Cognitive Control in Young and Older Adults: Does Mood Matter?
Abstract
:1. Introduction
2. Experiment 1
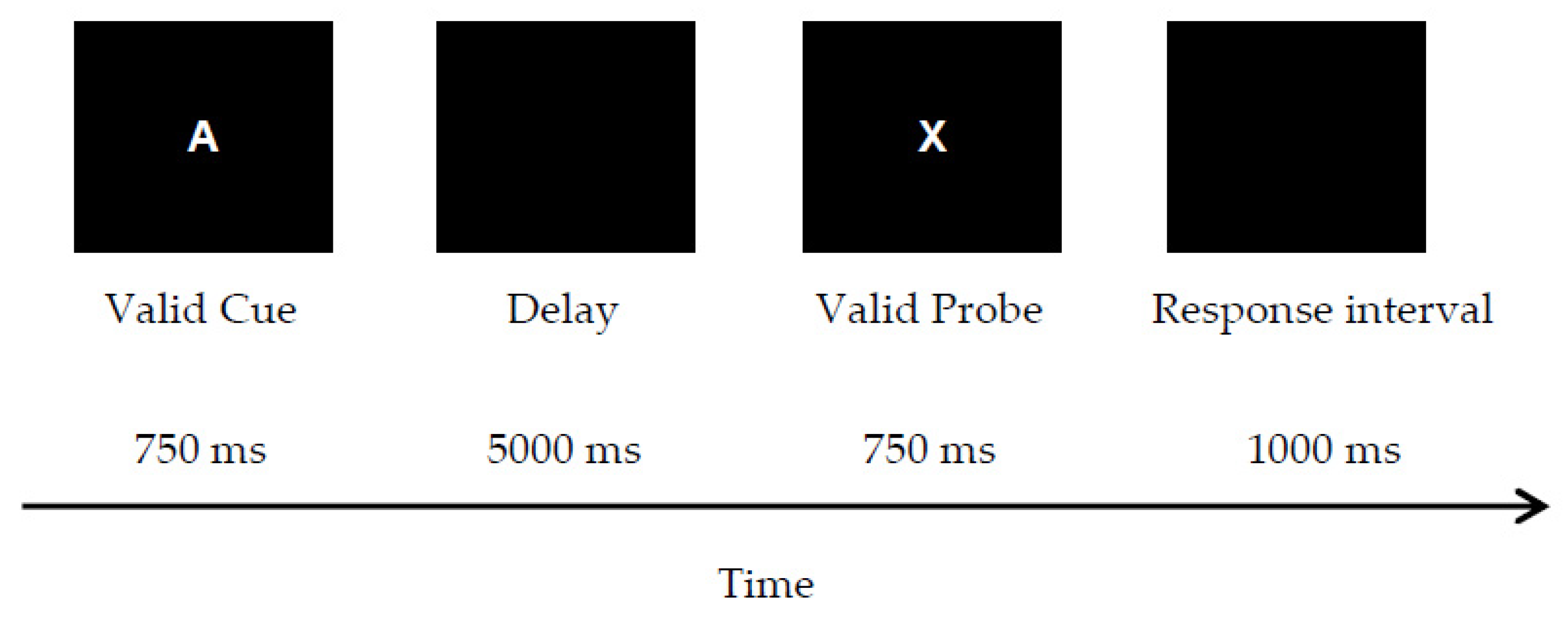
