Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice
Abstract
1. Introduction
2. Materials and Methods
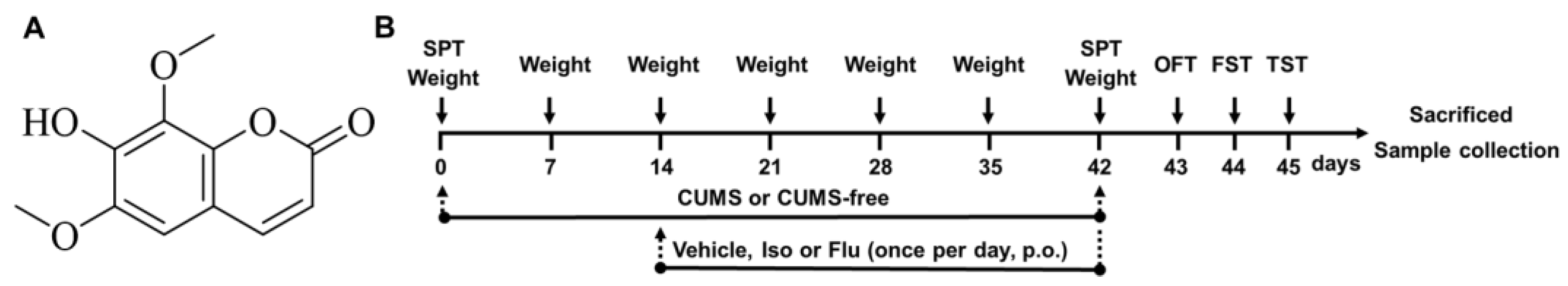
2.1. Materials Chemicals and Reagents
2.2. Animals and Treatments
2.3. CUMS
2.4. Behavioral Tests
2.4.1. Sucrose Preference Test (SPT)
2.4.2. Open Field Test (OFT)
2.4.3. Forced Swimming Test (FST)
2.4.4. Tail-Suspension Test (TST)
2.5. Sample Collections
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
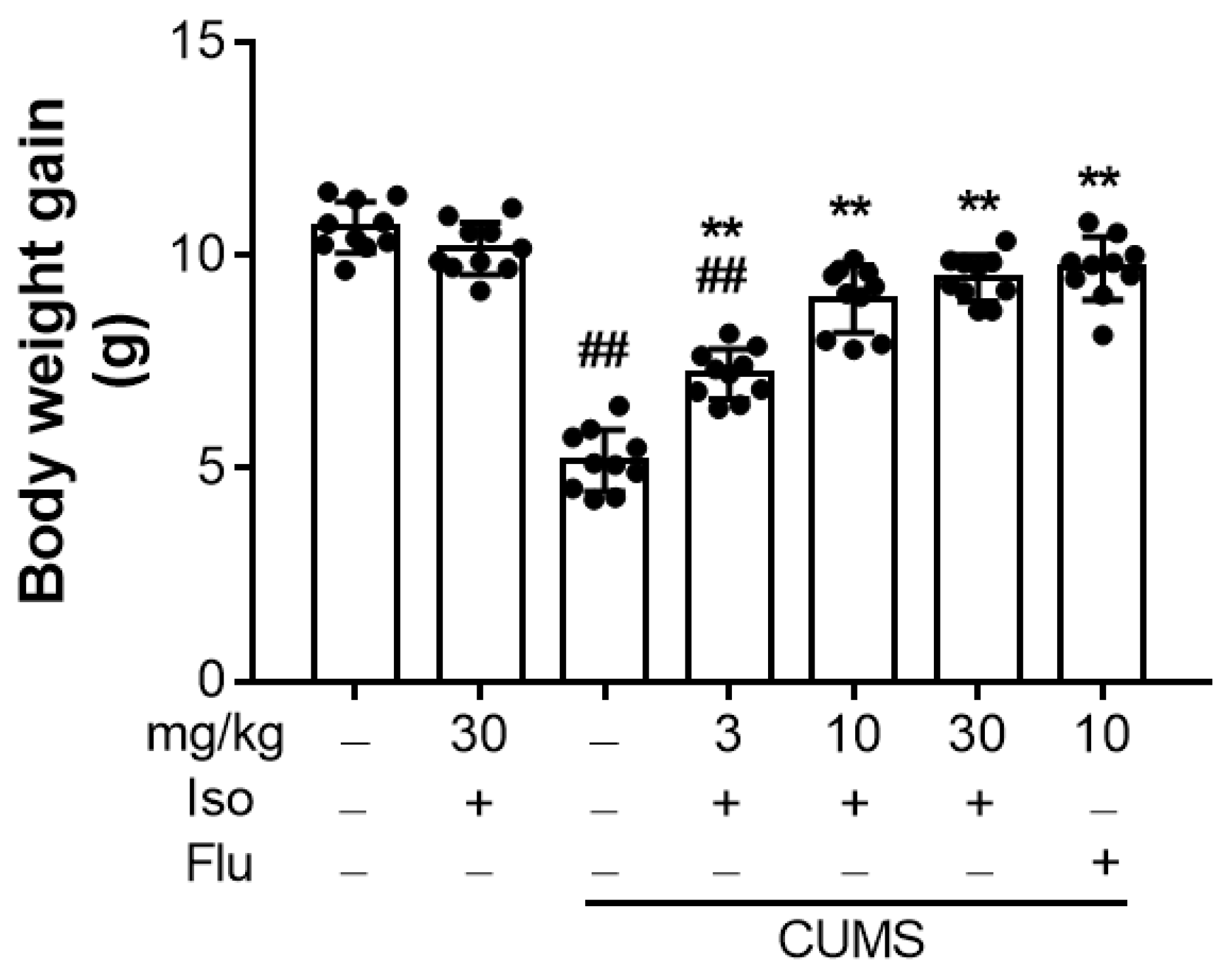
3.1. Isofraxidin Reverses CUMS-Induced Decrease in Body Weight Gain
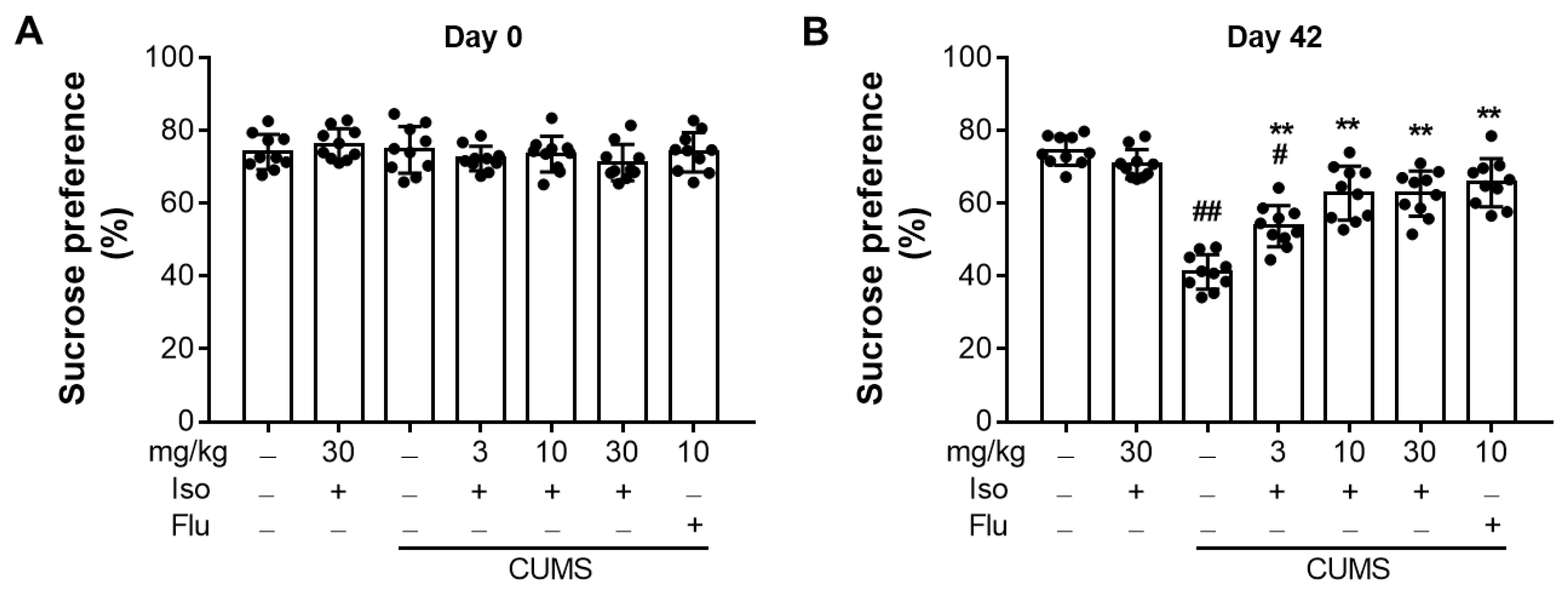
3.2. Isofraxidin Restores CUMS-Induced Decrease in Sucrose Consumption
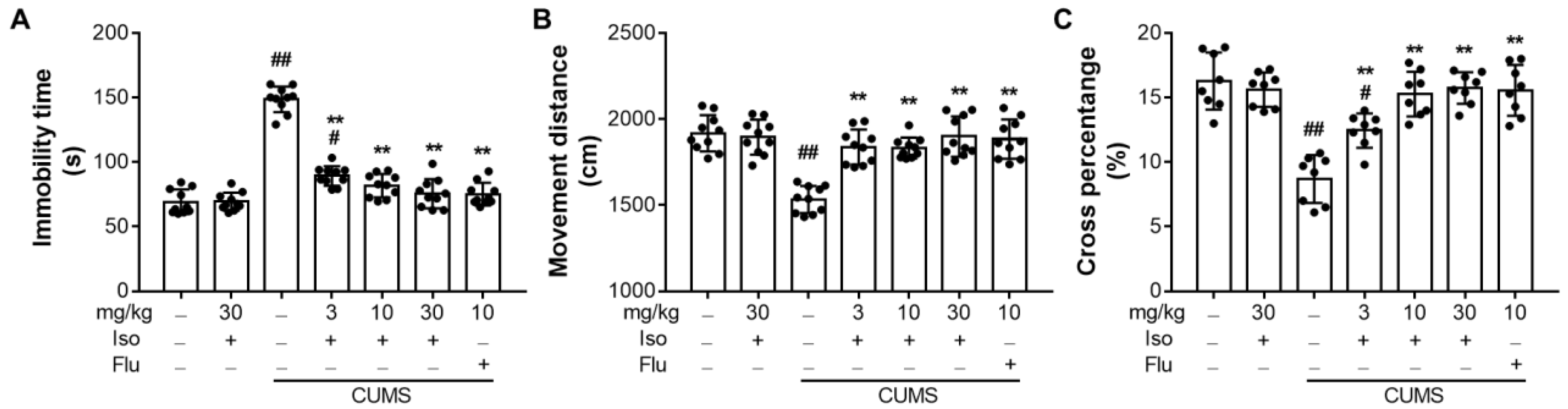
3.3. Isofraxidin Increases CUMS-Induced Decrease of Locomotor Activity in OFT
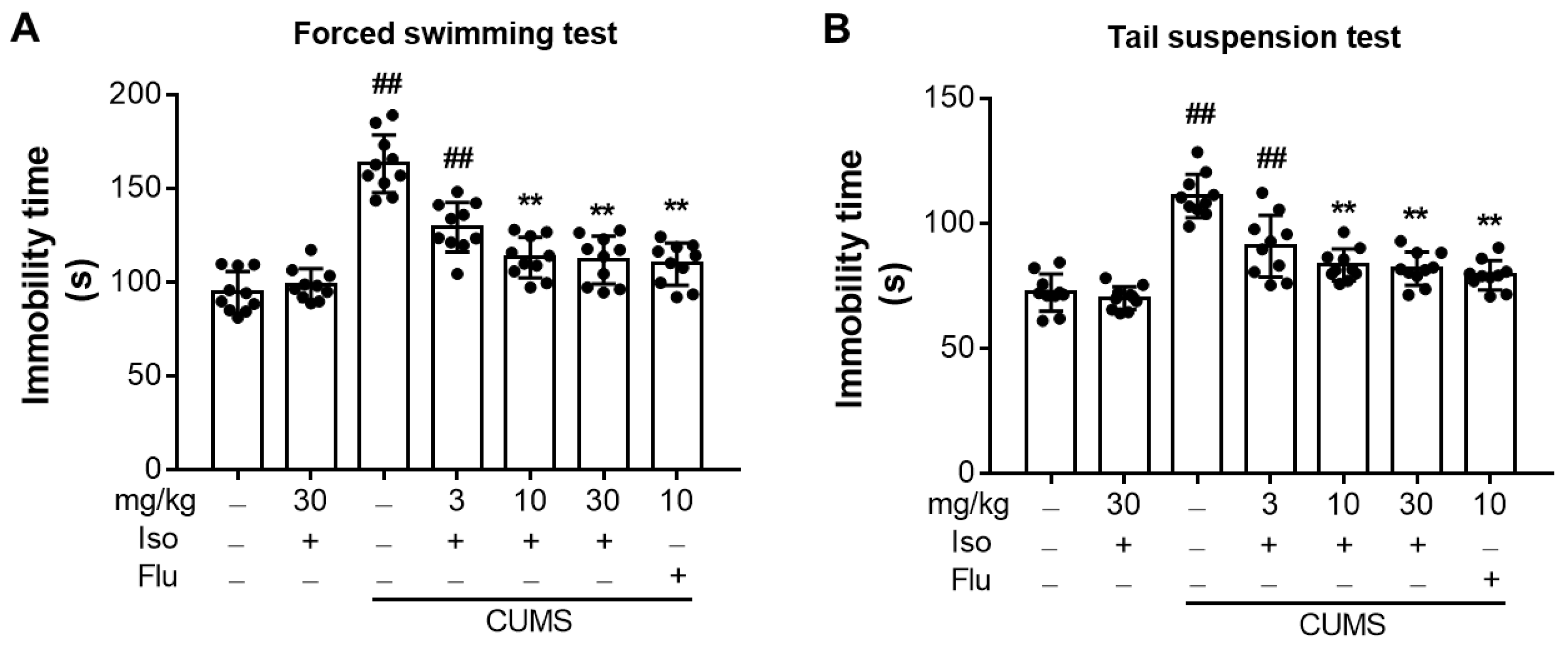
3.4. Isofraxidin Alleviates CUMS-Induced Increase of Immobility Duration in FST and TST
3.5. Isofraxidin Attenuates CUMS-Induced Hyperactivity of the Hypothalamic-Pituitary-Adrenal Axis
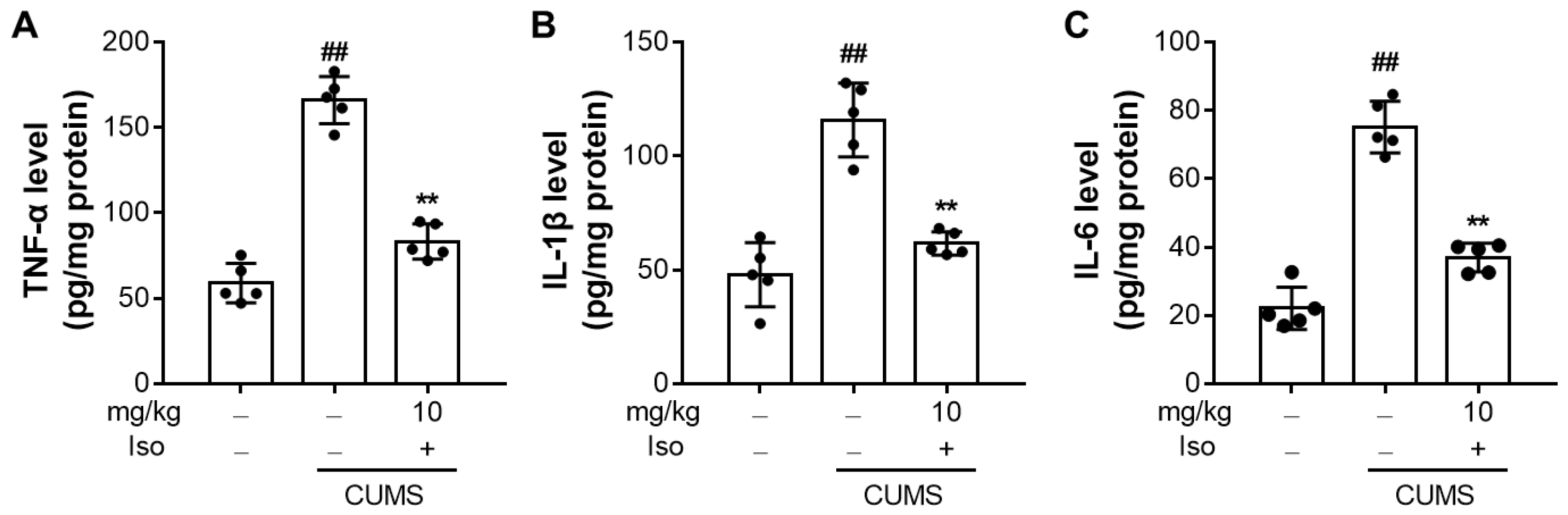
3.6. Isofraxidin Suppresses CUMS-Induced Increased Expression of Pro-Inflammatory Cytokines
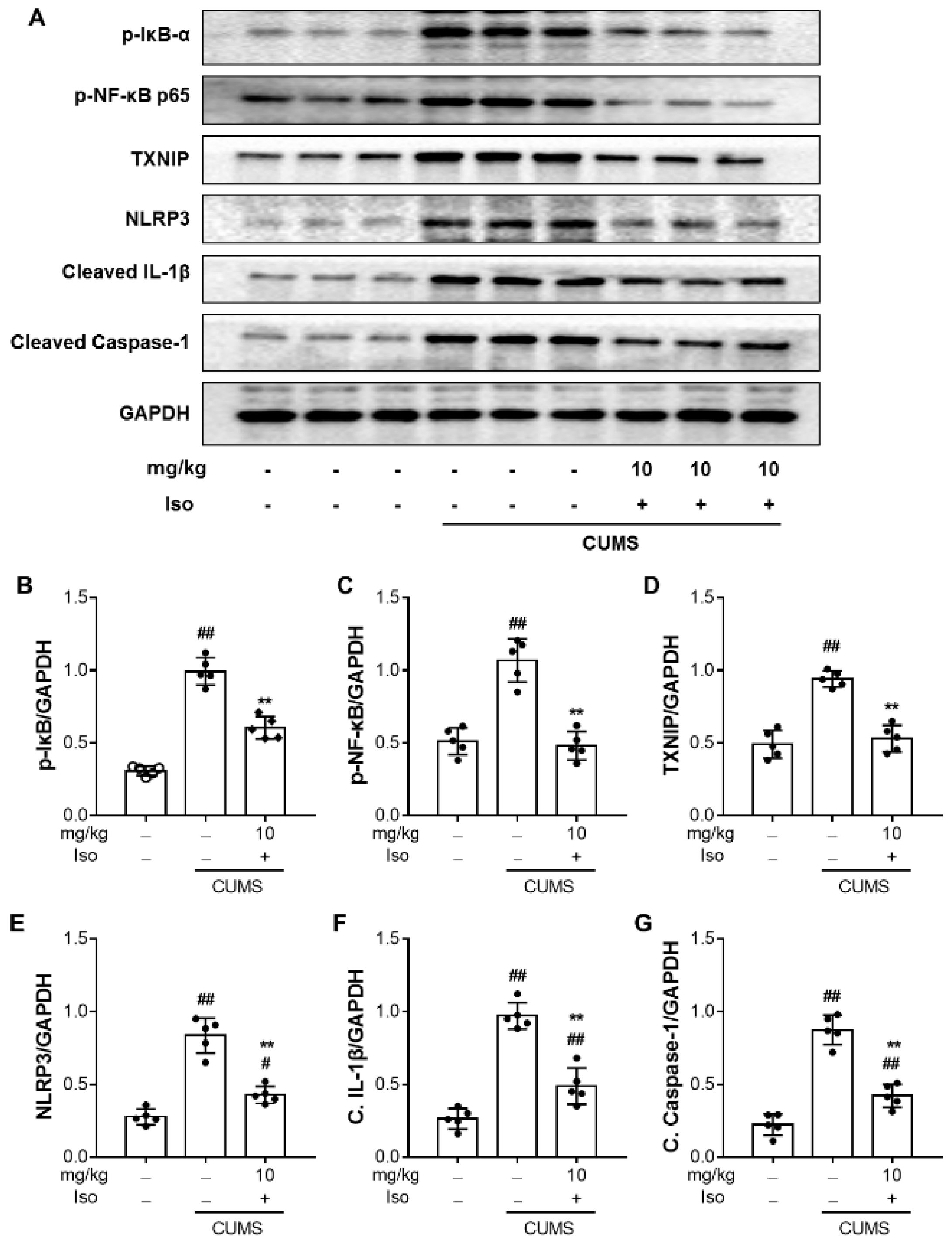
