Abstract
We evaluated the association between cardiorespiratory fitness (CRF) and executive function (EF) in young adults and the mediating effects of GMV on this relationship. This study involved 217 college students. An incremental load exercise program was used to evaluate VO2max. EF was estimated by the Flanker task, the 2-back task, and the more-odd shifting task, while structural magnetic resonance and region-based morphometry (RBM) were used to analyze GMV. The high CRF group had a shorter updating reaction time (RT) (p ≤ 0.05). CRF was positively correlated with the right orbital part of the middle frontal gyrus (ORBmid.R) GMV (p ≤ 0.05). ORBmid.R GMV was negatively correlated with updating RT (p ≤ 0.05). Model 4 in SPSS was used to assess the mediating effects of ORBmid.R GMV between CRF and updating RT. ORBmid.R GMV was established to have a partially mediating role between CRF and updating RT, which accounted for 19.6% of the total effect value. These findings indicate that the negative correlation between CRF and EF was significant, and ORBmid.R GMV played a mediating role in the relationship between CRF and EF, providing new evidence toward comprehensively revealing that CRF promotes EF performance.
1. Introduction
Throughout life, brain and cognition health among adolescents and young adults can influence academic achievement and overall health [1,2], which necessitates the identification of the predictors and modifiers of brain health at a young age [3]. Due to advances in the social economy, there has been a growing interest in the influence of lifestyle factors, such as regular physical exercise, in the promotion of a relatively high level of cardiorespiratory fitness (CRF) and health (e.g., brain health) [2,4,5,6]. CRF is operationalized by maximal oxygen consumption (VO2max) and is correlated with the functions of different physiological systems [7,8,9]. It affects the development of executive function (EF). Increasing physical activity levels and improving CRF are beneficial for the healthy development of EF [10,11]. Low levels of CRF in early adulthood are associated with higher risks of cardiovascular disease in late life [12,13,14] and negatively affect psychological functions (resulting in depression and anxiety) and EF, becoming evident in accelerated cognitive decline and brain atrophy in later years [15,16,17,18,19].
Physiologically, EF plays a pivotal role in cognitive functions [20], including: (i) inhibition (i.e., resisting habits, temptations, or distractions); (ii) updating (i.e., retaining and using information); and (iii) cognitive flexibility [21,22]. Various neuropsychological paradigms, such as the Flanker task, the 2-back task, and the more-odd shifting task, can be used to measure the performance of EF. Improvements in CRF have been shown to effectively enhance children’s working memory, further modifying their cognitive flexibility [23,24,25] and optimizing inhibition control among the elderly to slow down the degradation of visuospatial memory functions due to aging [26,27,28]. High CRF and physical activity (PA) levels have been consistently associated with the maintenance of cognitive functions in life, including a reduced risk of developing Alzheimer’s disease and a slow progression of cognitive problems in cognitively impaired patients [29,30,31].
However, the relationship between CRF and EF among adults has not been conclusively established [32]. Findings from previous studies [33,34,35] are inconsistent, which may be due to the big age gap among study participants. Therefore, it is necessary to comprehensively assess the relationship between CRF and the three sub-functions of young adults’ EF.
Advances in brain imaging technologies have facilitated the evaluation of underlying physiological mechanisms involved in the relationship between CRF and EF. Structural plasticity features of the brain mediate this relationship. At any age, CRF has important effects on human brain health [36] and acts as a protective factor against gray matter atrophy among the elderly [32,37,38,39,40,41]. There is a positive association between CRF and gray matter volume (GMV), especially among the elderly [42,43,44,45,46,47]. Elevated levels of CRF are associated with increased hippocampal and prefrontal cortex volume as well as better cognitive performance among the elderly [48]. CRF and cortical volume (i.e., frontal cortex), as well as EF (i.e., updating), are positively correlated among children [47,49,50].
College students are in the early adulthood phase in which the GMV in each brain area gradually increases and can effectively predict the development of its EF. The larger the GMV in the frontal lobe region, the better the EF [51]. Most studies have focused on children and older adults, while younger adults have not been assessed [52]. The relationships between CRF, brain structure, and EF, which have not been fully described in younger adults, are necessary for the assessment of exercise–cognition interactions [53]. We hypothesized that GMV plays an intermediary role in the influence of CRF on EF.
Based on the available scientific evidence, our hypotheses are: GMVs are used as intermediary variables for the effect of CRF on EF to construct an intermediary mode. We reveal that GMV is a potential neural pathway through which CRF affects an individual’s EF and provides a new perspective for the comprehensive understanding of the relationship between CRF and EF.
