Stereotactic Radiofrequency Ablation for Treatment-Refractory Depression: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- (1)
- The intervention had to be one of anterior cingulotomy or anterior capsulotomy. Studies that included multiple or combinations of these treatments were included if outcomes from single procedures were available. Where outcomes were for multiple procedures, these patients were not included.
- (2)
- The surgical indication was depressive illness. Studies that reported on depressive symptoms in the context of other primary diagnoses were excluded.
- (3)
- Measures of depressive symptoms were reported using validated scales at baseline and at least six months after surgery.
- (4)
- The study reported outcomes for at least eight patients, reducing risk of statistical anomalies during meta-analysis.
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Primary Outcomes
2.5. Data Analysis
2.6. Patient Level Analysis
2.7. Role of the Funding Source
3. Results
3.1. Risk of Bias
3.2. Meta-Analysis: Reported Data Only
3.3. Meta-Analysis: LOCF Analysis
3.4. Patient-Level Analysis
3.5. Adverse Effects
4. Discussion
4.1. Summary of Main Findings
4.2. GRADE Recommendation
4.3. Study Strengths
4.4. Study Limitations
4.5. Comparison to Deep Brain Stimulation
4.6. The Future of SRA for TRD
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 3 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | 3–4 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | 4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and, if applicable, details of automation tools used in the process. | 4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses) and, if not, the methods used to decide which results to collect. | 4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and, if applicable, details of automation tools used in the process. | 4 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 4 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 4 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics or data conversions. | 4 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 4 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 4 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 4 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | 4 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 4 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 4 |
