Differential Effects of Exercise on fMRI of the Midbrain Ascending Arousal Network Nuclei in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) in a Model of Postexertional Malaise (PEM)
Abstract
:1. Introduction
2. Methods
2.1. Ethics
2.2. Demographics
2.3. Exercise Provocation
2.4. Orthostatic Postural Tachycardia Phenotypes
- (i)
- Postural orthostatic tachycardia (POTS) was defined by ∆HR ≥ 30 beats per minute at least 4 time points before exercise and during each postexercise measurement period.
- (ii)
- Stress Test Activated Reversible Tachycardia (START) was defined by a normal ∆HR before exercise, but at least 2 episodes with ∆HR ≥ 30 beats per minute after exercise. The phenomenon was transient as postural tachycardia returned to normal within 36 to 48 h.
- (iii)
2.5. Verbal Working Memory Task
2.6. MRI Data Acquisition, Preprocessing and Analysis
3. Results
3.1. Demographics and Questionnaires
3.2. Partial Correlations
3.3. ANOVA
3.4. Multivariate General Linear Models (mGLM)
3.5. Light and Sound Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Fukuda, K.; Nisenbaum, R.; Stewart, G.; Thompson, W.W.; Robin, L.; Washko, R.M.; Noah, D.L.; Barrett, D.H.; Randall, B.; Herwaldt, B.L.; et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. J. Am. Med. Assoc. 1998, 280, 981–988. [Google Scholar] [CrossRef]
- Steele, L. Prevalence and patterns of Gulf War illness in Kansas veterans: Association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 2000, 152, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.A.; Henry, B.E.; Peterson, D.L.; Swarts, R.L.; Bastien, S.; Thomas, R.S. Chronic Fatigue Syndrome in Northern Nevada. Clin. Infect. Dis. 1991, 13, S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, F.; Bateman, L.; Bested, A.; Davenport, T.; Friedman, K.; Gurwitt, A.; Jason, L.; Lappp, C.; Stevens, S.; Underhill, R.; et al. Me/CFS: Primer for Clinical Practitioners; International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: Stony Brook, NY, USA, 2014; pp. 1–541. [Google Scholar]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015; ISBN 0309316898.
- White, R.F.; Steele, L.; O’Callaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C.; et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex Factors in the Etiology of Gulf War Illness: Wartime Exposures and Risk Factors in Veteran Subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef]
- Johnson, M.K. Organophosphorus esters causing delayed neurotoxic effects: Mechanism of action and structure activity studies. Arch. Toxicol. 1975, 34, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Nolte, C.T.; Jackrel, J.; Grob, D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am. J. Med. 1971, 50, 475–492. [Google Scholar] [CrossRef]
- Richardson, R.J.; Fink, J.K.; Glynn, P.; Hufnagel, R.B.; Makhaeva, G.F.; Wijeyesakere, S.J. Neuropathy target esterase (NTE/PNPLA6) and organophosphorus compound-induced delayed neurotoxicity (OPIDN). Adv. Neurotoxic. 2020, 4, 1–78. [Google Scholar] [CrossRef]
- Washington, S.D.; Rayhan, R.U.; Garner, R.; Provenzano, D.; Zajur, K.; Addiego, F.M.; VanMeter, J.W.; Baraniuk, J.N. Exercise alters brain activation in Gulf War Illness and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain Commun. 2020, 2, fcaa070. [Google Scholar] [CrossRef]