2. Materials and Methods
2.1. Participants
This study involved 221 freshmen aged between 18–20 years from Yangzhou University. The participants came from comparable sociocultural environments and followed a commonly prescribed syllabus as well as examination evaluation patterns. Before testing, participants were only asked to indicate whether they had sports habits rather than specific sports events and durations in their lives. The inclusion criteria were: (i) no history of mental or genetic disorders; (ii) test visual acuity or corrected visual acuity > 0.8, no color blindness or color deficiency; (iii) no serious physical illness, no history of brain trauma or nervous system disease, and no history of drug and alcohol dependence or other diseases that may affect the structure and function of the brain; (iv) right-handed; (v) college students with abnormal intelligence, as revealed by Raven’s Standard Progressive Matrices (SPM) test, were excluded; (vi) participants who met the conditions for magnetic resonance scanning, such as the absence of implanted metals (including metal dentures, etc.) and electronic, magnetic, or mechanical equipment (such as pacemakers) in the body. According to the above criteria, 4 participants were knocked out (3 males with MRI data missing and 1 female who exited). Our pooled dataset eventually included 217 participants (including 98 males and 119 females). The experiment was conducted in Yangzhou, China, with approval from the Ethical and Human Protection Committee of the Affiliated Hospital of Yangzhou University (2017-YKL045-01). Participants signed an informed consent form. All study procedures were in accordance with the latest version of the Declaration of Helsinki.
2.2. Cardiorespiratory Fitness Testing
VO2max was tested using an increasing load exercise protocol [54], i.e., CRF. Elmed EGT 1000 was used to increase the load, while VO2max was measured using the Cortex Metalyze R-II benchtop gas metabolism analyzer (Germany). The increasing load exercise scheme was as follows: starting load was 50 w, treadmill rhythm was 55~60 r/min, and increment was 50 w every 3 min until exhaustion.
Before the test, all participants completed a physical activity preparation questionnaire to ensure that they had not performed high-intensity exercises the day before nor taken drugs or drinks that were stimulating or inhibiting the nerves. They were prepared for 3 to 5 min before the formal test to prevent sports-associated injuries. The basal heart rate was measured, after which exercises were started when the heart rate returned to a quiet state. A polar heart rate band was used to monitor changes in heart rate during the exercise. All participants who met any of the following four indicators achieved VO2max: (i) with increasing load, oxygen uptake remained unchanged or slightly decreased (1500 mL/min); (ii) respiratory quotient >1.1; (iii) HR > 180 b/min; (iv) despite repeated encouragement, participants could not maintain a cycling rate of 55 to 60 r/min. After the completion of the test, VO2max was recorded, followed by resting for about 5 min. In the case that the participants’ bodies had no abnormal reactions, they were left alone. CRF was obtained by multiplying the VO2max value (L/kg/min) by 1000 and dividing it by body weight (kg).
As previously reported [55], participants with CRF < 30% were assigned to the low CRF group (65 people; 29 males and 36 females), while those with CRF > 70% were assigned to the high CRF group (65 people; 39 males and 26 females).
2.3. Executive Function Assessment
In this study, the Flanker task, the 2-back task, and the more-odd shifting task were used to evaluate updating, inhibition, and cognitive flexibility, respectively. The test tool has high reliability and validity and is unanimously recognized by peer experts [25]. Test indices were reaction time/ms and accuracy rate/%. The shorter the reaction time, the higher the operation efficiency of the function. The higher the accuracy rate, the better the function performance.
2.3.1. Flanker Task
The Flanker task was assessed by modified Eriksen [25]. In brief, a range of English letters appeared on the screen under congruent or incongruent conditions. Participants needed to discriminate the letter in the middle of the screen as soon as they could by pressing the “F” or “L” keys. The two conditions were equally represented and randomly presented. The test was made of two parts, and each part contained 48 trials, in which the duration of letter presentation was 1000 ms, the stimulation interval was 2000 ms, and the maximal reaction time was 2000 ms. If participants did not finish within 2000 ms, the trial RT was still recorded as 2000 ms.
2.3.2. 2-Back Task
The 2-back task [56,57,58] was designed to assess updating. Briefly, a series of numbers would appear on the screen (i.e., 1, 2, 3, and 4). Each 2-back test was composed of 13 figures in a random sequential lineup. The participants were asked to remember the second and third numerals in the sequence of appearance. When the fourth stimulus appeared, they needed to judge whether it was the same as the second by pressing the “A” key for yes or the “B” key for no with both hands on the keyboard. The stimulation interval was 2000 ms and the maximal reaction time was 2000 ms. If participants did not finish within 2000 ms, the trial RT was still recorded as 2000 ms.