| RESULTS | |||
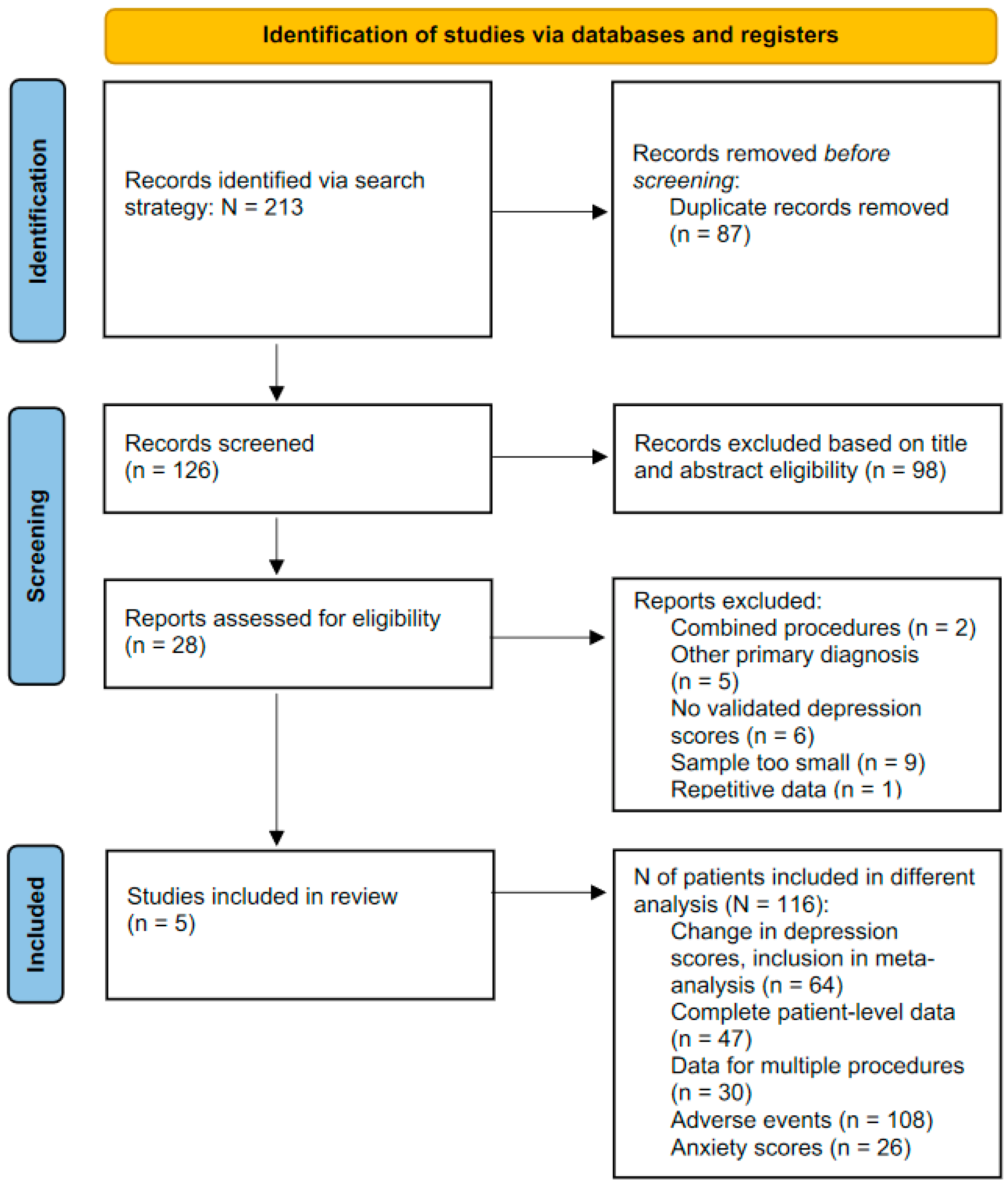
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 4–5 |
| 16b | Cite studies that might appear to meet the inclusion criteria but which were excluded and explain why they were excluded. | 5 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 5 |
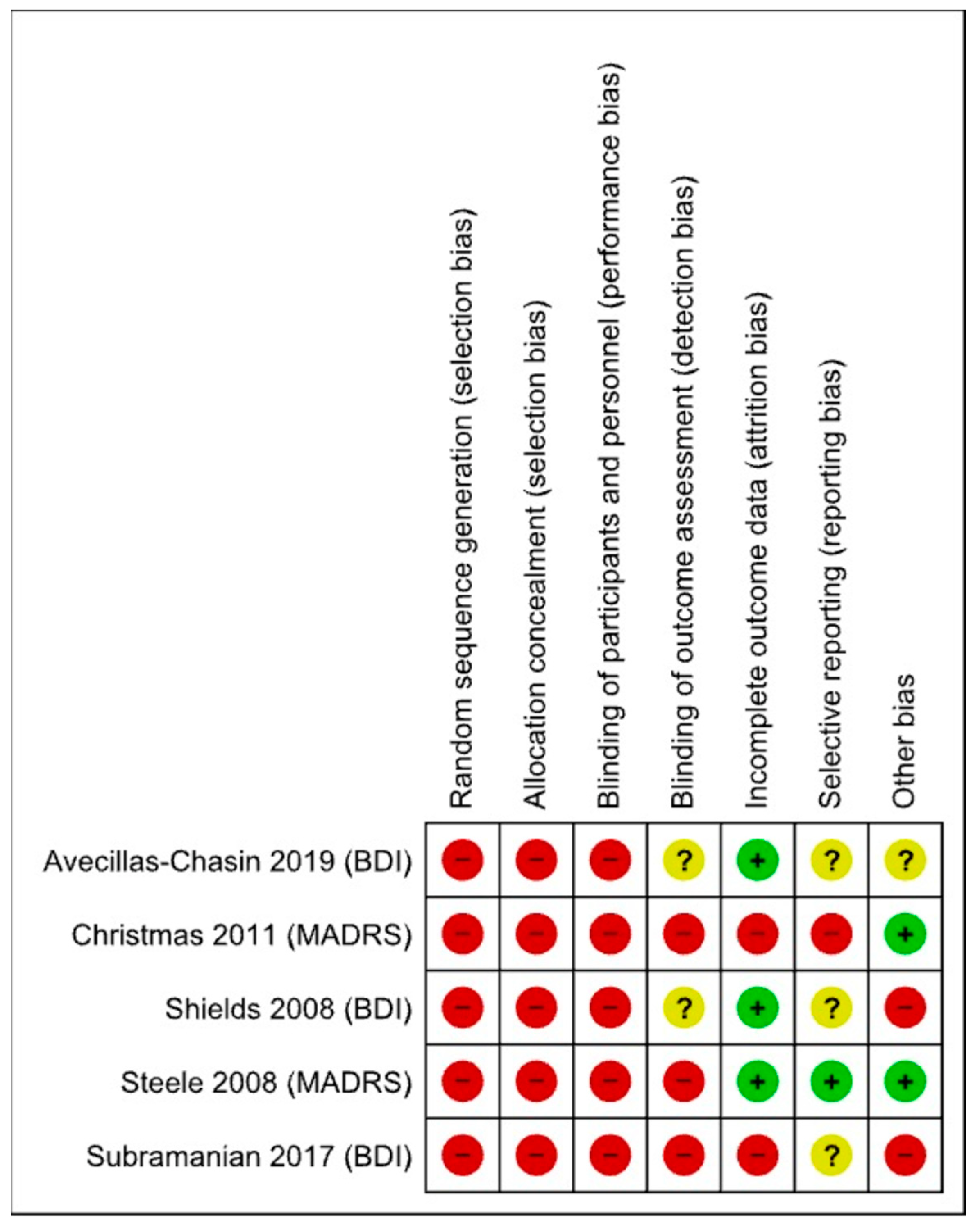
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 5–6 |
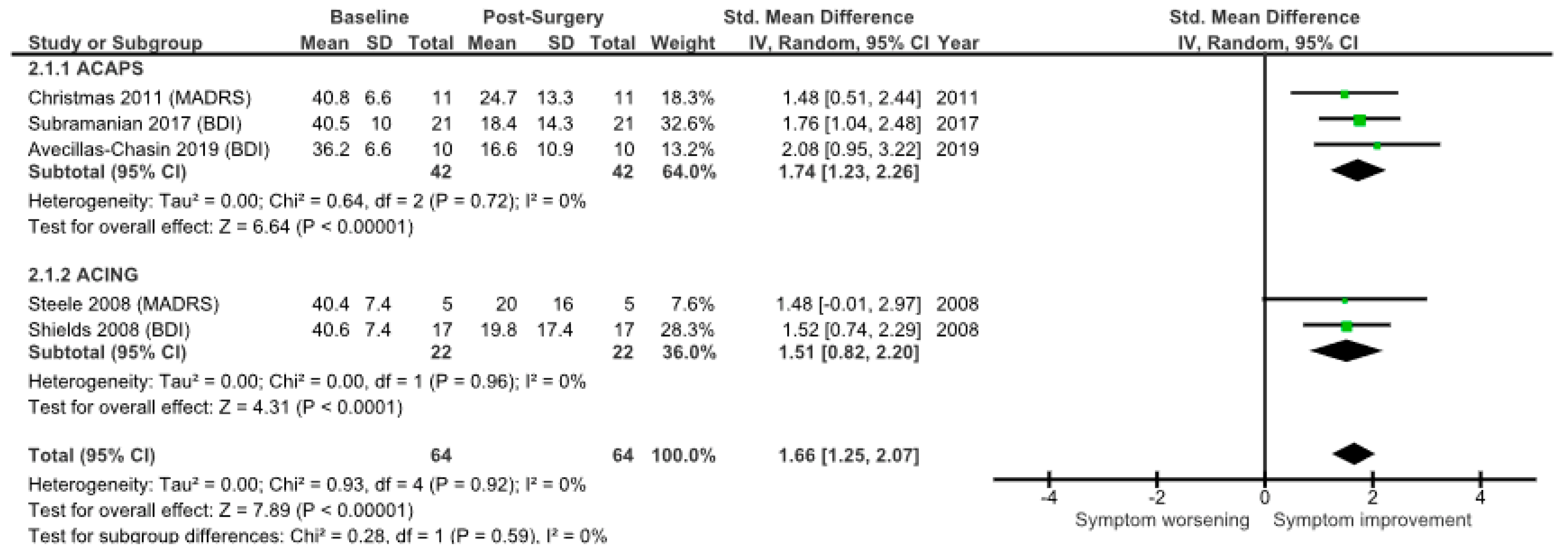
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 6–10 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | 6–10 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 6–10 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 6–10 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | 6–10 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | 5–6 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | 6–10 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 10–12 |
| 23b | Discuss any limitations of the evidence included in the review. | 10–11 | |
| 23c | Discuss any limitations of the review processes used. | 10–11 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 11 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 3 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | / | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | / | |
| Support | 25 | Describe sources of financial or non-financial support for the review and the role of the funders or sponsors in the review. | 12 |
| Competing interests | 26 | Declare any competing interests of review authors. | 12 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | / |
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Mrazek, D.A.; Hornberger, J.C.; Altar, C.A.; Degtiar, I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv. 2014, 65, 977–987. [Google Scholar] [CrossRef]
- Berlim, M.T.; Turecki, G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur. Neuropsychopharmacol. 2007, 17, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Sachdev, J. Long-Term Outcome of Neurosurgery for the Treatment of Resistant Depression. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 478–485. [Google Scholar] [CrossRef]