- Stouten, B. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv. Res. 2005, 5, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.F.; Lin, J.-M.S.; Maloney, E.M.; Boneva, R.S.; Nater, U.M.; Unger, E.R.; Reeves, W.C. An evaluation of exclusionary medical/psychiatric conditions in the definition of chronic fatigue syndrome. BMC Med. 2009, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nater, U.M.; Lin, J.M.S.; Maloney, E.M.; Jones, J.F.; Hao, T.; Boneva, R.S.; Raison, C.L.; Reeves, M.W.C.; Heim, C. Psychiatric comorbidity in persons with chronic fatigue syndrome identified from the georgia population. Psychosom. Med. 2009, 71, 557–565. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999, 282, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Baraniuk, J.N.; Adewuyi, O.; Merck, S.J.; Ali, M.; Ravindran, M.K.; Timbol, C.R.; Rayhan, R.; Zheng, Y.; Le, U.; Esteitie, R.; et al. A Chronic Fatigue Syndrome (CFS) severity score based on case designation criteria. Am. J. Transl. Res. 2013, 5, 53–68. [Google Scholar]
- McHorney, C.A.; Ware, J.E.; Rachel Lu, J.F.; Sherbourne, C.D. The MOS 36-ltem Short-Form Health Survey (SF-36): III. Tests of Data Quality, Scaling Assumptions, and Reliability across Diverse Patient Groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- Cella, M.; Chalder, T. Measuring fatigue in clinical and community settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Revicki, D.A.; Harding, G.; Coyne, K.S.; Peirce-Sandner, S.; Bhagwat, D.; Everton, D.; Burke, L.B.; Cowan, P.; et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009, 144, 35–42. [Google Scholar] [CrossRef]
- Naranch, K.; Park, Y.-J.; Repka-Ramirez, M.S.; Velarde, A.; Clauw, D.; Baraniuk, J.N. A Tender Sinus does not Always Mean Rhinosinusitis. Otolaryngol. Neck Surg. 2002, 127, 387–397. [Google Scholar] [CrossRef]
- Surian, A.A.; Baraniuk, J.N. Systemic Hyperalgesia in Females with Gulf War Illness, Chronic Fatigue Syndrome and Fibromyalgia. Sci. Rep. 2020, 10, 5751. [Google Scholar] [CrossRef] [Green Version]
- Garner, R.; Baraniuk, J.N. Orthostatic intolerance in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 185. [Google Scholar] [CrossRef]
- Garner, R.S.; Rayhan, R.U.; Baraniuk, J.N. Verification of exercise-induced transient postural tachycardia phenotype in Gulf War Illness. Am. J. Transl. Res. 2018, 10, 3254–3264. [Google Scholar]
- Gracely, R.H.; Kwilosz, D.M. The Descriptor Differential Scale: Applying psychophysical principles to clinical pain assessment. Pain 1988, 35, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Rayhan, R.U.; Washington, S.D.; Garner, R.; Zajur, K.; Martinez Addiego, F.; Vanmeter, J.W.; Baraniuk, J.N. Exercise challenge alters Default Mode Network dynamics in Gulf War Illness. BMC Neurosci. 2019, 20, 7. [Google Scholar] [CrossRef] [Green Version]
- Washington, S.D.; Rayhan, R.U.; Garner, R.; Provenzano, D.; Zajur, K.; Addiego, F.M.; VanMeter, J.W.; Baraniuk, J.N. Exercise alters cerebellar and cortical activity related to working memory in phenotypes of Gulf War Illness. Brain Commun. 2020, 2, fcz039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayhan, R.U.; Stevens, B.W.; Raksit, M.P.; Ripple, J.A.; Timbol, C.R.; Adewuyi, O.; VanMeter, J.W.; Baraniuk, J.N. Exercise Challenge in Gulf War Illness Reveals Two Subgroups with Altered Brain Structure and Function. PLoS ONE 2013, 8, e63903. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.C.; Collins, D.L.; Mills, S.R.; Brown, E.D.; Kelly, R.L.; Peters, T.M. 3D statistical neuroanatomical models from 305 MRI volumes. In Proceedings of the 1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference, San Francisco, CA, USA, 31 October–6 November 1993; pp. 1813–1817. [Google Scholar]
- SPM12 Software—Statistical Parametric Mapping. Available online: https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ (accessed on 16 February 2021).
- Edlow, B.L.; Takahashi, E.; Wu, O.; Benner, T.; Dai, G.; Bu, L.; Grant, P.E.; Greer, D.M.; Greenberg, S.M.; Kinney, H.C.; et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 2012, 71, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Athinoula, A. Martinos Center for Biomedical Imaging. Harvard Ascending Arousal Network Atlas. Available online: https://www.nmr.mgh.harvard.edu/resources/aan-atlas (accessed on 1 February 2020).