2.3.3. More-Odd Shifting Task
The more-odd shifting task was assessed by modified Hambrick [59]. In a nutshell, a series of numbers from either 1 to 4 or 6 to 9 would appear on the screen. Each more-odd shifting task consisted of 3 parts. The A part involved 16 homogeneous trials in which the numbers were printed on the screen. Participants used their left or right finger to indicate whether the presented number was greater than or less than 5 by pressing the “F” or “L” keys, respectively. The B part also involved 16 homogeneous trials in which green numbers were presented on the screen. Participants used their left or right finger to indicate whether the presented number was odd or even by pressing the “F” or “L” keys, respectively. The C part involved 32 homogeneous trials in which the numbers printed on the screen were from both the A and B trials. Participants needed to identify if the presented number was greater or less than 5 in black and if the presented number was odd or even in green. Pressing the wrong button and failing to respond within 150–1000 ms for homogeneous trials or within 300–1500 ms for heterogeneous trials were considered incorrect responses.
The stimulation interval was 2000 ms, and the stimulus-onset asynchrony was 2000 ms. The shifting index used in this study was the global switch cost, which was calculated as differences in response time between heterogeneous (i.e., the average of the C parts) and homogeneous (i.e., the average of the A and B parts) blocks.
2.4. MRI Data Acquisition
2.4.1. T1-Weighted Image Data Acquisition
GE Discovery MR750W 3.0 T magnetic resonance imaging was used for image acquisition. The T1-MPRAGE sequence structure image scan parameters were: TR/TE = 7.20/3.06 ms, TI = 450 ms, slice thickness = 1.00 mm, flip angle = 12°, acquisition matrix = 256 × 256, and field of view = 256 × 256 mm.
2.4.2. GMV Data Pre-Processing
The MRI data were processed using SPM12 implemented in MATLAB. SPM12 was originally used to analyze the MRIs obtained from all participants and gross anatomical abnormalities and to exclude artifacts. The MRIs were artificially adjusted to the anterior commissure to raise registration. Then, every MRI was divided into 3 parts (i.e., gray matter, white matter, and cerebrospinal fluid) by employing the toolbox function [56]. Finally, an 8 mm full-width-at-half-maximum Gaussian kernel was used to smooth the regulated images and to enhance the quality of the signal-to-noise ratio. The rex plug-in was used to extract the GMV of significantly changed areas. The threshold was set at p < 0.05, the voxel threshold at p < 0.01, and the cluster size at >50 voxels with FDR correction.
2.5. Statistical Analysis
The Statistical Package for Social Sciences (SPSS; SPSS Inc., Chicago, IL, USA) version 26.0 for Windows was used for the analyses. The chi-square test and the independent sample t-test were used to compare differences in the demographic variables and the CRF and EF of the high and low CRF groups, while a multivariate analysis of variance (MANOVA) was used to investigate differences between GMVs of the two groups; on this basis, brain regions with significant differences in GMV were further assessed. Using partial correlation analysis to control gender, age, and BMI, correlations between CRF, GMV (brain area with significant differences), and EF were used in process plug-in Model 4 to test the mediating effects of GMV on the relationship between CRF and EF. The deviation-corrected percentile bootstrap method was used to estimate 95% confidence intervals of the mediating effects by sampling 5000 bootstrap samples. p ≤ 0.05 was the threshold for significance.
3. Results
3.1. Demographics, CRF, and EF of the Two Groups
The gender, age, and BMI of college students affect their EF [57,58]. Therefore, we controlled for the above variables and the gender of the two groups (χ2 = 0.050, p > 0.05). The independent sample t-test was used to analyze differences in age, BMI, VO2max, relative VO2max, and EF subdomain. The obtained results indicated that there were significant differences in BMI (t(128) = 3.443, p ≤ 0.001), VO2max (t(128) = −19.587, p ≤ 0.001), relative VO2max (t(128) = −34.014, p ≤ 0.001), inhibition RT (t(128) = 2.356, p ≤ 0.05), updating RT (t(128) = 2.473, p ≤ 0.05), cognitive flexibility RT (t(128) = 6.514, p ≤ 0.001), and updating accuracy rate (t(128) = −2.344, p ≤ 0.05) between the two groups. However, there were insignificant differences in the inhibition accuracy rate (t(128) = −0.798, p > 0.05) and cognitive flexibility accuracy rate (t(128) = −0.693, p > 0.05), indicating that the high CRF was only better in updating (Table 1). The Kolmogorov–Smirnov test was used to assess normal distribution in sub-EF RT (p > 0.05). The numbers over third standard deviations were regarded as outliers [59]. The split-half reliability test also showed the significant trustworthiness of the Flanker task (r = 0.964) and the more-odd shifting task (r = 0.666).

Table 1.
Participants’ demographics, cardiorespiratory fitness, and executive function (M ± SD).
