The Efficacy and Tolerability of Electroconvulsive Therapy in Psychiatric Patients with Arachnoid Cysts: A Retrospective Chart Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.2.1. Clinical Global Impression-Severity (CGI-S)
2.2.2. Positive and Negative Syndrome Scale (PANSS)
2.2.3. Hamilton Depression Scale 17 (HAMD17)
2.2.4. Young Manic Rating Scale (YMRS)
2.2.5. Treatment Emergent Symptom Scale (TESS)
2.3. Electroconvulsive Therapy (ECT)
2.4. Pharmacological Treatment
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Clinical Profile
3.3. Other Subgroups
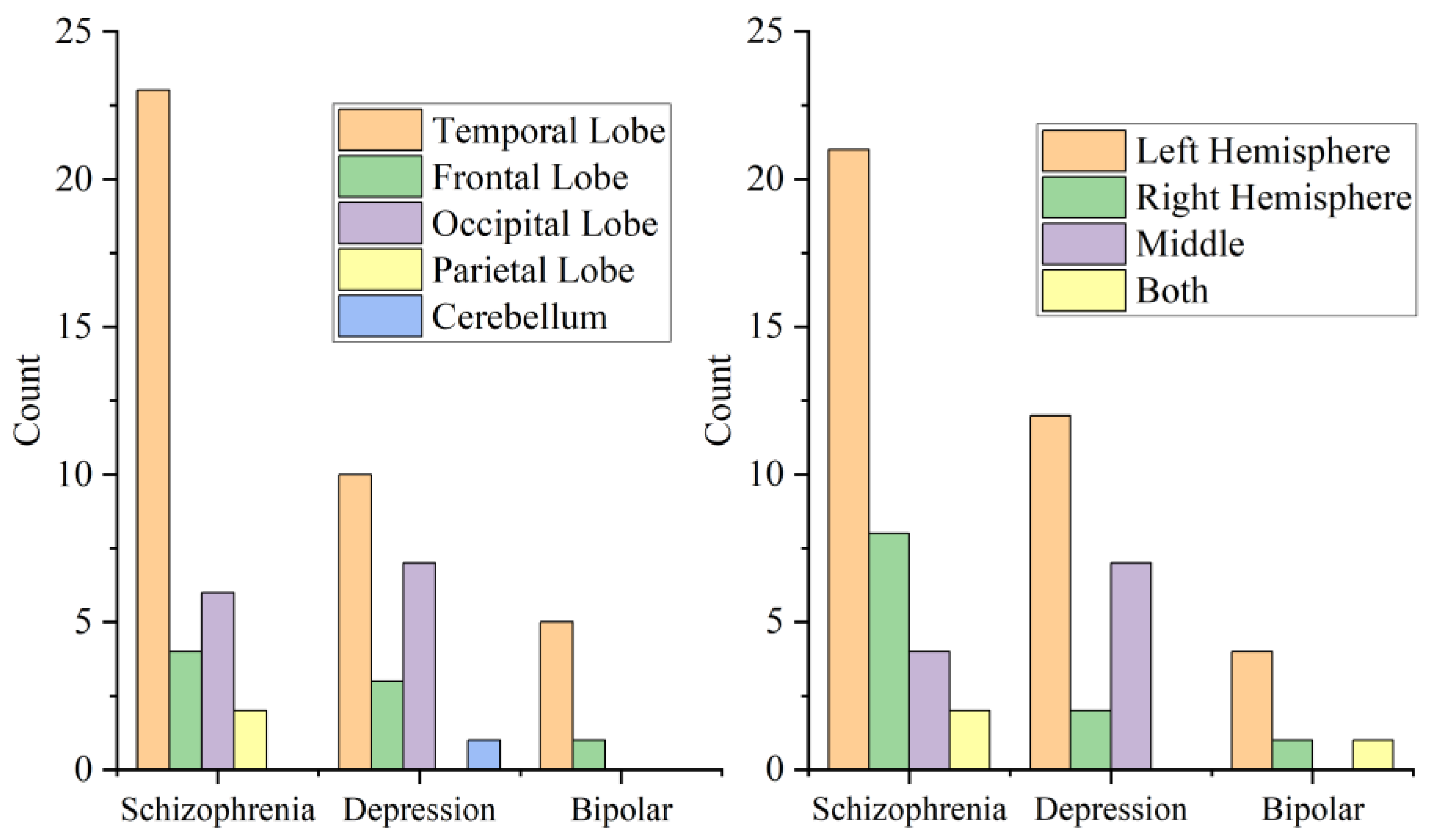
3.4. Location and Side of Cysts
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westermaier, T.; Schweitzer, T.; Ernestus, R.I. Arachnoid cysts. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 724, pp. 37–50. [Google Scholar] [CrossRef]
- Gosalakkal, J.A. Intracranial arachnoid cysts in children: A review of pathogenesis, clinical features, and management. Pediatr. Neurol. 2002, 26, 93–98. [Google Scholar] [CrossRef]
- Gelabert-González, M. Intracranial arachnoid cysts. Rev. Neurol. 2004, 39, 1161–1166. [Google Scholar] [PubMed]
- Wester, K. Gender Distribution and Sidedness of Middle Fossa Arachnoid Cysts. Neurosurgery 1992, 31, 940–944. [Google Scholar] [CrossRef]
- Carbone, J.; Sadasivan, A.P. Intracranial arachnoid cysts: Review of natural history and proposed treatment algorithm. Surg. Neurol. Int. 2021, 12, 621. [Google Scholar] [CrossRef]
- Rabiei, K.; Jaraj, D.; Marlow, T.; Jensen, C.; Skoog, I.; Wikkelsø, C. Prevalence and symptoms of intracranial arachnoid cysts: A population-based study. J. Neurol. 2016, 263, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sunny, D.E.; Amoo, M.; Al Breiki, M.; Teng, E.D.W.; Henry, J.; Javadpour, M. Prevalence of incidental intracranial findings on magnetic resonance imaging: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Sönksen, S.-E.; Jakobs, F.; Zschommler, Y.; Weber, F. Do arachnoid cysts grow? A retrospective volumetric study. J. Neurol. 2021, 268, 3777–3780. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Jallo, G. Arachnoid cysts: Case series and review of the literature. Neurosurg. Focus 2007, 22, 1–4. [Google Scholar] [CrossRef]
- Lindner, U.; Bubl, B.; Steudel, W.-I.; Papanagiotou, P.; Käsmann-Kellner, B.; Gortner, L.; Shamdeen, M.G. Increased Intracranial Pressure Caused by Non-Space-Occupying Arachnoid Cysts: Report of Two Patients. Neuropediatrics 2009, 40, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Vidrih, B.; Karlović, D.; Pasić, M.B. Arachnoid cyst as the cause of bipolar affective disorder: Case report. Acta Clin. Croat. 2012, 51, 655–659. [Google Scholar] [PubMed]
- Wong, C.-W.; Ko, S.-F.; Wai, Y.-Y. Arachnoid Cyst of the Lateral Ventricle Manifesting Positional Psychosis. Neurosurgery 1993, 32, 841–843; discussion 843. [Google Scholar] [CrossRef]
