Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache
Abstract
1. Introduction
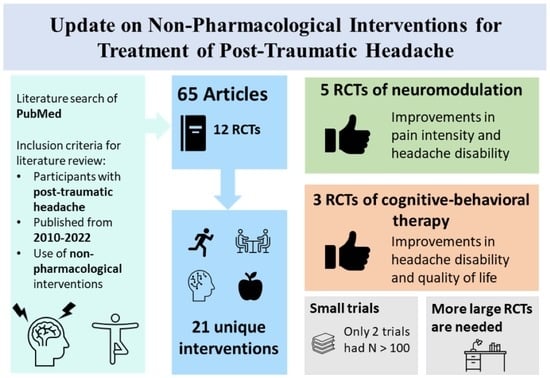
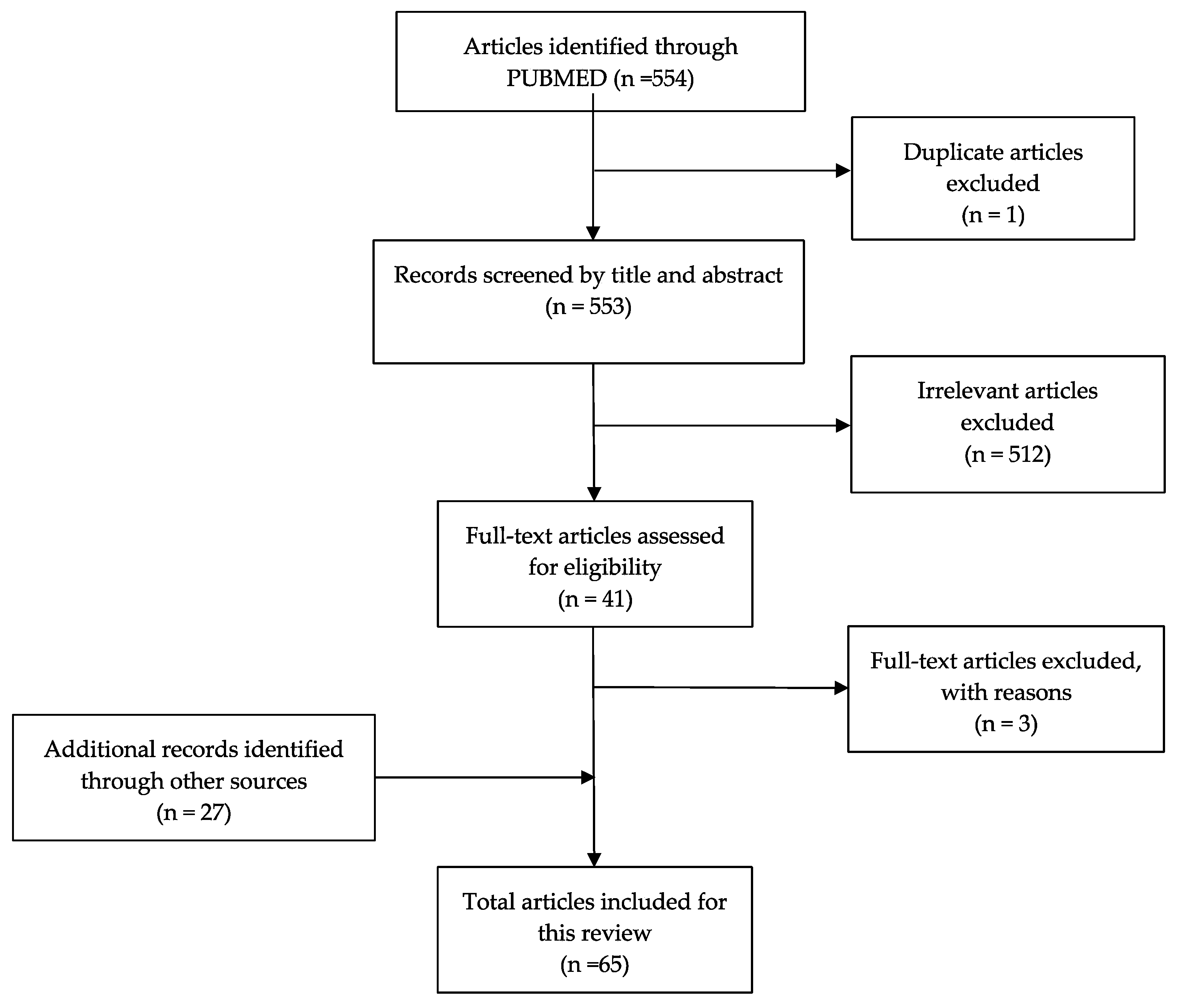
2. Methods
3. Cognitive and Behavioral Modification
4. Acupuncture
5. Lifestyle Modifications and Physical Therapy
6. Nutraceuticals
7. Neuromodulation
8. Osteopathic Manipulation Treatment (OMT)
9. Interdisciplinary Treatments
10. Treatment Recommendations
11. Limitations and Future Directives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Control. Traumatic Brain Injury & Concussion: TBI Data. Available online: https://www.cdc.gov/traumaticbraininjury/data/index.html (accessed on 4 September 2022).