3.2. Effects of CRF on the GMV of the Two Groups
The stochastic effect model of SPM12 was used to analyze the GMV of the two groups and to explore the relationship between CRF and GMV. The obtained results indicated that there were significant differences in the right frontal-mid-orb (ORBmid.R), right parahippocampal (PHG.R), left caudate (CAU.L), left putamen (PUT.L), right putamen (PUT.R), left thalamus (THA.L), and right thalamus (THA.R) (Table 2, Figure 1). All results were corrected by FDR (p = 0.05).

Table 2.
Significant changes in GMV between groups.
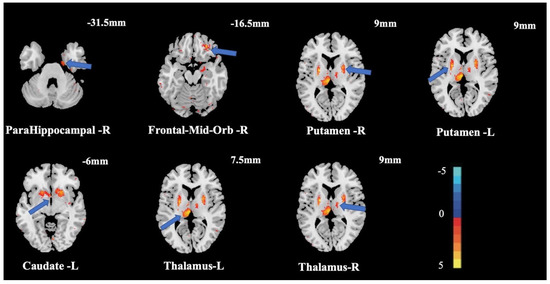
Figure 1.
Changes in GMV between groups. Note: Numbers in the figure represent coordinates of this section in the vertical axis (Z-axis); The light band and numbers in the lower right corner show correspondence between light and dark areas in the brain.
BMI is a covariate that should be included in the assessment of the impact of CRF on GMV in ROI. A multivariate analysis of variance analysis showed that there were significant differences (Wilks’ lambda = 0.834, F(8,121) = 3.018, p ≤ 0.05) between the groups. Between-group analyses revealed significant differences in ORBmid.R GMV (Wilks’ lambda = 0.881, F(2,127) = 8.596, p ≤ 0.05). For multiple corrections, Bonferroni corrections were applied (α = 0.014). Based on this analysis, we found that a high CRF increases ORBmid.R GMV (Table 3).

Table 3.
Differences in GMV between the groups (M ± SD).
3.3. Analysis of CRF, GMV, and EF
Age, sex, and BMI were used as controls for partial correlation analysis of correlations between CRF, GMV, and each sub-function of ACC and RT. It was found that: (i) CRF was positively correlated with ORBmid.R GMV (r = 0.230, p ≤ 0.05) (Figure 2A); (ii) ORBmid.R GMV was negatively correlated with updating RT (r = −0.186, p ≤ 0.05) (Figure 2B); and (iii) CRF was negatively correlated with updating RT (r = −0.184, p ≤ 0.05) (Figure 2C), positively correlated with updating accuracy rate (r = 0.202, p ≤ 0.05) (Figure 2D), and positively correlated with cognitive flexibility RT (r = −0.490, p ≤ 0.001) (Figure 2E).
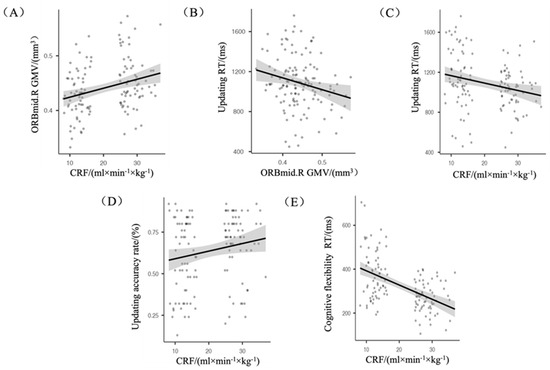
Figure 2.
(A) CRF was positively correlated with ORBmid.R GMV; (B) ORBmid.R GMV was negatively correlated with updating RT; (C) CRF was negatively correlated with updating RT; (D) CRF was positively correlated with the updating accuracy rate; (E) CRF was negatively correlated with cognitive flexibility RT.
3.4. Mediating Role of GMV between CRF and Updating RT
There were significant correlations between CRF, ORBmid.R GMV, and updating RT. Model 4 (simple mediating model) in the Hayes SPSS macro was used to control for sex, age, and BMI, after which the mediating effects of CRF, ORBmid.R GMV, and updating RT were assessed. It was found that CRF had significant predictive effects on updating RT (B = −0.240, t = −2.793, p ≤ 0.05). After the insertion of the mediating variable, the direct predictive effect of CRF on updating RT was still significant (B = −0.193, t = −2.177, p ≤ 0.05). The positive predictive effects of CRF and ORBmid.R GMV were significant (B = 0.288, t = 3.400, p ≤ 0.05), and the negative predictive effect of ORBmid.R GMV on updating RT was also significant (B = −0.161, t = −1.814, p ≤ 0.05). The upper and lower limits of the bootstrap 95% confidence interval for the effects of CRF and updating RT and the mediating effect of ORBmid.R GMV did not contain 0. This shows that CRF can directly predict updating RT and can also predict updating RT through the mediating effects of ORBmid.R GMV. These direct (−0.193) and mediating (−0.047) effects respectively accounted for 80.4% and 19.6% of the total effect (−0.240) (Table 4 and Table 5, Figure 3).