- Kuhnley, E.J.; White, D.H.; Granoff, A.L. Psychiatric presentation of an arachnoid cyst. J. Clin. Psychiatry 1981, 42, 167–168. [Google Scholar] [PubMed]
- Vakis, A.F.; Koutentakis, D.I.; Karabetsos, D.A.; Kalostos, G.N. Psychosis-like syndrome associated with intermittent intracranial hypertension caused by a large arachnoid cyst of the left temporal lobe. Br. J. Neurosurg. 2006, 20, 156–159. [Google Scholar] [CrossRef]
- Baquero, G.A.; Molero, P.; Pla, J.; Ortuño, F. A schizophrenia-like psychotic disorder secondary to an arachnoid cyst remitted with neurosurgical treatment of the cyst. Open Neuroimaging J. 2014, 8, 1–4. [Google Scholar] [CrossRef]
- Kellner, C.H.; Obbels, J.; Sienaert, P. When to consider electroconvulsive therapy (ECT). Acta Psychiatr. Scand. 2020, 141, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Ingram, A.; Saling, M.M.; Schweitzer, I. Cognitive Side Effects of Brief Pulse Electroconvulsive Therapy. J. ECT 2008, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Maltbie, A.A.; Wingfield, M.S.; Volow, M.R.; Weiner, R.D.; Sullivan, J.L.; Cavenar, J.O., Jr. Electroconvulsive therapy in the presence of brain tumor. Case reports and an evaluation of risk. J. Nerv. Ment. Dis. 1980, 168, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Buday, J.; Albrecht, J.; Mareš, T.; Podgorná, G.; Horáčková, K.; Kališová, L.; Raboch, J.; Anders, M. Brain Tumors and Electroconvulsive Therapy: A Literature Overview of the Last 80 Years. Front. Neurol. 2020, 11, 723. [Google Scholar] [CrossRef]
- Prasad, S.; Avery, R.A.; de Alba Campomanes, A.; Sutton, L.N.; Liu, G.T. Symptomatic increased intracranial pressure due to arachnoid cysts. Pediatr. Neurol. 2011, 44, 377–380. [Google Scholar] [CrossRef]
- Perry, C.L.; Lindell, E.P.; Rasmussen, K.G. ECT in patients with arachnoid cysts. J. ECT 2007, 23, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Hanretta, A.T.; Akra, I.; Malek-Ahmadi, P. Electroconvulsive Therapy and Arachnoid Cysts. J. ECT 2007, 23, 126–127. [Google Scholar] [CrossRef]
- Wachtel, L.E.; Baranano, K.; Reti, I.M. Electroconvulsive Therapy for Catatonia in a Boy With Hydrocephalus and an Arachnoid Cyst. Pediatr. Neurol. 2010, 43, 73–75. [Google Scholar] [CrossRef]
- Desseilles, M.; Thiry, J.-C.; Monville, J.-F.; Ansseau, M.; Makhinson, M. Electroconvulsive Therapy for Depression in a Patient with an Intracranial Arachnoid Cyst. J. ECT 2009, 25, 64–66. [Google Scholar] [CrossRef]
- Escalona, P.R.; Coffey, C.E.; Maus-Feldman, J. Electroconvulsive Therapy in a Depressed Patient with an Intracranial Arachnoid Cyst: A Brain Magnetic Resonance Imaging Study. Convuls. Ther. 1991, 7, 133–138. [Google Scholar]
- Kastenholz, K.J.; Rosenthal, L.J.; Dinwiddie, S.H. Electroconvulsive Therapy in a Patient with Catatonia and an Intracranial Arachnoid Cyst. J. ECT 2014, 30, e53–e54. [Google Scholar] [CrossRef]