- Horn, A.; Kühn, A.A. Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage 2015, 107, 127–135. [Google Scholar] [CrossRef]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019, 184, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, A.; Nguyen, T.A.-K.; Tinkhauser, G.; Petermann, K.; Debove, I.; Wiest, R.; Pollo, C. Accuracy of different three-dimensional subcortical human brain atlases for DBS -lead localisation. NeuroImage. Clin. 2018, 20, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Brett, M. Region of interest analysis using an SPM toolbox. Neuroimage 2002, 13, 210–217. [Google Scholar]
- Brett, M. MarsBaR region of interest toolbox for SPM. Neuroimage 2002, 16, 769–1198. [Google Scholar]
- Puelles, L. Comments on the limits and internal structure of the mammalian midbrain. Anatomy 2016, 10, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Puelles, L. Survey of Midbrain, Diencephalon, and Hypothalamus Neuroanatomic Terms Whose Prosomeric Definition Conflicts with Columnar Tradition. Front. Neuroanat. 2019, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, C.; Bartholomaeus, C.; Puelles, L. Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons from Developmental Gene Patterns. Front. Neuroanat. 2019, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Kinomura, S.; Larsson, J.; Gulyás, B.; Roland, P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996, 271, 512–515. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Griebel, G.; Pobbe, R.; Blanchard, R.J. Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 2011, 35, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.C. Translating dynamic defense patterns from rodents to people. Neurosci. Biobehav. Rev. 2017, 76, 22–28. [Google Scholar] [CrossRef]
- Bracha, H.S. Freeze, flight, fight, fright, faint: Adaptationist perspectives on the acute stress response spectrum. CNS Spectr. 2004, 9, 679–685. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Gladwin, T.E.; de Valk, N.M.; Zhang, W.; Kaldewaij, R.; van Ast, V.; Koch, S.B.J.; Klumpers, F.; Roelofs, K. Neural Dynamics of Shooting Decisions and the Switch from Freeze to Fight. Sci. Rep. 2019, 9, 4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Ranaldi, R. Dopamine and reward seeking: The role of ventral tegmental area. Rev. Neurosci. 2014, 25, 621–630. [Google Scholar] [CrossRef]
- Michelsen, K.A.; Prickaerts, J.; Steinbusch, H.W.M. The dorsal raphe nucleus and serotonin: Implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog. Brain Res. 2008, 172, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.D.; Lowry, C.A. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J. Psychopharmacol. 2013, 27, 1090–1106. [Google Scholar] [CrossRef]