- Moffitt, T.E.; Harrington, H.; Caspi, A.; Kim-Cohen, J.; Goldberg, D.; Gregory, A.M.; Poulton, R. Depression and Generalized Anxiety Disorder: Cumulative and Sequential Comorbidity in a Birth Cohort Followed Prospectively to Age 32 Years. Arch. Gen. Psychiatry 2007, 64, 651–660. [Google Scholar] [CrossRef]
- Kopell, B.H.; Greenberg, B.; Rezai, A.R. Deep brain stimulation for psychiatric disorders. J. Clin. Neurophysiol. 2004, 21, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Zrinzo, L. The role of imaging in the surgical treatment of movement disorders. Neuroimaging Clin. 2010, 20, 125–140. [Google Scholar] [CrossRef]
- Organ, L.W. Electrophysiologic principles of radiofrequency lesion making. Appl. Neurophysiol. 1976, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.C.; Asaad, W.; Eskandar, E.N.; Jain, F.A.; Cosgrove, G.R.; Flaherty, A.W.; Cassem, E.H.; Price, B.H.; Rauch, S.L.; Dougherty, D.D. Prospective assessment of stereotactic ablative surgery for intractable major depression. Biol. Psychiatry 2008, 64, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Nuttin, B.; Wu, H.; Mayberg, H.; Hariz, M.; Gabriëls, L.; Galert, T.; Merkel, R.; Kubu, C.; Vilela-Filho, O.; Matthews, K.; et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Noblesse, L.H.; Temel, Y.; Ackermans, L.; Lim, L.W.; Steinbusch, H.W.; Visser-Vandewalle, V. Neurostimulatory and ablative treatment options in major depressive disorder: A systematic review. Acta Neurochir. 2010, 152, 565–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freeman, C.; Crossley, D.; Eccleston, D. Neurosurgery for Mental Disorder. Report from the Neurosurgery Working Group of the Royal College of Psychiatrists; Council Report CR89; Royal College of Psychiatrists: London, UK, 2000. [Google Scholar]