- Grover, S.; Aneja, J.; Singh, A.; Singla, N. Use of electroconvulsive therapy in the presence of arachnoid cyst: A case report and review of existing literature. J. ECT 2013, 29, e38–e39. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Berk, M.; Ng, F.; Dodd, S.; Callaly, T.; Campbell, S.; Bernardo, M.; Trauer, T. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J. Eval. Clin. Pract. 2008, 14, 979–983. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Wu, B.-J.; Lan, T.-H.; Hu, T.-M.; Lee, S.-M.; Liou, J.-Y. Validation of a five-factor model of a Chinese Mandarin version of the Positive and Negative Syndrome Scale (CMV-PANSS) in a sample of 813 schizophrenia patients. Schizophr. Res. 2015, 169, 489–490. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhao, J.P.; Phillips, M.; Liu, J.B.; Cai, M.F.; Sun, S.Q.; Huang, M.F. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br. J. Psychiatry J. Ment. Sci. 1988, 152, 660–664. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Zou, Y.-F.; Wang, Y.; Liu, P.; Feng, X.-L.; Wang, B.-Y.; Zang, T.-H.; Yu, X.; Wei, J.; Liu, Z.-C.; Liu, Y.; et al. Association of brain-derived neurotrophic factor genetic Val66Met polymorphism with severity of depression, efficacy of fluoxetine and its side effects in Chinese major depressive patients. Neuropsychobiology 2010, 61, 71–78. [Google Scholar] [CrossRef]
- Gaines, G.Y., 3rd; Rees, D.I. Electroconvulsive therapy and anesthetic considerations. Anesth. Analg. 1986, 65, 1345–1356. [Google Scholar] [CrossRef]
- Gjerde, P.B.; Litleskare, S.; Lura, N.G.; Tangen, T.; Helland, C.A.; Wester, K. Anxiety and Depression in Patients with Intracranial Arachnoid Cysts–A Prospective Study. World Neurosurg. 2019, 132, e645–e653. [Google Scholar] [CrossRef]
- Davison, K. Schizophrenia-like psychoses associated with organic cerebral disorders: A review. Psychiatr. Dev. 1983, 1, 1–33. [Google Scholar]
- Caetano, S.C.; Fonseca, M.; Hatch, J.P.; Olvera, R.L.; Nicoletti, M.; Hunter, K.; Lafer, B.; Pliszka, S.R.; Soares, J.C. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci. Lett. 2007, 427, 142–147. [Google Scholar] [CrossRef]
- Ramezani, M.; Johnsrude, I.; Rasoulian, A.; Bosma, R.; Tong, R.; Hollenstein, T.; Harkness, K.; Abolmaesumi, P. Temporal-lobe morphology differs between healthy adolescents and those with early-onset of depression. NeuroImage Clin. 2014, 6, 145–155. [Google Scholar] [CrossRef]
- Shimada, H.; Park, H.; Makizako, H.; Doi, T.; Lee, S.; Suzuki, T. Depressive symptoms and cognitive performance in older adults. J. Psychiatr. Res. 2014, 57, 149–156. [Google Scholar] [CrossRef]
- Jones, L.D.; Payne, M.E.; Messer, D.F.; Beyer, J.L.; MacFall, J.R.; Krishnan, K.R.; Taylor, W.D. Temporal lobe volume in bipolar disorder: Relationship with diagnosis and antipsychotic medication use. J. Affect. Disord. 2009, 114, 50–57. [Google Scholar] [CrossRef]