- Mobbs, D.; Petrovic, P.; Marchant, J.L.; Hassabis, D.; Weiskopf, N.; Seymour, B.; Dolan, R.J.; Frith, C.D. When Fear Is Near: Threat Imminence Elicits Prefrontal-Periaqueductal Gray Shifts in Humans. Science 2007, 317, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Barnden, L.R.; Shan, Z.Y.; Staines, D.R.; Marshall-Gradisnik, S.; Finegan, K.; Ireland, T.; Bhuta, S. Hyperintense sensorimotor T1 spin echo MRI is associated with brainstem abnormality in chronic fatigue syndrome. NeuroImage Clin. 2018, 20, 102–109. [Google Scholar] [CrossRef]
- Barnden, L.R.; Kwiatek, R.; Crouch, B.; Burnet, R.; Del Fante, P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. NeuroImage Clin. 2016, 11, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, L.S.; McCall, J.G.; Charney, D.S.; Murrough, J.W. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci. Adv. 2020, 4, 2398212820930321. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2017, 11, 130. [Google Scholar] [CrossRef]
- Naegeli, C.; Zeffiro, T.; Piccirelli, M.; Jaillard, A.; Weilenmann, A.; Hassanpour, K.; Schick, M.; Rufer, M.; Orr, S.P.; Mueller-Pfeiffer, C. Locus Coeruleus Activity Mediates Hyperresponsiveness in Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bracha, H.S. Postmortem Locus Coeruleus Neuron Count in Three American Veterans with Probable or Possible War-Related PTSD. J. Neuropsychiatr. 2005, 17, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bordia, T.; Zahr, N.M. The Inferior Colliculus in Alcoholism and Beyond. Front. Syst. Neurosci. 2020, 14, 606345. [Google Scholar] [CrossRef]
- Brandão, M.L.; Melo, L.L.; Cardoso, S.H. Mechanisms of defense in the inferior colliculus. Behav. Brain Res. 1993, 58, 49–55. [Google Scholar] [CrossRef]
- Parham, K.; Willott, J.F. Effects of inferior colliculus lesions on the acoustic startle response. Behav. Neurosci. 1990, 104, 831–840. [Google Scholar] [CrossRef]
- Heeringa, A.N.; van Dijk, P. The immediate effects of acoustic trauma on excitation and inhibition in the inferior colliculus: A Wiener-kernel analysis. Hearth Res. 2016, 331, 47–56. [Google Scholar] [CrossRef]