- Seifert, T.D.; Evans, R.W. Posttraumatic Headache: A Review. Curr. Pain Headache Rep. 2010, 14, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.D. Sports Concussion and Associated Post-Traumatic Headache. Headache J. Head Face Pain 2013, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.K.; Bell, K.R.; Walker, W.C.; Schomer, K. Systematic Review of Interventions for Post-traumatic Headache. PM&R 2012, 4, 129–140. [Google Scholar] [CrossRef]
- Labastida-Ramírez, A.; Benemei, S.; Albanese, M.; D’Amico, A.; Grillo, G.; Grosu, O.; Ertem, D.H.; Mecklenburg, J.; Fedorova, E.P.; Řehulka, P.; et al. Persistent post-traumatic headache: A migrainous loop or not? The clinical evidence. J. Headache Pain 2020, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Kamins, J. Models for Treating Post-traumatic Headache. Curr. Pain Headache Rep. 2021, 25, 52. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.F.; Eggertsen, P.P.; Frederiksen, O.V.; Thastum, M.M.; Svendsen, S.W.; Tuborgh, A.; Næss-Schmidt, E.T.; Rask, C.U.; Schröder, A.; Kasch, H.; et al. Characterization of persistent post-traumatic headache and management strategies in adolescents and young adults following mild traumatic brain injury. Sci. Rep. 2022, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Boubour, A.; Walia, H.; Barr, W. Post-Concussive Syndrome: A Focus on Post-Traumatic Headache and Related Cognitive, Psychiatric, and Sleep Issues. Curr. Neurol. Neurosci. Rep. 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Riechers, R.G.; Walker, M.F.; Ruff, R.L. Post-traumatic headaches. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, pp. 567–578. [Google Scholar] [CrossRef]
- Blume, H.K. Headaches after Concussion in Pediatrics: A Review. Curr. Pain Headache Rep. 2015, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Register-Mihalik, J.K.; DeFreese, J.D.; Callahan, C.E.; Carneiro, K. Utilizing the Biopsychosocial Model in Concussion Treatment: Post-Traumatic Headache and beyond. Curr. Pain Headache Rep. 2020, 24, 44. [Google Scholar] [CrossRef]
- Seifert, T. Post-Traumatic Headache Therapy in the Athlete. Curr. Pain Headache Rep. 2016, 20, 41. [Google Scholar] [CrossRef]
- Brown, A.W.; Watanabe, T.K.; Hoffman, J.M.; Bell, K.R.; Lucas, S.; Dikmen, S. Headache After Traumatic Brain Injury: A National Survey of Clinical Practices and Treatment Approaches. PM&R 2015, 7, 3–8. [Google Scholar] [CrossRef]
- Howard, L.; Schwedt, T.J. Posttraumatic headache: Recent progress. Curr. Opin. Neurol. 2020, 33, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Levyim, D.; Choe, M.; Taraman, S.; Langdon, R. Survey of Child Neurologists on Management of Pediatric Post-traumatic Headache. J. Child Neurol. 2019, 34, 739–747. [Google Scholar] [CrossRef]
- Lucas, S. Post-Traumatic Headache. In Headache and Migraine Biology and Management; Elsevier: Amsterdam, The Netherlands, 2015; pp. 161–174. [Google Scholar] [CrossRef]
- Kacperski, J.; Arthur, T. Management of post-traumatic headaches in children and adolescents. Headache J. Head Face Pain 2016, 56, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M.; Naegel, S.; Bosche, B.; Holle, D. An update on the management of post-traumatic headache. Ther. Adv. Neurol. Disord. 2015, 8, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Mitsikostas, D.D.; Mantovani, E.; Litsardopoulos, P.; Panagiotopoulos, V.; Tamburin, S. An updated brief overview on post-traumatic headache and a systematic review of the non-pharmacological interventions for its management. Expert Rev. Neurother. 2021, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.; Jinich, S.; Ellett, G.V. Behavioral Therapies and Mind-Body Interventions for Posttraumatic Headache and Post-Concussive Symptoms: A Systematic Review. Headache J. Head Face Pain 2019, 59, 151–163. [Google Scholar] [CrossRef]