3.7. Isofraxidin Inhibits CUMS-Induced Activation of NF-κB and NLRP3 Inflammasome Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, H.J.; van den Berg, J.F.; Dekker, M.J.H.J.; van Tuijl, H.R.; Otte, W.; Smit, F.; Hofman, A.; Stricker, B.H.C.; Tiemeier, H. Incidence and Recurrence of Late-Life Depression. Arch. Gen. Psychiatary 2008, 65, 1394–1401. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Eiden, L.E. Activation of the HPA axis and depression of feeding behavior induced by restraint stress are separately regulated by PACAPergic neurotransmission in the mouse. Stress 2016, 19, 374–382. [Google Scholar] [CrossRef]
- Lu, S.; Gao, W.; Huang, M.; Li, L.; Xu, Y. In search of the HPA axis activity in unipolar depression patients with childhood trauma: Combined cortisol awakening response and dexamethasone suppression test. J. Psychiatr. Res. 2016, 78, 24–30. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawronska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain. Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.L.; Jaime, H.B.; Villanueva, M.D.E.; Padilla, M.B.; Palacios, G.V.; Aguilar, F.J.A. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol. Behav. 2016, 161, 15–23. [Google Scholar] [CrossRef]
- Adler, U.C.; Marques, A.H.; Calil, H.M. Inflammatory aspects of depression. Inflamm. Allergy Drug Targets 2008, 7, 19–23. [Google Scholar] [CrossRef]