- Xiong, B.; Alkharabsheh, A.; Manohar, S.; Chen, G.-D.; Yu, N.; Zhao, X.; Salvi, R.; Sun, W. Hyperexcitability of inferior colliculus and acoustic startle reflex with age-related hearing loss. Hearth Res. 2017, 350, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Orr, S.P.; Lasko, N.B.; Shalev, A.Y.; Pitman, R.K. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 1995, 104, 75–82. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Patrick Neary, J.; Roberts, A.D.W.; Leavins, N.; Harrison, M.F.; Croll, J.C.; Sexsmith, J.R. Prefrontal cortex oxygenation during incremental exercise in chronic fatigue syndrome. Clin. Physiol. Funct. Imaging 2008, 28, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Bowen, A.; Schier, L.A.; Tupone, D.; Uddin, O.; Heinricher, M.M. Parabrachial Complex: A Hub for Pain and Aversion. J. Neurosci. 2019, 39, 8225–8230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, K.W.; Bluthé, R.-M.; Dantzer, R.; Zhou, J.-H.; Shen, W.-H.; Johnson, R.W.; Broussard, S.R. Cytokine-induced sickness behavior. Brain. Behav. Immun. 2003, 17 (Suppl. S1), S112–S118. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Ciric, R.; Roalf, D.R.; Davatzikos, C.; Bassett, D.S.; Wolf, D.H. Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Hum. Brain Mapp. 2019, 40, 2033–2051. [Google Scholar] [CrossRef] [Green Version]
- Parkes, L.; Fulcher, B.; Yücel, M.; Fornito, A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 2018, 171, 415–436. [Google Scholar] [CrossRef]
- Terem, I.; Dang, L.; Champagne, A.; Abderezaei, J.; Pionteck, A.; Almadan, Z.; Lydon, A.-M.; Kurt, M.; Scadeng, M.; Holdsworth, S.J. 3D amplified MRI (aMRI). Magn. Reson. Med. 2021, 86, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front. Neurol. 2018, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
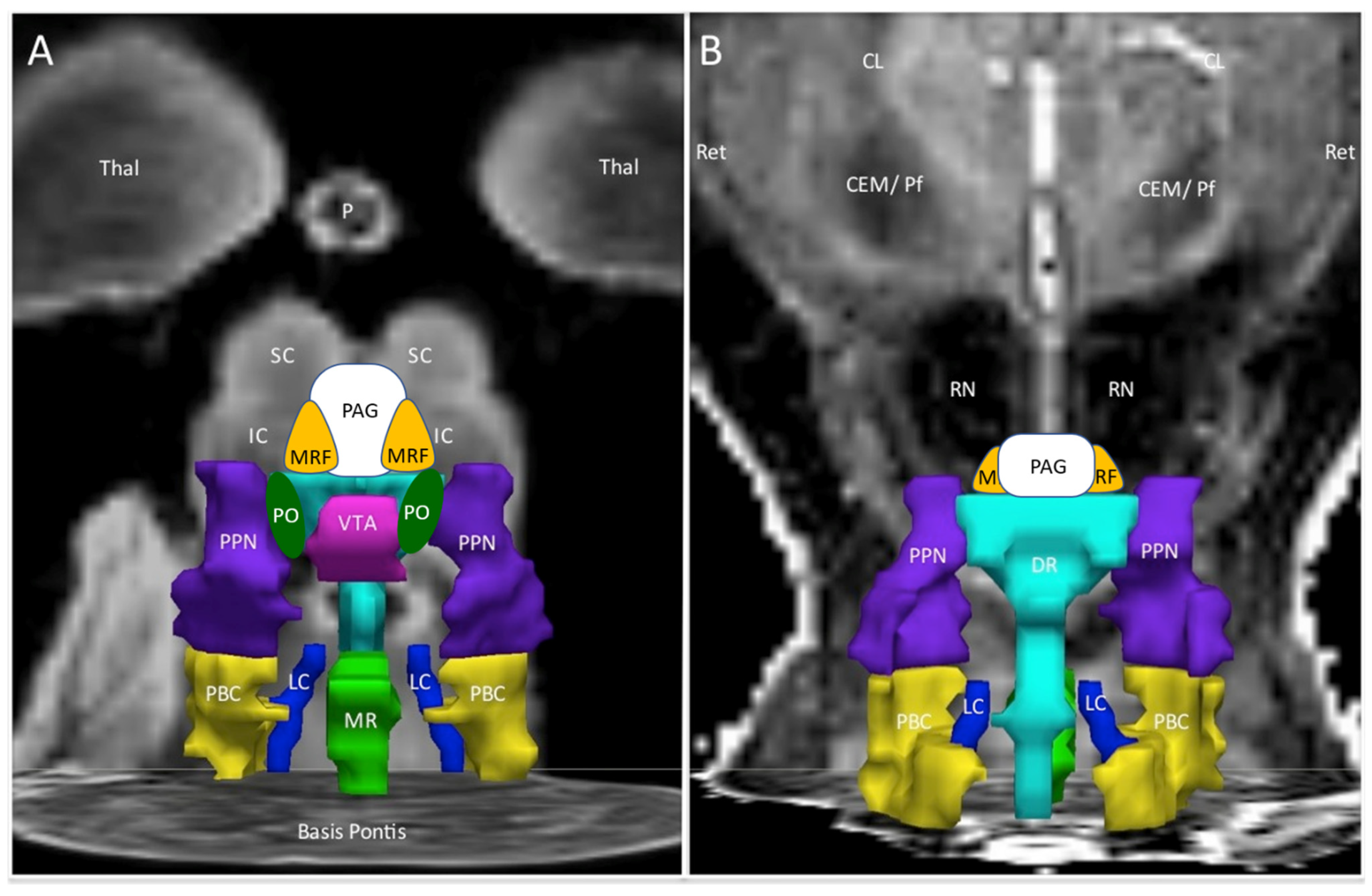

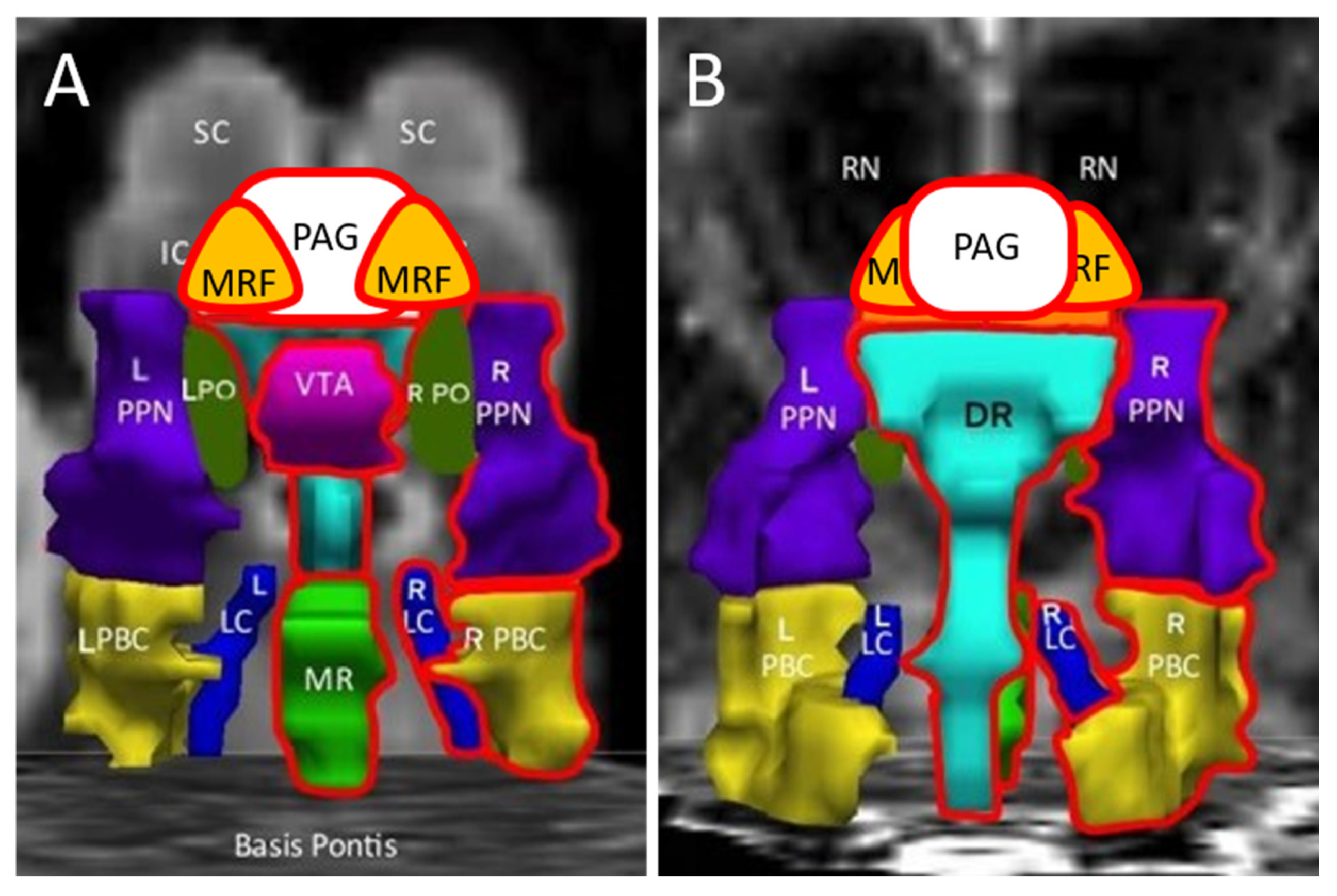


| Abbreviation | Nuclei | Color |
|---|---|---|
| Thal CEM/Pf Ret CL P RN | Background nuclei for orientation thalamus centromedian/parafascicular nucleus reticular nucleus central lateral nucleus of the thalamus pineal midbrain red nuclei Basis Pontis—arbitrary section through the rostral pons to form the “floor” of the figure | grey scale |
| Midbrain and Isthmus (superior to inferior) | ||
| SC | superior colliculus | grey |
| IC | inferior colliculus | grey |
| PAG | periaquequctal grey | white |
| MRF | midbrain reticular formation | orange |
| VTA | ventral tegmental area | violet |
| DR | dorsal raphe | cyan |
| MR | median raphe | lime green |
| PTN | pedunculotegmental nuclei (PTN, formerly PPN pedunculopontine nuclei) | navy blue |
| PO | pontis oralis | dark green |
| PBC | parabrachial complex | yellow |
| LC | locus coeruleus | royal blue |
| Control | ME/CFS | GWI | Control vs. ME/CFS | Control vs. GWI | ME/CFS vs. GWI | |
|---|---|---|---|---|---|---|
| N | 31 | 36 | 78 | |||