- Twamley, E.W.; Jak, A.J.; Delis, D.C.; Bondi, M.W.; Lohr, J.B. Cognitive Symptom Management and Rehabilitation Therapy (CogSMART) for Veterans with traumatic brain injury: Pilot randomized controlled trial. J. Rehabil. Res. Dev. 2014, 51, 59–70. [Google Scholar] [CrossRef]
- Kacperski, J.; Hung, R.; Blume, H.K. Pediatric Posttraumatic Headache. Semin. Pediatr. Neurol. 2016, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.; Balcer, L.; Galetta, S.; Minen, M. Feasibility of Smartphone-Delivered Progressive Muscle Relaxation in Persistent Post-Traumatic Headache Patients. J. Neurotrauma 2021, 38, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, M.; Scheid, A.; Fine, E.; Zoffness, R. Review of the Management of Pediatric Post-Concussion Syndrome—A Multi-Disciplinary, Individualized Approach. Curr. Rev. Muscul. Med. 2019, 12, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fraser, F.; Matsuzawa, Y.; Lee, Y.S.C.; Minen, M. Behavioral Treatments for Post-Traumatic Headache. Curr. Pain Headache Rep. 2017, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Perucchini, D.; Cleland, J.A.; Barbieri, C.; de Lima e Sá Resende, F.; Negrini, S. The effectiveness of a cognitive behavioral exercise approach (CBEA) compared to usual care in patients with a Whiplash Associated Disorder: A quasi-experimental clinical trial. BMR 2017, 30, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kjeldgaard, D.; Forchhammer, H.B.; Teasdale, T.W.; Jensen, R.H. Cognitive behavioural treatment for the chronic post-traumatic headache patient: A randomized controlled trial. J. Headache Pain 2014, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- McGeary, D.D.; Resick, P.A.; Penzien, D.B.; McGeary, C.A.; Houle, T.T.; Eapen, B.C.; Jaramillo, C.A.; Nabity, P.S.; Reed, D.E.; Moring, J.C.; et al. Cognitive Behavioral Therapy for Veterans with Comorbid Posttraumatic Headache and Posttraumatic Stress Disorder Symptoms: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 746–757. [Google Scholar] [CrossRef]
- McCarty, C.A.; Zatzick, D.; Stein, E.; Wang, J.; Hilt, R.; Rivara, F.P. Collaborative Care for Adolescents with Persistent Postconcussive Symptoms: A Randomized Trial. Pediatrics 2016, 138, e20160459. [Google Scholar] [CrossRef] [PubMed]
- Khusid, M.A. Clinical Indications for Acupuncture in Chronic Post-Traumatic Headache Management. Mil. Med. 2015, 180, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.B.; Bellanti, D.M.; Paat, C.F.; Boyd, C.C.; Duncan, A.; Price, A.; Zhang, W.; French, L.M.; Chae, H. A Randomized Exploratory Study to Evaluate Two Acupuncture Methods for the Treatment of Headaches Associated with Traumatic Brain Injury. Med. Acupunct. 2016, 28, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Pinchefsky, E.; Dubrovsky, A.S.; Friedman, D.; Shevell, M. Part II—Management of Pediatric Post-traumatic Headaches. Pediatr. Neurol. 2015, 52, 270–280. [Google Scholar] [CrossRef]