- Sinha, S.; McGovern, R.A.; Mikell, C.B.; Banks, G.P.; Sheth, S.A. Ablative Limbic System Surgery: Review and Future Directions. Curr. Behav. Neurosci. Rep. 2015, 2, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Steele, J.D.; Christmas, D.; Eljamel, M.S.; Matthews, K. Anterior cingulotomy for major depression: Clinical outcome and relationship to lesion characteristics. Biol. Psychiatry 2008, 63, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Christmas, D.; Eljamel, M.S.; Butler, S.; Hazari, H.; MacVicar, R.; Steele, J.D.; Livingstone, A.; Matthews, K. Long term outcome of thermal anterior capsulotomy for chronic, treatment refractory depression. J. Neurol. Neurosurg. Psychiatry 2011, 82, 594–600. [Google Scholar] [CrossRef]
- Subramanian, L.; Bracht, T.; Jenkins, P.; Choppin, S.; Linden, D.E.; Phillips, G.; Simpson, B.A. Clinical improvements following bilateral anterior capsulotomy in treatment-resistant depression. Psychol. Med. 2017, 47, 1097–1106. [Google Scholar] [CrossRef]
- Avecillas-Chasin, J.M.; Hurwitz, T.A.; Bogod, N.M.; Honey, C.R. An Analysis of Clinical Outcome and Tractography following Bilateral Anterior Capsulotomy for Depression. Stereotact. Funct. Neurosurg. 2019, 97, 369–380. [Google Scholar] [CrossRef]
- Volpini, M.; Giacobbe, P.; Cosgrove, G.R.; Levitt, A.; Lozano, A.M.; Lipsman, N. The History and Future of Ablative Neurosurgery for Major Depressive Disorder. Stereotact. Funct. Neurosurg. 2017, 95, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Bakar, B.; Cetin, C.; Oppong, J.; Erdogan, A.M. Current Ablation Type Surgical Treatment Modalities In Treatment-Resistant Major Depression: Review of The Recent Major Surgical Series. J. Basic Clin. Health Sci. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13681. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer program], version 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014.
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Hamer, R.M.; Simpson, P.M. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am. J. Psychiatry 2009, 166, 639–641. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Wang, F.C.; Lane, H.Y. Percentage reduction of depression severity versus absolute severity after initial weeks of treatment to predict final response or remission. Psychiatry Clin. Neurosci. 2013, 67, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.M.; Rohan, K.J.; Langenberg, P.; Snitker, S.; Postolache, T.T. Calibration of response and remission cut-points on the Beck Depression Inventory-Second Edition for monitoring seasonal affective disorder treatment outcomes. J. Affect. Disord. 2012, 138, 123–127. [Google Scholar] [CrossRef]