- Alcocer-Gomez, E.; Cordero, M.D. NLRP3 Inflammasome: A New Target in Major Depressive Disorder. Cns. Neurosci. Ther. 2014, 20, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gomez, E.; de Miguel, M.; Casas-Barquero, N.; Nunez-Vasco, J.; Sanchez-Alcazar, J.A.; Fernandez-Rodriguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Henkel, T. Function Amd Activation of Nf-Kappa-B in the Immune-System. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-kappa B activity. Annu. Rev. Immunol. 2000, 18, 621. [Google Scholar] [CrossRef]
- Dionisie, V.; Ciobanu, A.M.; Toma, V.A.; Manea, M.C.; Baldea, I.; Olteanu, D.; Sevastre-Berghian, A.; Clichici, S.; Manea, M.; Riga, S.; et al. Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int. J. Mol. Sci. 2021, 22, 7483. [Google Scholar] [CrossRef] [PubMed]
- Rafało-Ulińska, A.; Brański, P.; Pałucha-Poniewiera, A. Combined Administration of (R)-Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Induces Rapid and Sustained Effects in the CUMS Model of Depression via a TrkB/BDNF-Dependent Mechanism. Pharmaceuticals 2022, 15, 125. [Google Scholar] [CrossRef]
- Zhang, M.M.; Huo, G.M.; Cheng, J.; Zhang, Q.P.; Li, N.Z.; Guo, M.X.; Liu, Q.; Xu, G.H.; Zhu, J.X.; Li, C.F.; et al. Gypenoside XVII, an Active Ingredient from Gynostemma Pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice. Nutrients 2022, 14, 2418. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Deng, M.Z.; Gao, Z.J.; Dang, Y.Y.; Zheng, G.D.; Yang, X.J.; Chao, Y.X.; Cai, Y.F.; Wu, X.L. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct 2020, 11, 4416–4427. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Mojarrab, M.; Kazemi-Afrakoti, S.; Farzaei, M.H. Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 2020, 25, 2040. [Google Scholar] [CrossRef]
- Lian, B.; Gu, J.; Zhang, C.; Zou, Z.; Yu, M.; Li, F.; Wu, X.; Zhao, A.Z. Protective effects of isofraxidin against scopolamine-induced cognitive and memory impairments in mice involve modulation of the BDNF-CREB-ERK signaling pathway. Metab. Brain Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, B. Isofraxidin Inhibits Receptor Activator of Nuclear Factor-kappaB Ligand-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages Isolated from Sprague-Dawley Rats by Regulating NF-kappaB/NFATc1 and Akt/NFATc1 Signaling Pathways. Cell Transpl. 2021, 30, 963689721990321. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mu, Q.; Li, W.; Xing, W.; Zhang, H.; Fan, T.; Yao, H.; He, L. Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes. Immunobiology 2015, 220, 406–413. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Lin, D.; Xu, P. Isofraxidin Alleviates Myocardial Infarction Through NLRP3 Inflammasome Inhibition. Inflammation 2020, 43, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Q.L.; Wu, L.H.; Jawaid, P.; Jiao, Y.F.; Kadowaki, M.; Kondo, T. Isofraxidin, a potent reactive oxygen species (ROS) scavenger, protects human leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in p53-independent manner. Apoptosis 2014, 19, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Ko, H.H.; Jin, Y.J.; Yang, S.Z.; Shih, Y.A.; Chen, I.S. Dihydrochalcone glucosides and antioxidant activity from the roots of Anneslea fragrans var. lanceolata. Phytochemistry 2012, 78, 120–125. [Google Scholar] [CrossRef]
- Hall, S.; Arora, D.; Anoopkumar-Dukie, S.; Grant, G.D. Effect of Coffee in Lipopolysaccharide-Induced Indoleamine 2,3-Dioxygenase Activation and Depressive-like Behavior in Mice. J. Agric. Food Chem. 2016, 64, 8745–8754. [Google Scholar] [CrossRef]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 Ameliorates Behavioral Abnormalities and Modulates the Hippocampal Proteomic Change in Triple Transgenic Mice of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef]
- Wu, X.; Tang, B.; Liao, X.; Su, Z.; Lee, S.M.; Cai, Y.; Li, C. Suppressive effects of the supercritical-carbon dioxide fluid extract of Chrysanthemum indicum on chronic unpredictable mild stress-induced depressive-like behavior in mice. Food Funct. 2019, 10, 1212–1224. [Google Scholar] [CrossRef]
- Monteiro, S.; Roque, S.; de Sa-Calcada, D.; Sousa, N.; Correia-Neves, M.; Cerqueira, J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 2015, 6, 6. [Google Scholar] [CrossRef]
- Lippi, S.L.P. Chronic Mild Unpredictable Stress and High-Fat Diet Given during Adolescence Impact Both Cognitive and Noncognitive Behaviors in Young Adult Mice. Brain Sci. 2021, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, G.K.; Hindi, E.A.; Zayed, M.A.; Abd El-Aziz, G.S.; Alturkistani, H.A.; Ibrahim, R.F.; Al-Thepyani, M.A.; Bakhlgi, R.; Alzahrani, N.A.; Ashraf, G.M.; et al. The Impact of Chronic Unpredictable Mild Stress-Induced Depression on Spatial, Recognition and Reference Memory Tasks in Mice: Behavioral and Histological Study. Behav. Sci. 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.I.; Abdul Aziz, N.H.K.; Noor, D.A.M. Antidepressant-like Effects of Polygonum minus Aqueous Extract in Chronic Ultra-Mild Stress-Induced Depressive Mice Model. Behav. Sci. 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yan, X.B.; Hofman, M.A.; Swaab, D.F.; Zhou, J.N. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci. Bull. 2010, 26, 297–303. [Google Scholar] [CrossRef]