- Baker, V.B.; Eliasen, K.M.; Hack, N.K. Lifestyle modifications as therapy for medication refractory post-traumatic headache (PTHA) in the military population of Okinawa. J. Headache Pain 2018, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.P.; Shah, R.; Irwin, S.L.; Greene, K.; Szperka, C.L. Acute and chronic management of posttraumatic headache in children: A systematic review. Headache 2021, 61, 1475–1492. [Google Scholar] [CrossRef] [PubMed]
- Conidi, F.X. Interventional Treatment for Post-traumatic Headache. Curr. Pain Headache Rep. 2016, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Kirkwood, M.W.; Potter, M.N.; Wilson, P.E.; Provance, A.J.; Howell, D.R. Early physical activity and clinical outcomes following pediatric sport-related concussion. J. Clin. Transl. Res. 2020, 5, 161–168. [Google Scholar] [PubMed]
- Grabowski, P.; Wilson, J.; Walker, A.; Enz, D.; Wang, S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: A retrospective analysis on safety and feasibility. Phys. Ther. Sport 2017, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kamins, J.; Charles, A. Posttraumatic Headache: Basic Mechanisms and Therapeutic Targets. Headache J. Head Face Pain 2018, 58, 811–826. [Google Scholar] [CrossRef]
- Schneider, K.J.; Meeuwisse, W.H.; Nettel-Aguirre, A.; Barlow, K.; Boyd, L.; Kang, J.; Emery, C.A. Cervicovestibular rehabilitation in sport-related concussion: A randomised controlled trial. Br. J. Sports Med. 2014, 48, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Schwedt, T.J. Post-traumatic headache due to mild traumatic brain injury: Current knowledge and future directions. Cephalalgia 2021, 41, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Monsour, D.A.; Lay, C.; Ansari, T.; Lagman-Bartolome, A.M. Post-Traumatic Headache in Children and Adolescents: A Narrative Review with a Focus on Management. Curr. Neurol. Neurosci. Rep. 2020, 20, 53. [Google Scholar] [CrossRef]
- Scrimgeour, A.G.; Condlin, M.L.; Loban, A.; DeMar, J.C. Omega-3 Fatty Acids and Vitamin D Decrease Plasma T-Tau, GFAP, and UCH-L1 in Experimental Traumatic Brain Injury. Front. Nutr. 2021, 8, 685220. [Google Scholar] [CrossRef]
- Colombo, B.; Saraceno, L.; Comi, G. Riboflavin and migraine: The bridge over troubled mitochondria. Neurol. Sci. 2014, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Trojian, T.H.; Wang, D.H.; Leddy, J.J. Nutritional Supplements for the Treatment and Prevention of Sports-Related Concussion—Evidence Still Lacking. Curr. Sport. Med. Rep. 2017, 16, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, A.; Crawford, S.; Bodell, L.; Dewey, D.; Barlow, K.M. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: A prospective cohort. Dev. Med. Child Neurol. 2013, 55, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Piantino, J.; Lim, M.M.; Newgard, C.D.; Iliff, J. Linking Traumatic Brain Injury, Sleep Disruption and Post-Traumatic Headache: A Potential Role for Glymphatic Pathway Dysfunction. Curr. Pain Headache Rep. 2019, 23, 62. [Google Scholar] [CrossRef]
- Standiford, L.; O’Daniel, M.; Hysell, M.; Trigger, C. A randomized cohort study of the efficacy of PO magnesium in the treatment of acute concussions in adolescents. Am. J. Emerg. Med. 2021, 44, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Suchindran, C.M.; MacIntosh, B.A.; Gaylord, S.; Ringel, A.; Hibbeln, J.R.; Feldstein, A.E.; Mori, T.A.; et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain 2013, 154, 2441–2451. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; MacIntosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.-X.; Miller, V.; Lynch, C.; Honvoh, G.; et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ 2021, 374, n1448. [Google Scholar] [CrossRef]