| Age | 43.2 ± 16.5 | 47.3 ± 13.1 | 47.1 ± 7.4 | |||
| Female * | 38.7% | 69.4% | 21.8% | 0.029 | 0.0001 | |
| BMI | 28.3 ± 4.5 | 26.2 ± 5.6 | 29.4 ± 5.3 | 0.008 | ||
| Dolorimetry (kg) | 4.6 ± 2.4 | 3.9 ± 1.9 | 4.2 ± 2.1 | |||
| PTSD * | 9.7% | 13.8% | 43.6% | 0.001 | 0.004 | |
| Migraine * | 13.3% | 41.7% | 63.5% | <0.00001 | ||
| Chalder Fatigue (0 to 33) | 12.3 ± 5.4 | 23.3 ± 6.2 | 25.3 ± 4.7 | <0.00001 | <0.00001 | |
| McGill pain (0 to 45) | 3.6 ± 6.4 | 13.4 ± 11.0 | 23.8 ± 9.0 | 0.00012 | <0.00001 | <0.00001 |
| CFS Severity Questionnaire (0 no symptom to 4 severe) | ||||||
| Fatigue | 1.3 ± 1.2 | 3.4 ± 0.8 | 3.5 ± 0.7 | <0.00001 | <0.00001 | |
| Postexertional Malaise | 0.6 ± 1.1 | 3.5 ± 0.8 | 3.4 ± 1.0 | <0.00001 | <0.00001 | |
| Sleep | 1.7 ± 1.4 | 3.2 ± 0.9 | 3.5 ± 0.8 | <0.00001 | <0.00001 | |
| Memory, Concentration | 1.2 ± 1.2 | 2.9 ± 0.9 | 3.1 ± 0.8 | <0.00001 | <0.00001 | |
| Muscle Pain | 0.6 ± 0.9 | 2.5 ± 1.3 | 3.1 ± 1.0 | <0.00001 | <0.00001 | 0.011 |
| Joint Pain | 0.8 ± 1.0 | 1.9 ± 1.4 | 3.2 ± 1.0 | 0.00026 | <0.00001 | <0.00001 |
| Headache | 1.0 ± 1.2 | 2.0 ± 1.3 | 2.7 ± 1.2 | 0.0022 | <0.00001 | 0.025 |
| Sore Throat | 0.3 ± 0.7 | 1.0 ± 1.0 | 1.4 ± 1.2 | 0.031 | 0.000013 | |
| Lymph Nodes | 0.1 ± 0.4 | 1.0 ± 1.1 | 1.5 ± 1.3 | 0.004 | <0.00001 | |
| SF-36 (100 best to 0 worst) | ||||||
| Physical Function | 85.2 ± 24.2 | 44.4 ± 26.5 | 46.9 ± 24.6 | <0.00001 | <0.00001 | |
| Role Physical | 80.0 ± 36.8 | 10.0 ± 25.9 | 9.5 ± 24.8 | <0.00001 | <0.00001 | |
| Bodily Pain | 82.9 ± 20.1 | 47.2 ± 27.7 | 29.5 ± 18.3 | <0.00001 | <0.00001 | 0.00024 |
| General Health | 69.8 ± 22.8 | 33.2 ± 22.9 | 26.4 ± 19.2 | <0.00001 | <0.00001 | |
| Vitality | 60.2 ± 20.7 | 18.0 ± 15.9 | 16.6 ± 15.3 | <0.00001 | <0.00001 | |
| Social Function | 80.0 ± 25.1 | 30.7 ± 27.0 | 30.8 ± 24.5 | <0.00001 | <0.00001 | |
| Role Emotional | 86.7 ± 31.1 | 70.5 ± 44.1 | 30.7 ± 38.4 | <0.00001 | <0.00001 | |
| Mental Health | 73.6 ± 16.8 | 67.8 ± 17.4 | 54.8 ± 22.3 | 0.000084 | 0.0056 | |
| Control | ME/CFS | GWI | Control > ME/CFS | GWI > ME/CFS | |
|---|---|---|---|---|---|
| L_MRF | 0.266 ± 0.164 | 0.123 ± 0.124 | 0.197 ± 0.103 | ||
| R_MRF | 0.331 ± 0.181 | 0.216 ± 0.119 | 0.172 ± 0.103 | ||
| PAG | 0.234 ± 0.145 | 0.061 ± 0.101 | 0.222 ± 0.089 | ||
| VTA | 0.279 ± 0.127 | 0.103 ± 0.089 | 0.193 ± 0.061 | p = 0.034, g = 0.58 | |
| DR | 0.275 ± 0.118 | 0.086 ± 0.103 | 0.233 ± 0.081 | ||
| MR | 0.295 ± 0.124 | 0.131 ± 0.123 | 0.249 ± 0.087 | ||
| L_PO | 0.263 ± 0.134 | 0.061 ± 0.126 | 0.257 ± 0.078 | p = 0.019, g = 0.56 | |
| R_PO | 0.290 ± 0.134 | 0.103 ± 0.136 | 0.252 ± 0.087 | ||