- Kunugi, H.; Hori, H.; Adachi, N.; Numakawa, T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin. Neurosci. 2010, 64, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.J.S.; Neal, S.J.; Hughes, Z.A.; Beyna, M.; Rosenzweig-Lipson, S.; Moss, S.J.; Brandon, N.J. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl. Psychiatry 2012, 2, e199. [Google Scholar] [CrossRef]
- Capuron, L.; Neurauter, G.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Fuchs, D.; Miller, A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 2003, 54, 906–914. [Google Scholar] [CrossRef]
- Bot, M.; Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Mann, D.L. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. J. Psychosom. Res. 2011, 71, 13–17. [Google Scholar] [CrossRef][Green Version]
- Sutcigil, L.; Oktenli, C.; Musabak, U.; Bozkurt, A.; Cansever, A.; Uzun, O.; Sanisoglu, S.Y.; Yesilova, Z.; Ozmenler, N.; Ozsahin, A.; et al. Pro- and anti-inflammatory cytokine balance in major depression: Effect of sertraline therapy. Clin. Dev. Immunol. 2007, 2007, 76396. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, X.Y.; Zhang, Q.Y.; Kong, L.D. Microglial NLRP3 inflammasome activation mediates IL-1 beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav. Immun. 2014, 41, 90–100. [Google Scholar] [CrossRef]
- Tung, T.H.; Chen, Y.C.; Lin, Y.T.; Huang, S.Y. N-3 PUFA Ameliorates the Gut Microbiota, Bile Acid Profiles, and Neuropsychiatric Behaviours in a Rat Model of Geriatric Depression. Biomedicines 2022, 10, 1594. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Gu, J.; Zou, Z.; Yu, M.; Zhang, C.; Xiao, Q.; Chen, X.; Li, C. Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sci. 2022, 12, 1376. https://doi.org/10.3390/brainsci12101376
Wu X, Gu J, Zou Z, Yu M, Zhang C, Xiao Q, Chen X, Li C. Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sciences. 2022; 12(10):1376. https://doi.org/10.3390/brainsci12101376
Chicago/Turabian StyleWu, Xiaoli, Jingwen Gu, Zhicong Zou, Meng Yu, Chen Zhang, Qinghui Xiao, Xin Chen, and Chuwen Li. 2022. "Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice" Brain Sciences 12, no. 10: 1376. https://doi.org/10.3390/brainsci12101376
APA StyleWu, X., Gu, J., Zou, Z., Yu, M., Zhang, C., Xiao, Q., Chen, X., & Li, C. (2022). Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sciences, 12(10), 1376. https://doi.org/10.3390/brainsci12101376