- Faurot, K.R.; Cole, W.R.; MacIntosh, B.A.; Dunlap, M.; Moore, C.B.; Roberson, B.; Guerra, M.; Domenichiello, A.F.; Palsson, O.; Rivera, W.; et al. Targeted dietary interventions to reduce pain in persistent post-traumatic headache among service members: Protocol for a randomized, controlled parallel-group trial. Contemp. Clin. Trials 2022, 119, 106851. [Google Scholar] [CrossRef]
- Mollica, A.; Safavifar, F.; Fralick, M.; Giacobbe, P.; Lipsman, N.; Burke, M.J. Transcranial Magnetic Stimulation for the Treatment of Concussion: A Systematic Review. Neuromodul. Technol. Neural Interface 2021, 24, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Greben, R.; Oriuwa, C.; Siddiqi, S.H.; Burke, M.J. Neuromodulation Treatments for Mild Traumatic Brain Injury and Post-concussive Symptoms. Curr. Neurol. Neurosci. Rep. 2022, 22, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Shukla, S.; Fallah, A.; Song, D.; Lin, L.; Golshan, S.; Tsai, A.; Jak, A.; Polston, G.; Lee, R. Repetitive Transcranial Magnetic Stimulation in Managing Mild Traumatic Brain Injury-Related Headaches. Neuromodul. Technol. Neural Interface 2016, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Metzger-Smith, V.; He, Y.; Cordero, J.; Ehlert, B.; Song, D.; Lin, L.; Golshan, S.; Tsai, A.; Vaninetti, M.; et al. Left Dorsolateral Prefrontal Cortex rTMS in Alleviating MTBI Related Headaches and Depressive Symptoms. Neuromodul. Technol. Neural Interface 2018, 21, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Stilling, J.M.; Paxman, E.; Mercier, L.J.; Gan, L.S.; Wang, M.; Amoozegar, F.; Dukelow, S.P.; Monchi, O.; Debert, C.T. Treatment of Persistent Post-Traumatic Headache and Post-Concussion Symptoms Using Repetitive Transcranial Magnetic Stimulation: A Pilot, Double-Blind, Randomized Controlled Trial. J. Neurotrauma 2020, 37, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.; Skinner, K.; Prabhakaran, V.; Kaczmarek, K.; Danilov, Y. Translingual Neurostimulation for the Treatment of Chronic Symptoms Due to Mild-to-Moderate Traumatic Brain Injury. Arch. Rehabil. Res. Clin. Transl. 2019, 1, 100026. [Google Scholar] [CrossRef]
- Ptito, A.; Papa, L.; Gregory, K.; Folmer, R.L.; Walker, W.C.; Prabhakaran, V.; Wardini, R.; Skinner, K.; Yochelson, M. A Prospective, Multicenter Study to Assess the Safety and Efficacy of Translingual Neurostimulation Plus Physical Therapy for the Treatment of a Chronic Balance Deficit Due to Mild-to-Moderate Traumatic Brain Injury. Neuromodul. Technol. Neural Interface 2021, 24, 1412–1421. [Google Scholar] [CrossRef]
- Carlson, J.; Ross, G.W. Neurofeedback Impact on Chronic Headache, Sleep, and Attention Disorders Experienced by Veterans with Mild Traumatic Brain Injury: A Pilot Study. Biofeedback 2021, 49, 2–9. [Google Scholar] [CrossRef]
- Srihagulang, C.; Vongsfak, J.; Vaniyapong, T.; Chattipakorn, N.; Chattipakorn, S.C. Potential roles of vagus nerve stimulation on traumatic brain injury: Evidence from in vivo and clinical studies. Exp. Neurol. 2022, 347, 113887. [Google Scholar] [CrossRef]
