤ 100 mL | ADCth: 600 × 10−6 mm2/s | Vh/Vi ≥ 1.8 Vh−Vi ≥ 15 mL Vi < 70 mL | For patients with ICA or MCA occlusions and target mismatches on multimodal CT or MR imaging, endovascular therapy in a time window of 6 to 16 h may be beneficial |
| 2017 [13] | Jovin TG et al. | DAWN | Baseline MRI or multimodal CT scan was obtained before treatment of patients. Endovascular treatment within 6–24 h from TLSW, followed by analysis with RAPID software to evaluate ischemic core size | Use of NIHSS as an indicator of tissue at risk in lieu of perfusion studies (CCM) | Age ≥ 80 y, Vi < 21 cm3; Age < 80 y, Vi < 31 cm3; Age < 80 y, 31 ≤ Vi ≤ 51 cm3 | Age ≥ 80 year, NIHSS ≥ 10, Vi < 21 cm3; Age < 80 year, NIHSS ≥ 10, Vi < 31 cm3; Age < 80 year, NIHSS ≥ 20, 31 ≤ Vi ≤ 51 cm3 | Compared with subjects treated with standard medical therapy alone, there were better outcomes and substantial areas of salvageable brain based on age-adjusted clinical core mismatches of patients who could experience endovascular treatment within 6–24 h from TLSW |
| 2017 [14] | Mokin M | Review SWIFT PRIME | Use RAPID software to analyze cases of both intravenous tPA only and endovascular treatment in DEFUSE | VP10 ≤ 100 mL | rCBF < 30% | Vh/Vi ≥ 1.8 Vh-Vi ≥ 15 mL Vi ≤ 50 mL | The most accurate thresholds for predicting the final 27 h infarct volume were rCBF 0.30~0.34 or rCBV 0.32~0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Song, S.; Li, Z.; Huang, B.; Geng, Y.; Zhang, L. The Application of Software “Rapid Processing of Perfusion and Diffusion” in Acute Ischemic Stroke. Brain Sci. 2022, 12, 1451. https://doi.org/10.3390/brainsci12111451
Zhang Y, Song S, Li Z, Huang B, Geng Y, Zhang L. The Application of Software “Rapid Processing of Perfusion and Diffusion” in Acute Ischemic Stroke. Brain Sciences. 2022; 12(11):1451. https://doi.org/10.3390/brainsci12111451
Chicago/Turabian StyleZhang, Yudi, Shuang Song, Zhenzhong Li, Boyuan Huang, Yanlu Geng, and Lihong Zhang. 2022. "The Application of Software “Rapid Processing of Perfusion and Diffusion” in Acute Ischemic Stroke" Brain Sciences 12, no. 11: 1451. https://doi.org/10.3390/brainsci12111451
APA StyleZhang, Y., Song, S., Li, Z., Huang, B., Geng, Y., & Zhang, L. (2022). The Application of Software “Rapid Processing of Perfusion and Diffusion” in Acute Ischemic Stroke. Brain Sciences, 12(11), 1451. https://doi.org/10.3390/brainsci12111451