- Shenton, M.E.; Kikinis, R.; Jolesz, F.A.; Pollak, S.D.; LeMay, M.; Wible, C.G.; Hokama, H.; Martin, J.; Metcalf, D.; Coleman, M.; et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N. Engl. J. Med. 1992, 327, 604–612. [Google Scholar] [CrossRef]
- Holinger, D.P.; Shenton, M.E.; Wible, C.G.; Donnino, R.; Kikinis, R.; Jolesz, F.A.; McCarley, R.W. Superior temporal gyrus volume abnormalities and thought disorder in left-handed schizophrenic men. Am. J. Psychiatry 1999, 156, 1730–1735. [Google Scholar] [CrossRef]
- Hecht, D. Depression and the hyperactive right-hemisphere. Neurosci. Res. 2010, 68, 77–87. [Google Scholar] [CrossRef]
- Steyn, P.J.; Van den Heuvel, L.L. Cut it out or wait it out? Case series of middle fossa arachnoid cysts presenting with psychiatric symptoms and a discussion of the ethics of neurosurgical management. Gen. Psychiatry 2021, 34, e100523. [Google Scholar] [CrossRef]
- Khan, A.H.; Ahmed, S.E. Arachnoid Cyst and Psychosis. Cureus 2017, 9, e1707. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Alves, A.; Talina, M.; Carreiro, S.; Guimarães, J.; Xavier, M. Arachnoid cyst in a patient with psychosis: Case report. Ann. Gen. Psychiatry 2007, 6, 16. [Google Scholar] [CrossRef]
- Bahk, W.M.; Pae, C.U.; Chae, J.H.; Jun, T.Y.; Kim, K.S. A case of brief psychosis associated with an arachnoid cyst. Psychiatry Clin. Neurosci. 2002, 56, 203–205. [Google Scholar] [CrossRef]
- Mace, C.J.; Trimble, M.R. Psychosis following temporal lobe surgery: A report of six cases. J. Neurol. Neurosurg. Psychiatry 1991, 54, 639–644. [Google Scholar] [CrossRef]
| Characteristics | ECT N = 43 | No-ECT N = 19 | p | |
|---|---|---|---|---|
| Age | Mean ± SD | 25.65 ± 10.712 | 28.63 ± 12.855 | 0.541 * |
| Range | 12~49 | 14~56 | ||
| Frequency of ECT | Mean ± SD | 9.95 ± 7.575 | 0(0) | |
| Range | 2~32 | 0(0) | ||
| Sex | Male (%) | 22(51.2) | 11(57.9) | 0.624 † |
| Female (%) | 21(48.8) | 8(42.1) | ||
| Diagnose | Schizophrenia (%) | 24(55.8) | 11(57.9) | 1.000 † |
| Depression (%) | 15(34.9) | 6(31.6) | ||
| Bipolar disorder (%) | 4(9.3) | 2(10.5) | ||
| Location | Temporal Lobe (%) | 23(53.5) | 15(78.9) | 0.284 ‡ |
| Frontal Lobe (%) | 7(16.3) | 1(5.3) | ||
| Occipital Lobe (%) | 11(25.6) | 2(10.5) | ||
| Parietal Lobe (%) | 1(2.3) | 1(5.3) | ||
| Cerebellar (%) | 1(2.3) | 0(0) | ||
| Side | Left hemisphere (%) | 23(53.5) | 14(73.7) | 0.491 ‡ |
| Right hemisphere (%) | 8(18.6) | 3(15.8) | ||
| Middle (%) | 9(20.9) | 2(10.5) | ||
| Both (%) | 3(7) | 0(0) | ||
| Other chronic disease | Hypertension (%) | 1(2.3) | 1(5.3) | |
| Diabetes (%) | 1(2.3) | 1(5.3) | ||
| Hyperlipoidemia (%) | 2(4.7) | 0(0) | ||
| lung diseases (%) | 2(4.7) | 1(5.3) | ||
| Family history | 8(18.6) | 5(26.3) | 0.513 * |
| Scale | ECT | NO-ECT | p | |
|---|---|---|---|---|
| PANSS | Pre-treatment | 118.96 ± 24.880 | 145.73 ± 20.804 | 0.466 * |
| Post-treatment | 55.67 ± 12.596 | 86.64 ± 26.269 | <0.001 * | |