Table 4.
Indirect model test of ORBmid.R GMV.

Table 5.
Decompositions list of total, direct and intermediate effects.
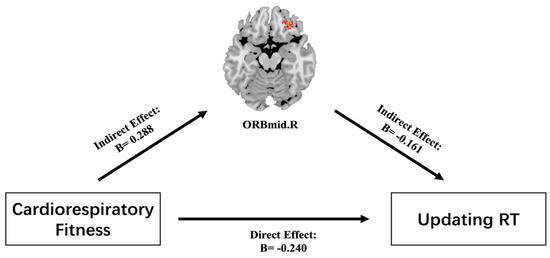
Figure 3.
Mediating effect of ORBmid.R GMV on the relationship between CRF and updating RT. Note: The red area is ORBmid.R GMV, while the values are standardized β coefficients for the mediating test.
4. Discussion
ORBmid.R GMVs are significant mediators in the link between CRF and updating RT, which validates our hypothesis. The higher the CRF, the shorter the updating RT, indicating that CRF has a significant negative predictive effect on updating RT among college students. The direct effect size was 80.4%. Previous studies on the relationship between CRF and EF showed that higher CRF tended to higher efficiency in the frontal parietal network. Meanwhile, higher efficiency in the frontal parietal network was associated with superior EF. Therefore, the relationship between CRF and EF can be adjusted by having better efficiencies in the frontal parietal network of people.
This study further used PROCESS to establish the mediating effect based on correlations among CRF, ORBmid.R GMV, and updating. It was established that the ORBmid.R GMV of college students played a partial mediating role between CRF and updating. First, higher CRF resulted in bigger ORBmid.R GMV, thereby affecting neural connections in areas such as the frontal lobe and improving the activities of underlying neural networks, which might lift the performance of EF. Second, a bigger ORBmid.R GMV exerts direct effects on their receptors within the prefrontal cortex and hippocampus and improves the performance of EF, at least in part via those mechanisms. In this study, ORBmid.R GMV was responsible for 19.6% of the CRF-associated effects on updating. This indicates that CRF affects updating through ORBmid.R GMV. Therefore, CRF-related ORBmid.R GMV plays a role in CRF-related updating.
Differently, CRF was positively correlated with motor area GMV (i.e., putamen, caudate, thalamus, and parahippocampal regions). However, it was not found that the above four regions were related to the three sub-functions of EF. The putamen, caudate, and thalamus are the most concentrated parts of motor conduction and integration coordination, and they play an important role in CRF but are less related to EF [60]. The parahippocampal region may be the only one of four regions associated with EF. Previous research has reported that compared with the elderly, parahippocampal GMV is positively correlated with EF. It is stronger in the elderly than in young adults [61,62,63]. We have not come to the same conclusion in this study, which may be related to lower direct correspondence between the parahippocampal GMV and the EF of college students in early adulthood.
The mediation model was used in our study for the first time to investigate the mediating roles of ORBmid.R GMV in the relationship between CRF and EF, and the potential neural pathways of this relationship were found. Nevertheless, this study is limited by several factors. First, despite the large sample size, this study only covers the effects of CRF on GMV and EF in young and fit healthy adults. Second, the study only collects BMI data; it is important for us to control for obesity using other indexes such as waist-to-hip ratios in later life. Finally, the study mainly understands the relationship between CRF and EF from cerebral cortex development but not by analyzing serum concentration. We look forward to uncovering the association between CRF and EF from the molecular perspective in the future. Our findings are consistent with the theory that CRF is important in EF. Several studies have revealed associations between CRF and EF, implying that enhanced EF promotes decision-making with regard to exercise, thereby contributing to healthy outcomes.
5. Conclusions
Using a sample drawn from a study of college students, we found that higher CRF was related to increased ORBmid.R GMV, which was itself related to shorter updating RT. We examined this potential role and found that ORBmid.R GMV mediated the association between CRF and EF. CRF-related ORBmid.R GMV may be a new pathway underpinning the link between CRF and EF. These findings suggest that even in younger adults, CRF can predict neurological differences leading to structural brain changes, such as greater volume in the ORBmid.R, which may, in turn, forecast a more active and efficient use of EF. Therefore, a future study in this area will be essential in developing more effective exercise programs for the wider population and exploring the causal relationship between CRF and EF on different levels (e.g., brain, behavior). This may help establish a scientific and effective theoretical basis and core technology for the formulation of exercise intervention programs for the coordinated development of “body, mind, and brain”.