- Rytter, H.M.; Graff, H.J.; Henriksen, H.K.; Aaen, N.; Hartvigsen, J.; Hoegh, M.; Nisted, I.; Næss-Schmidt, E.T.; Pedersen, L.L.; Schytz, H.W.; et al. Nonpharmacological Treatment of Persistent Postconcussion Symptoms in Adults: A Systematic Review and Meta-analysis and Guideline Recommendation. JAMA Netw. Open 2021, 4, e2132221. [Google Scholar] [CrossRef]
- Murray, T.R.; Ferderer, T.; Gehred, A.; Rose, S.C. Treatment of Post-traumatic Headaches in Children: A Systematic Review. Semin. Pediatr. Neurol. 2021, 40, 100935. [Google Scholar] [CrossRef]
- Davis, L.; Hanson, B.; Gilliam, S. Pilot study of the effects of mixed light touch manual therapies on active duty soldiers with chronic post-traumatic stress disorder and injury to the head. J. Bodyw. Mov. Ther. 2016, 20, 42–51. [Google Scholar] [CrossRef]
- Esterov, D.; Thomas, A.; Weiss, K. Osteopathic manipulative medicine in the management of headaches associated with postconcussion syndrome. J. Osteopath. Med. 2021, 121, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Rytter, H.M.; Westenbaek, K.; Henriksen, H.; Christiansen, P.; Humle, F. Specialized interdisciplinary rehabilitation reduces persistent post-concussive symptoms: A randomized clinical trial. Brain Inj. 2019, 33, 266–281. [Google Scholar] [CrossRef] [PubMed]
| Intervention Type | Examples | Summary of Findings |
|---|---|---|
| Cognitive and Behavioral Modification |
|
|
| Acupuncture |
|
|
| Lifestyle Modification |
|
|
| Physical Activity |
|
|
| Nutraceuticals |
|
|
| Neuromodulation |
|
|
| Interdisciplinary |
|
|
| Study | Study Design | Intervention | Participants | Headache Outcome Measure(s) | Results | Limitations |
|---|---|---|---|---|---|---|
| Leung et al. (2016) [54] | Sham, randomized controlled trial | rTMS | rTMS Group:
SHAM Group:
| Headache exacerbation composite score (sum of headache intensity rating/duration) |
|
|
| Leung et al. (2018) [55] | Sham, randomized controlled trial | rTMS | rTMS Group:
SHAM Group:
| Average daily headache intensity Occurrences of daily headaches Debilitating headache composite score |
|
|
| Stilling et al. (2020) [56] | Pilot, double-blinded randomized controlled trial | rTMS | rTMS Group:
SHAM Group:
| Headache severity and frequency Headache Impact Test-6 (HIT-6) |
|
|
| Tyler et al. (2019) [57] | Double-blinded randomized controlled trial | Translingual neurostimulation | High-Frequency Group:
Low-Frequency Group:
| Headache Disability Index |
|
|
| Ptito et al. (2021) [58] | Double-blinded randomized controlled trial | Translingual neurostimulation + physical therapy | High-Frequency Group:
Low-Frequency Group:
| Headache Disability Index |
|
|
| Nelson et al. (2015) [59] | Pilot study | Infra-low neurofeedback |
| Headache Impact Test-6 (HIT-6) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Zhou, Y.; Greenwald, B.D. Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache. Brain Sci. 2022, 12, 1357. https://doi.org/10.3390/brainsci12101357
Lee MJ, Zhou Y, Greenwald BD. Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache. Brain Sciences. 2022; 12(10):1357. https://doi.org/10.3390/brainsci12101357
Chicago/Turabian StyleLee, Matthew J., Yi Zhou, and Brian D. Greenwald. 2022. "Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache" Brain Sciences 12, no. 10: 1357. https://doi.org/10.3390/brainsci12101357
APA StyleLee, M. J., Zhou, Y., & Greenwald, B. D. (2022). Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache. Brain Sciences, 12(10), 1357. https://doi.org/10.3390/brainsci12101357