- Hawley, C.J.; Gale, T.M.; Sivakumaran, T. Defining remission by cut off score on the MADRS: Selecting the optimal value. J. Affect. Disord. 2002, 72, 177–184. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Jackson-Koku, G. Beck Depression Inventory. Occup. Med. 2016, 66, 174–175. [Google Scholar] [CrossRef]
- Wikberg, C.; Nejati, S.; Larsson, M.E.H.; Petersson, E.-L.; Westman, J.; Ariai, N.; Kivi, M.; Eriksson, M.; Eggertsen, R.; Hange, D.; et al. Comparison between the Montgomery-Asberg Depression Rating Scale-Self and the Beck Depression Inventory II in Primary Care. Prim. Care Companion CNS Disord. 2015, 17, 26138. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mo, J.; Sui, L.; Zhang, J.; Hu, W.; Zhang, C.; Wang, Y.; Liu, C.; Zhao, B.; Wang, X.; et al. Deep Brain Stimulation in Treatment-Resistant Depression: A Systematic Review and Meta-Analysis on Efficacy and Safety. Front. Neurosci. 2021, 15, 655412. [Google Scholar] [CrossRef]
- Zrinzo, L.; Wilson, J.; Hariz, M.; Joyce, E.; Morris, J.; Schmidt, U. Exploring every ethical avenue. Commentary: The Moral Obligation to Prioritize Research Into Deep Brain Stimulation Over Brain Lesioning Procedures for Severe Enduring Anorexia Nervosa. Front. Psychiatry 2019, 10, 326. [Google Scholar] [CrossRef]
- Chang, J.G.; Jung, H.H.; Kim, S.J.; Chang, W.S.; Jung, N.Y.; Kim, C.H.; Chang, J.W. Bilateral thermal capsulotomy with magnetic resonance-guided focused ultrasound for patients with treatment-resistant depression: A proof-of-concept study. Bipolar Disord. 2020, 22, 771–774. [Google Scholar] [CrossRef]
- Davidson, B.; Hamani, C.; Rabin, J.S.; Goubran, M.; Meng, Y.; Huang, Y.; Baskaran, A.; Sharma, S.; Ozzoude, M.; Richter, M.A.; et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: Clinical and imaging results from two phase I trials. Mol. Psychiatry 2020, 25, 1946–1957. [Google Scholar] [CrossRef]
- Weijer, C. I Need a Placebo like I Need a Hole in the Head. J. Law Med. Ethics 2002, 30, 69–72. [Google Scholar] [CrossRef]