Author Contributions
Conceptualization, A.C.; data curation, L.Z. and X.D.; formal analysis and visualization, Y.L., L.Z., K.C. and X.X.; writing—original draft preparation, Y.L.; writing—review and editing, A.C., L.Z., Y.L. and Z.L.; supervision, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
We are thankful for the administrative and technical support from the Affiliated Hospital of Yangzhou University. This research was partially funded by the National Natural Science Foundation of China (No. 31771243; 7 January 2017).
Institutional Review Board Statement
The study protocol was approved by the ethics and human protection committees of Yangzhou University Affiliated Hospital (2017-YKL045-01). All participants provided written consent after receiving a detailed explanation of the experimental procedure. All research procedures were in line with the latest version of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the privacy of the participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Capouskova, K.; Kringelbach, M.L.; Deco, G. Modes of cognition: Evidence from metastable brain dynamics. Neuroimage 2022, 260, 119489. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.Y.; Hubbard, A.E.; Shaw, L.M.; Trojanowski, J.Q.; Petersen, R.C.; Aisen, P.S.; Weiner, M.W.; Jagust, W.J. Longitudinal change of biomarkers in cognitive decline. Arch. Neurol. 2011, 68, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Gracey, F.; Kelly, G.; Jim, J. A new model to guide identity-focused multidisciplinary rehabilitation for children and young people following acquired brain injury: I-FoRM. Neuropsychol. Rehabil. 2022, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.N.; Fares, C.; Bader, R. Relationship between emotional expressions and lifestyle changes among university students during COVID-19 lockdown in Lebanon. J. Infect. Dev. Ctries. 2022, 16, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Floody, P.; Soto-García, D.; Caamaño-Navarrete, F.; Carter-Thuillier, B.; Guzmán-Guzmán, I.P. Negative Physical Self-Concept Is Associated to Low Cardiorespiratory Fitness, Negative Lifestyle and Poor Mental Health in Chilean Schoolchildren. Nutrients 2022, 14, 2771. [Google Scholar] [CrossRef]
- Rodriguez, J.C.; Peterman, J.E.; Fleenor, B.S.; Whaley, M.H.; Kaminsky, L.A.; Harber, M.P. Cardiopulmonary Exercise Responses in Individuals with Metabolic Syndrome: The Ball State Adult Fitness Longitudinal Lifestyle Study. Metab. Syndr. Relat. Disord. 2022. [Google Scholar] [CrossRef]
- McAllister, M.J.; Gonzalez, D.E.; Leonard, M.; Martaindale, M.H.; Bloomer, R.J.; Pence, J.; Martin, S.E. Firefighters with higher cardiorespiratory fitness demonstrate lower markers of cardiovascular disease risk. J. Occup. Environ. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.E.; Vincent, H.K.; Mathews, A.E.; Cautela, B.G.; Sandoval, M.; Tremblay, A.; Langkamp-Henken, B. Impact of probiotic supplementation on exercise endurance among non-elite athletes: Study protocol for a randomized, placebo-controlled, double-blind, clinical trial. Trials 2022, 23, 603. [Google Scholar] [CrossRef]
- Birnbaumer, P.; Weiner, L.; Handl, T.; Tschakert, G.; Hofmann, P. Effects of Different Durations at Fixed Intensity Exercise on Internal Load and Recovery-A Feasibility Pilot Study on Duration as an Independent Variable for Exercise Prescription. J. Funct. Morphol. Kinesiol. 2022, 7, 54. [Google Scholar] [CrossRef]
- Petkus, A.J.; Jarrahi, B.; Holschneider, D.P.; Gomez, M.E.; Filoteo, J.V.; Schiehser, D.M.; Fisher, B.E.; Van Horn, J.D.; Jakowec, M.W.; McEwen, S.C.; et al. Thalamic volume mediates associations between cardiorespiratory fitness (VO(2)max) and cognition in Parkinson’s disease. Parkinsonism. Relat. Disord. 2021, 86, 19–26. [Google Scholar] [CrossRef]
- Carnevale, D.; Elferink-Gemser, M.; Filgueiras, A.; Huijgen, B.; Andrade, C.; Castellano, J.; SiIva, D.; Vasconcellos, F. Executive Functions, Physical Abilities, and Their Relationship with Tactical Performance in Young Soccer Players. Percept. Mot. Skills 2022, 129, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Catasta, G. Lifestyle behaviours predicting major cardiovascular diseases mortality in a practically extinct cohort of middle-aged men followed-up for 61 years. Acta Cardiol. 2022. [Google Scholar] [CrossRef]