| L_PTN | 0.316 ± 0.133 | 0.049 ± 0.101 | 0.264 ± 0.086 | p = 0.008, g = 0.81 | p = 0.009, g = 0.60 |
| R_PTN | 0.356 ± 0.126 | 0.125 ± 0.086 | 0.259 ± 0.080 | p = 0.014, g = 0.78 | |
| L_LC | 0.309 ± 0.147 | 0.142 ± 0.158 | 0.226 ± 0.103 | ||
| R_LC | 0.316 ± 0.181 | 0.143 ± 0.175 | 0.274 ± 0.126 | ||
| L_PBC | 0.331 ± 0.139 | 0.078 ± 0.135 | 0.231 ± 0.092 | p = 0.029, g = 0.65 | |
| R_PBC | 0.299 ± 0.148 | 0.088 ± 0.136 | 0.263 ± 0.101 |
| Control | ME/CFS | GWI | Control > GWI | ME/CFS > GWI | |
|---|---|---|---|---|---|
| N | 31 | 36 | 78 | ||
| L_MRF | 0.341 ± 0.146 | 0.206 ± 0.147 | 0.073 ± 0.094 | p = 0.008, g = 0.65 | |
| R_MRF | 0.364 ± 0.149 | 0.301 ± 0.138 | 0.077 ± 0.084 | p = 0.002, g = 0.75 | p = 0.014, g = 0.58 |
| PAG | 0.182 ± 0.141 | 0.271 ± 0.142 | 0.060 ± 0.078 | p = 0.016, g = 0.57 | |
| VTA | 0.252 ± 0.104 | 0.195 ± 0.099 | 0.078 ± 0.068 | p = 0.018, g = 0.58 | |
| DR | 0.229 ± 0.113 | 0.265 ± 0.126 | 0.068 ± 0.070 | p = 0.009, g = 0.60 | |
| MR | 0.231 ± 0.115 | 0.285 ± 0.139 | 0.092 ± 0.075 | p = 0.018, g = 0.54 | |
| L_PO | 0.178 ± 0.125 | 0.241 ± 0.141 | 0.129 ± 0.082 | ||
| R_PO | 0.248 ± 0.131 | 0.255 ± 0.135 | 0.101 ± 0.077 | ||
| L_PTN | 0.233 ± 0.124 | 0.208 ± 0.127 | 0.093 ± 0.084 | ||
| R_PTN | 0.308 ± 0.158 | 0.289 ± 0.104 | 0.126 ± 0.074 | p = 0.04, g = 0.50 | |
| L_LC | 0.223 ± 0.144 | 0.274 ± 0.146 | 0.097 ± 0.089 | ||
| R_LC | 0.276 ± 0.167 | 0.313 ± 0.150 | 0.077 ± 0.095 | p = 0.022, g = 0.55 | |
| L_PBC | 0.250 ± 0.133 | 0.250 ± 0.135 | 0.106 ± 0.091 | ||
| R_PBC | 0.240 ± 0.135 | 0.289 ± 0.139 | 0.110 ± 0.076 | p = 0.041, g = 0.50 |
| SC | ME/CFS | GWI | ME/CFS > GWI | |
|---|---|---|---|---|
| N | 31 | 36 | 78 | |
| L_MRFΔ | 0.075 ± 0.201 | 0.083 ± 0.163 | −0.124 ± 0.129 | |
| R_MRFΔ | 0.033 ± 0.216 | 0.085 ± 0.171 | −0.095 ± 0.122 | |
| PAGΔ | −0.052 ± 0.167 | 0.210 ± 0.175 | −0.162 ± 0.110 | p = 0.001, g = 0.75 |
| VTAΔ | −0.027 ± 0.138 | 0.092 ± 0.130 | −0.114 ± 0.080 | p = 0.016, g = 0.57 |
| DRΔ | −0.046 ± 0.139 | 0.179 ± 0.159 | −0.165 ± 0.103 | p = 0.001, g = 0.75 |
| MRΔ | −0.065 ± 0.153 | 0.155 ± 0.173 | −0.157 ± 0.113 | p = 0.005, g = 0.62 |
| L_POΔ | −0.085 ± 0.167 | 0.180 ± 0.181 | −0.128 ± 0.108 | p = 0.006, g = 0.62 |
| R_POΔ | −0.041 ± 0.178 | 0.152 ± 0.175 | −0.151 ± 0.123 | p = 0.014, g = 0.56 |
| L_PTNΔ | −0.083 ± 0.154 | 0.158 ± 0.166 | −0.171 ± 0.107 | p = 0.002, g = 0.69 |
| R_PTNΔ | −0.048 ± 0.168 | 0.164 ± 0.155 | −0.132 ± 0.100 | p = 0.004, g = 0.66 |
| L_LCΔ | −0.086 ± 0.172 | 0.133 ± 0.201 | −0.129 ± 0.130 | |
| R_LCΔ | −0.039 ± 0.218 | 0.169 ± 0.220 | −0.197 ± 0.165 | p = 0.024, g = 0.52 |