| p < 0.001 † | p = 0.865 † | |||
| HAMD | Pre-treatment | 23.33 ± 3.222 | 24.50 ± 2.510 | 0.438 ‡ |
| Post-treatment | 11.00 ± 1.813 | 13.17 ± 2.639 | 0.042 ‡ | |
| p < 0.001 § | p < 0.001 § | |||
| YMRS | Pre-treatment | 28.50 ± 4.359 | 35.50 ± 2.121 | 0.108 ‡ |
| Post-treatment | 8.75 ± 2.062 | 11.50 ± 2.121 | 0.201 ‡ | |
| p = 0.001 § | p = 0.079 § | |||
| CGIs | Pre-treatment | 4.98 ± 0.801 | 5.63 ± 0.597 | 1.000 * |
| Post-treatment | 2.35 ± 0.482 | 3.53 ± 0.964 | <0.001 * | |
| p < 0.001 † | p < 0.001 † | |||
| Reducing Score of CGIs | 1(%) | N = 1(2.3) | N = 7(36.8) | 0.001 ** |
| 2(%) | N = 21(48.8) | N = 4(21.1) | ||
| 3(%) | N = 14(32.6) | N = 7(36.8) | ||
| 4(%) | N = 7(16.3) | N = 1(5.3) | ||
| TESS | 36.44 ± 8.843 | 39.05 ± 9.366 | 0.297 ‡ |
| TESS Score | Reducing Score of CGIs | ||
|---|---|---|---|
| Sex | Male | 36.21 ± 8.880 | p = 0.765 † |
| Female | 38.41 ± 9.171 | ||
| p = 0.341 * | |||
| Diagnose | Schizophrenia | 35.60 ± 9.708 | p = 0.508 † |
| Depression | 36.95 ± 6.778 | ||
| Bipolar disorder | 47.83 ± 3.488 | ||
| p = 0.006 ‡ | |||
| Location of cyst | Temporal Lobe | 38.66 ± 8.666 | p = 0.413 † |
| Frontal Lobe | 39.63 ± 8.366 | ||
| Occipital Lobe | 32.00 ± 9.600 | ||
| Parietal Lobe | 34.50 ± 9.192 | ||
| Cerebellum | 38.00 | ||
| p = 0.204 ‡ | |||
| Side of cyst | Left Hemisphere | 37.68 ± 9.080 | p = 0.752 † |
| Right Hemisphere | 37.55 ± 8.478 | ||
| Middle | 33.45 ± 9.606 | ||
| Both | 44.67 ± 2.517 | ||
| p = 0.193 ‡ | |||
| Family history | YES | 35.08 ± 6.525 | p = 0.120 † |
| NO | 37.82 ± 9.536 | ||
| p = 0.334 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Tian, Y.; Gan, Y.; Fu, Y.; Chen, Q.; Zou, L.; Zhao, B.; Yan, Y.; Liu, S.; Chen, X.; et al. The Efficacy and Tolerability of Electroconvulsive Therapy in Psychiatric Patients with Arachnoid Cysts: A Retrospective Chart Study. Brain Sci. 2022, 12, 1393. https://doi.org/10.3390/brainsci12101393
Lu Y, Tian Y, Gan Y, Fu Y, Chen Q, Zou L, Zhao B, Yan Y, Liu S, Chen X, et al. The Efficacy and Tolerability of Electroconvulsive Therapy in Psychiatric Patients with Arachnoid Cysts: A Retrospective Chart Study. Brain Sciences. 2022; 12(10):1393. https://doi.org/10.3390/brainsci12101393
Chicago/Turabian StyleLu, Ying, Yu Tian, Yu Gan, Yixiao Fu, Qibin Chen, Lei Zou, Bangshu Zhao, Yu Yan, Shudong Liu, Xiaolu Chen, and et al. 2022. "The Efficacy and Tolerability of Electroconvulsive Therapy in Psychiatric Patients with Arachnoid Cysts: A Retrospective Chart Study" Brain Sciences 12, no. 10: 1393. https://doi.org/10.3390/brainsci12101393
APA StyleLu, Y., Tian, Y., Gan, Y., Fu, Y., Chen, Q., Zou, L., Zhao, B., Yan, Y., Liu, S., Chen, X., & Li, X. (2022). The Efficacy and Tolerability of Electroconvulsive Therapy in Psychiatric Patients with Arachnoid Cysts: A Retrospective Chart Study. Brain Sciences, 12(10), 1393. https://doi.org/10.3390/brainsci12101393