- Petersen, K.S.; Murphy, J.; Whitbread, J.; Clifton, P.M.; Keogh, J.B. The Effect of a Peanut-Enriched Weight Loss Diet Compared to a Low-Fat Weight Loss Diet on Body Weight, Blood Pressure, and Glycemic Control: A Randomized Controlled Trial. Nutrients 2022, 14, 2986. [Google Scholar] [CrossRef]
- Guio de Prada, V.; Ortega, J.F.; Ramirez-Jimenez, M.; Morales-Palomo, F.; Pallares, J.G.; Mora-Rodriguez, R. Training intensity relative to ventilatory thresholds determines cardiorespiratory fitness improvements in sedentary adults with obesity. Eur. J. Sport Sci. 2019, 19, 549–556. [Google Scholar] [CrossRef]
- Hammami, A.; Randers, M.B.; Kasmi, S.; Razgallah, M.; Tabka, Z.; Chamari, K.; Bouhlel, E. Effects of soccer training on health-related physical fitness measures in male adolescents. J. Sport Health Sci. 2018, 7, 169–175. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Park, J.; Lee, M.; Kim, J.Y.; Kim, K.C.; Kim, S.H.; Im, J.A.; Chu, S.H.; Suh, S.H.; et al. Association between cardiorespiratory fitness and the prevalence of metabolic syndrome among Korean adults: A cross sectional study. BMC Public Health 2014, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e101–e118. [Google Scholar] [CrossRef]
- Ruotsalainen, I.; Glerean, E.; Karvanen, J.; Gorbach, T.; Renvall, V.; Syväoja, H.J.; Tammelin, T.H.; Parviainen, T. Physical activity and aerobic fitness in relation to local and interhemispheric functional connectivity in adolescents’ brains. Brain Behav. 2021, 11, e01941. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Chad-Friedman, E.; Botdorf, M.; Riggins, T.; Dougherty, L.R. Early childhood cumulative risk is associated with decreased global brain measures, cortical thickness, and cognitive functioning in school-age children. Dev. Psychobiol. 2021, 63, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Saliba, B.J.; Raine, L.B.; Picchietti, D.L.; Hillman, C.H. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2013, 162, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, L.; Pontifex, M.B.; Hillman, C.H.; Kramer, A.F. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J. Int. Neuropsychol. Soc. 2011, 17, 975–985. [Google Scholar] [CrossRef]
- Chen, A.G.; Zhu, L.N.; Yan, J.; Yin, H.C. Neural Basis of Working Memory Enhancement after Acute Aerobic Exercise: fMRI Study of Preadolescent Children. Front. Psychol. 2016, 7, 1804. [Google Scholar] [CrossRef]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Malkowski, E.; Alves, H.; Kim, J.S.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 2010, 48, 1394–1406. [Google Scholar] [CrossRef]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef]
- Boots, E.A.; Schultz, S.A.; Oh, J.M.; Larson, J.; Edwards, D.; Cook, D.; Koscik, R.L.; Dowling, M.N.; Gallagher, C.L.; Carlsson, C.M.; et al. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. 2015, 9, 639–649. [Google Scholar] [CrossRef]
- Yaffe, K.; Fiocco, A.J.; Lindquist, K.; Vittinghoff, E.; Simonsick, E.M.; Newman, A.B.; Satterfield, S.; Rosano, C.; Rubin, S.M.; Ayonayon, H.N.; et al. Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology 2009, 72, 2029–2035. [Google Scholar] [CrossRef]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of tai chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Themanson, J.R.; Hillman, C.H. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience 2006, 141, 757–767. [Google Scholar] [CrossRef]
- Zou, L.; Loprinzi, P.D.; Yeung, A.S.; Zeng, N.; Huang, T. The Beneficial Effects of Mind-Body Exercises for People With Mild Cognitive Impairment: A Systematic Review With Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1556–1573. [Google Scholar] [CrossRef]
- Zukowski, L.A.; Tennant, J.E.; Iyigun, G.; Giuliani, C.A.; Plummer, P. Dual-tasking impacts gait, cognitive performance, and gaze behavior during walking in a real-world environment in older adult fallers and non-fallers. Exp. Gerontol. 2021, 150, 111342. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, C.E.; Strang, A.; Anderson, E.; Jurcsisn, J.; Johnson, E.; Das, T.; Kuchan, M.J.; Barbey, A.K. Author Correction: Enhanced physical and cognitive performance in active duty Airmen: Evidence from a randomized multimodal physical fitness and nutritional intervention. Sci. Rep. 2021, 11, 3820, Erratum in Sci. Rep. 2020, 10, 17826. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Saavedra, J.M. Exercise, health outcomes, and pædiatric obesity: A systematic review of meta-analyses. J. Sci. Med. Sport 2019, 22, 76–84. [Google Scholar] [CrossRef]