| L_PBCΔ | −0.081 ± 0.181 | 0.171 ± 0.198 | −0.125 ± 0.128 | p = 0.025, g = 0.52 |
| R_PBCΔ | −0.059 ± 0.167 | 0.202 ± 0.195 | −0.153 ± 0.129 | p = 0.005, g = 0.62 |
| POTS | START | STOPP | STOPP > START | |
|---|---|---|---|---|
| L_MRFΔ | −0.009 ± 0.315 | −0.093 ± 0.235 | −0.063 ± 0.174 | |
| R_MRFΔ | 0.003 ± 0.319 | −0.096 ± 0.239 | −0.051 ± 0.177 | |
| PAGΔ | 0.004 ± 0.271 | −0.140 ± 0.202 | −0.026 ± 0.150 | |
| VTAΔ | −0.062 ± 0.211 | −0.146 ± 0.157 | 0.034 ± 0.116 | |
| DRΔ | −0.090 ± 0.251 | −0.153 ± 0.188 | 0.028 ± 0.139 | |
| MRΔ | −0.164 ± 0.271 | −0.166 ± 0.203 | 0.050 ± 0.150 | |
| L_POΔ | −0.085 ± 0.274 | −0.170 ± 0.204 | 0.061 ± 0.151 | |
| R_POΔ | −0.048 ± 0.298 | −0.259 ± 0.222 | 0.072 ± 0.164 | |
| L_PTNΔ | −0.247 ± 0.256 | −0.098 ± 0.191 | −0.028 ± 0.142 | |
| R_PTNΔ | −0.053 ± 0.260 | −0.098 ± 0.194 | −0.008 ± 0.144 | |
| L_LCΔ | −0.219 ± 0.314 | −0.232 ± 0.235 | 0.102 ± 0.174 | |
| R_LCΔ | −0.070 ± 0.390 | −0.329 ± 0.291 | 0.156 ± 0.216 | p = 0.035, g = 0.42 |
| L_PBCΔ | −0.304 ± 0.302 | −0.268 ± 0.226 | 0.117 ± 0.167 | p = 0.029, g = 0.29 |
| R_PBCΔ | 0.020 ± 0.316 | −0.298 ± 0.236 | 0.110 ± 0.174 | p = 0.026, g = 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraniuk, J.N.; Amar, A.; Pepermitwala, H.; Washington, S.D. Differential Effects of Exercise on fMRI of the Midbrain Ascending Arousal Network Nuclei in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) in a Model of Postexertional Malaise (PEM). Brain Sci. 2022, 12, 78. https://doi.org/10.3390/brainsci12010078
Baraniuk JN, Amar A, Pepermitwala H, Washington SD. Differential Effects of Exercise on fMRI of the Midbrain Ascending Arousal Network Nuclei in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) in a Model of Postexertional Malaise (PEM). Brain Sciences. 2022; 12(1):78. https://doi.org/10.3390/brainsci12010078
Chicago/Turabian StyleBaraniuk, James N., Alison Amar, Haris Pepermitwala, and Stuart D. Washington. 2022. "Differential Effects of Exercise on fMRI of the Midbrain Ascending Arousal Network Nuclei in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) in a Model of Postexertional Malaise (PEM)" Brain Sciences 12, no. 1: 78. https://doi.org/10.3390/brainsci12010078
APA StyleBaraniuk, J. N., Amar, A., Pepermitwala, H., & Washington, S. D. (2022). Differential Effects of Exercise on fMRI of the Midbrain Ascending Arousal Network Nuclei in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) in a Model of Postexertional Malaise (PEM). Brain Sciences, 12(1), 78. https://doi.org/10.3390/brainsci12010078