| Procedure and Study | N | % | Follow up Timepoint/Months | Primary Outcome |
|---|---|---|---|---|
| ACAPS | 42 | 65.6 | ||
| Avecillas-Chasin et al. [21] | 10 | 15.6 | 12 | BDI |
| Subramanian et al. [20] | 21 | 32.8 | 6 (median) | BDI |
| Christmas et al. [19] | 11 | 17.2 | 12 | MADRS |
| ACING | 22 | 34.4 | ||
| Shields et al. [10] | 17 | 26.6 | 30 (mean) | BDI |
| Steele et al. [18] | 5 | 7.8 | 12 | MADRS |
| Total | 64 | 100 |
| Anterior Capsulotomy | Anterior Cingulotomy | |||
|---|---|---|---|---|
| Adverse Events * | Short-Term (%) | Long-Term (%) | Short-Term (%) | Long-Term (%) |
| Confusion/disorientation | 42.7 | - | - | - |
| Urinary incontinence | 41.3 | 4.0 | 12.1 | - |
| Fatigue | 22.7 | 4.0 | - | - |
| Headache | 12.0 | 4.0 | - | - |
| Memory problems | 9.3 | 13.3 | - | 3.0 |
| Apathy | 5.3 | 14.7 | - | - |
| Concentration/attention impairment | 5.3 | 10.7 | - | - |
| Motor weakness | 4.0 | - | - | - |
| Weight gain | 2.7 | 5.3 | - | - |
| Infection | 2.7 | - | 3.0 | - |
| Seizures | 1.3 | 2.7 | - | 3.0 |
| Personality change | - | 5.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Münchenberg, P.S.; Joyce, E.M.; Matthews, K.; Christmas, D.; Zrinzo, L. Stereotactic Radiofrequency Ablation for Treatment-Refractory Depression: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 1379. https://doi.org/10.3390/brainsci12101379
Münchenberg PS, Joyce EM, Matthews K, Christmas D, Zrinzo L. Stereotactic Radiofrequency Ablation for Treatment-Refractory Depression: A Systematic Review and Meta-Analysis. Brain Sciences. 2022; 12(10):1379. https://doi.org/10.3390/brainsci12101379
Chicago/Turabian StyleMünchenberg, Pauline Sarah, Eileen M. Joyce, Keith Matthews, David Christmas, and Ludvic Zrinzo. 2022. "Stereotactic Radiofrequency Ablation for Treatment-Refractory Depression: A Systematic Review and Meta-Analysis" Brain Sciences 12, no. 10: 1379. https://doi.org/10.3390/brainsci12101379
APA StyleMünchenberg, P. S., Joyce, E. M., Matthews, K., Christmas, D., & Zrinzo, L. (2022). Stereotactic Radiofrequency Ablation for Treatment-Refractory Depression: A Systematic Review and Meta-Analysis. Brain Sciences, 12(10), 1379. https://doi.org/10.3390/brainsci12101379