- Robinson, L.E.; Stodden, D.F.; Barnett, L.M.; Lopes, V.P.; Logan, S.W.; Rodrigues, L.P.; D’Hondt, E. Motor Competence and its Effect on Positive Developmental Trajectories of Health. Sports Med. 2015, 45, 1273–1284. [Google Scholar] [CrossRef]
- Scott, S.P.; MJ, D.E.S.; Koehler, K.; Petkus, D.L.; Murray-Kolb, L.E. Cardiorespiratory Fitness Is Associated with Better Executive Function in Young Women. Med. Sci. Sports Exerc. 2016, 48, 1994–2002. [Google Scholar] [CrossRef][Green Version]
- Carol, R.N.; Schreiber Compo, N. The effect of encoding duration on implicit and explicit eyewitness memory. Conscious. Cogn. 2018, 61, 117–128. [Google Scholar] [CrossRef]
- Herting, M.M.; Nagel, B.J. Differences in brain activity during a verbal associative memory encoding task in high- and low-fit adolescents. J. Cogn. Neurosci. 2013, 25, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 (Suppl 2), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.M.; Hayes, J.P.; Cadden, M.; Verfaellie, M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front. Aging Neurosci. 2013, 5, 31. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Wittfeld, K.; Jochem, C.; Dörr, M.; Schminke, U.; Gläser, S.; Bahls, M.; Markus, M.R.P.; Felix, S.B.; Leitzmann, M.F.; Ewert, R.; et al. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin. Proc. 2020, 95, 44–56. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Voss, M.W.; Prakash, R.S.; Chaddock, L.; Szabo, A.; White, S.M.; Wojcicki, T.R.; Mailey, E.; McAuley, E.; Kramer, A.F.; et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 2012, 26, 811–819. [Google Scholar] [CrossRef]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef]
- Schwarb, H.; Johnson, C.L.; Daugherty, A.M.; Hillman, C.H.; Kramer, A.F.; Cohen, N.J.; Barbey, A.K. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage 2017, 153, 179–188. [Google Scholar] [CrossRef]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; Gogtay, N.; Evans, A.; Rapoport, J.; Giedd, J. Intellectual ability and cortical development in children and adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Bolger, L.E.; Bolger, L.A.; O’Neill, C.; Coughlan, E.; O’Brien, W.; Lacey, S.; Burns, C.; Bardid, F. Global levels of fundamental motor skills in children: A systematic review. J. Sports Sci. 2021, 39, 717–753. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Welsman, J.R. Aerobic fitness: What are we measuring? Med. Sport Sci. 2007, 50, 5–25. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Erickson, K.I.; Kienzler, C.; King, M.; Pontifex, M.B.; Raine, L.B.; Hillman, C.H.; Kramer, A.F. The role of aerobic fitness in cortical thickness and mathematics achievement in preadolescent children. PLoS ONE 2015, 10, e0134115. [Google Scholar] [CrossRef]
- Hillman, C.H.; Kramer, A.F.; Belopolsky, A.V.; Smith, D.P. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. Int. J. Psychophysiol. 2006, 59, 30–39. [Google Scholar] [CrossRef]
- Anderheim, L.; Holter, H.; Bergh, C.; Möller, A. Does psychological stress affect the outcome of in vitro fertilization? Hum. Reprod. 2005, 20, 2969–2975. [Google Scholar] [CrossRef][Green Version]
- An, Y.; Yi, S.; Fitzpatrick, A.; Gupta, V.; Prak, P.R.; Oum, S.; Logerfo, J.P. Appropriate body mass index and waist circumference cutoff for overweight and central obesity among adults in Cambodia. PLoS ONE 2013, 8, e77897. [Google Scholar] [CrossRef]
- Ratcliff, R. Methods for dealing with reaction time outliers. Psychol. Bull. 1993, 114, 510–532. [Google Scholar] [CrossRef]
- Hanna, C.; Hamilton, J.; Arnavut, E.; Blum, K.; Thanos, P.K. Brain Mapping the Effects of Chronic Aerobic Exercise in the Rat Brain Using FDG PET. J. Pers. Med. 2022, 12, 860. [Google Scholar] [CrossRef]
- Hou, J.H.; Ou, Y.N.; Xu, W.; Zhang, P.F.; Tan, L.; Yu, J.T. Association of peripheral immunity with cognition, neuroimaging, and Alzheimer’s pathology. Alzheimers Res. Ther. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Pinasco, C.; Roca, M.; Ferrari, J.; Couto, B.; García-Cordero, I.; Ibañez, A.; Cruz, F.; Reyes, P.; Matallana, D.; et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia 2019, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, L.; Cheng, X.; Ge, H.; Hu, G.; Xue, C.; Qi, W.; Xu, W.; Chen, S.; Gao, R.; et al. Functional Integrity of Executive Control Network Contributed to Retained Executive Abilities in Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 710172. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).