2.1. Method
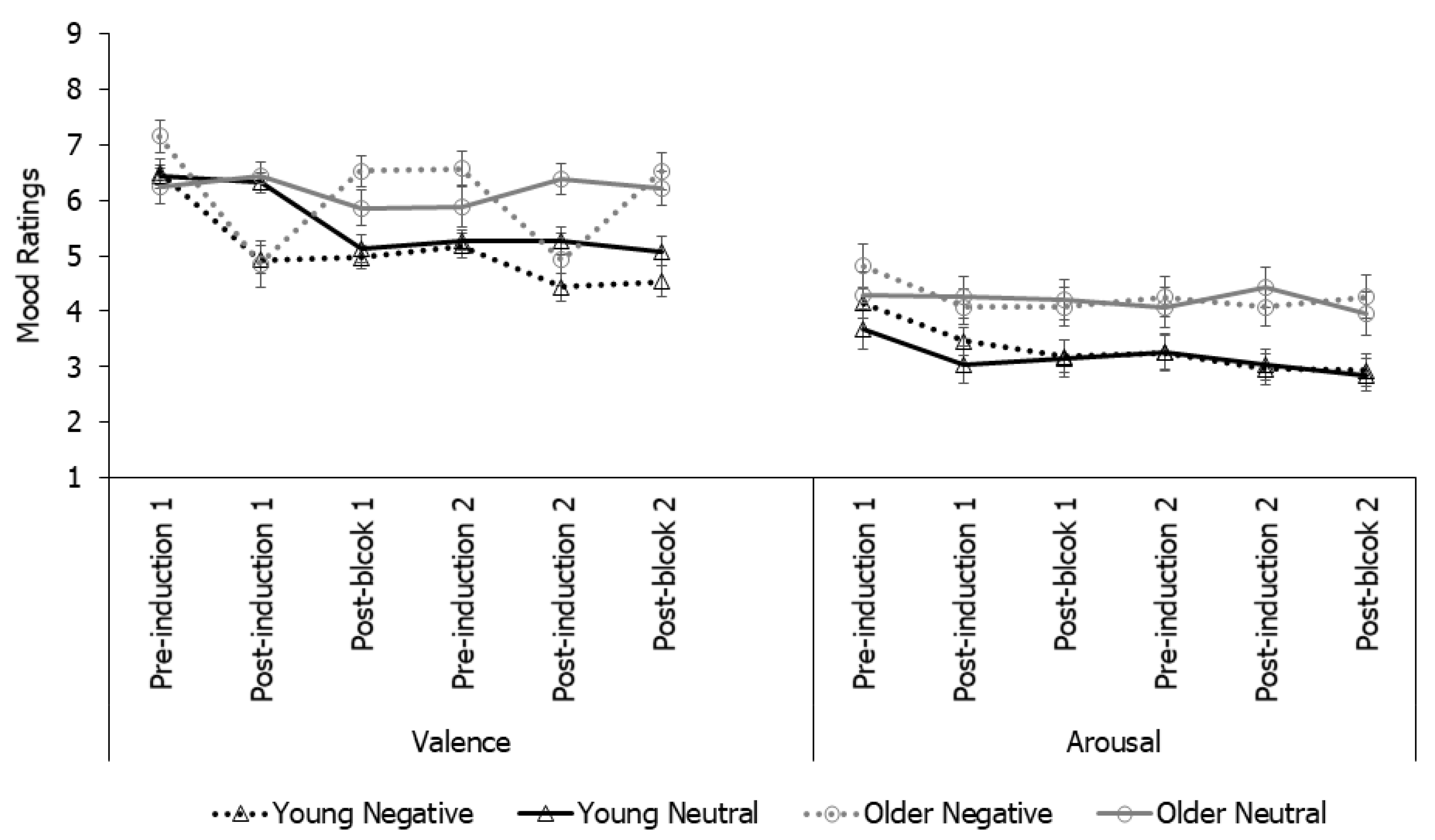
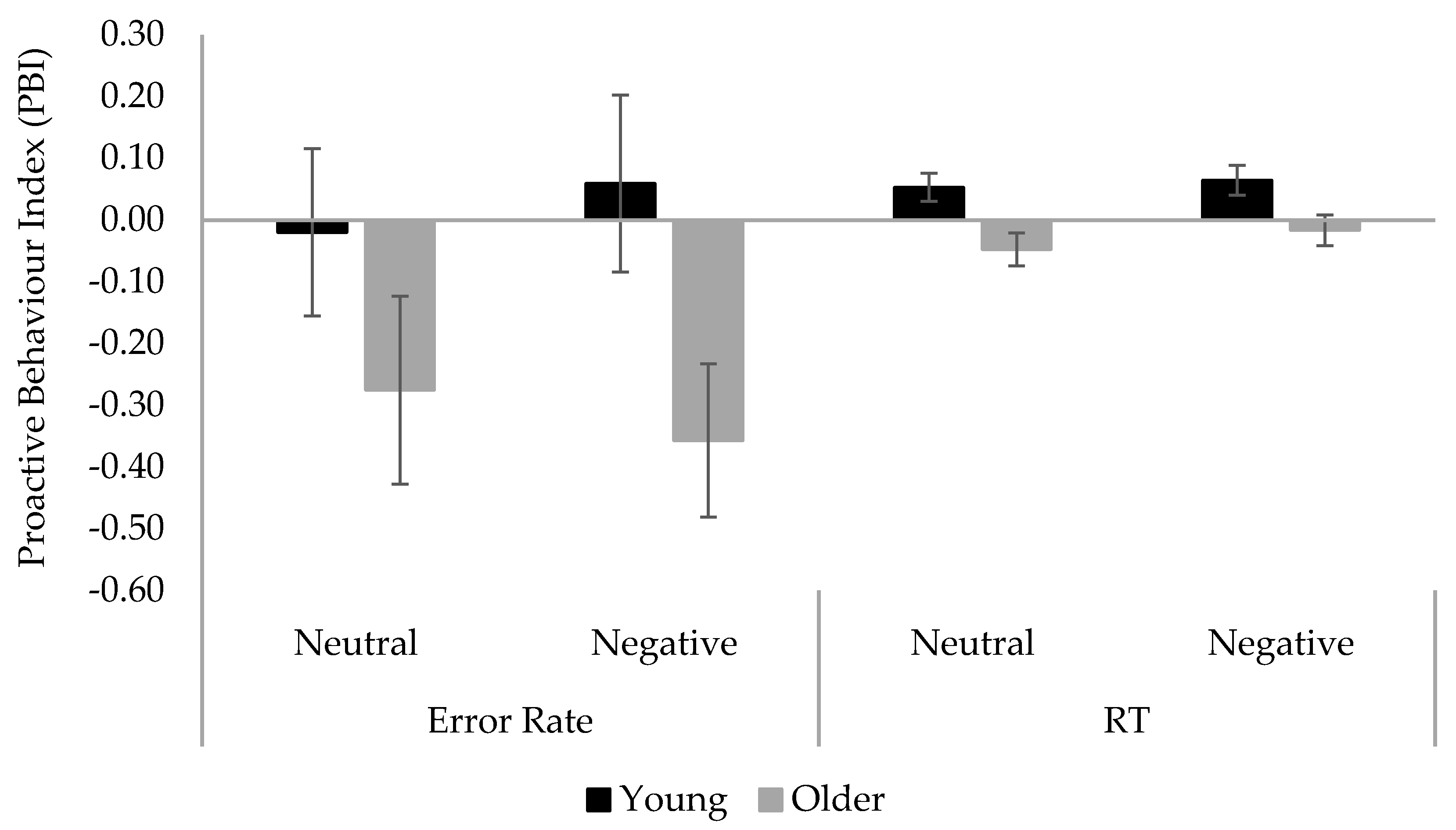
2.2. Results
2.3. Discussion
3. Experiment 2
3.1. Method
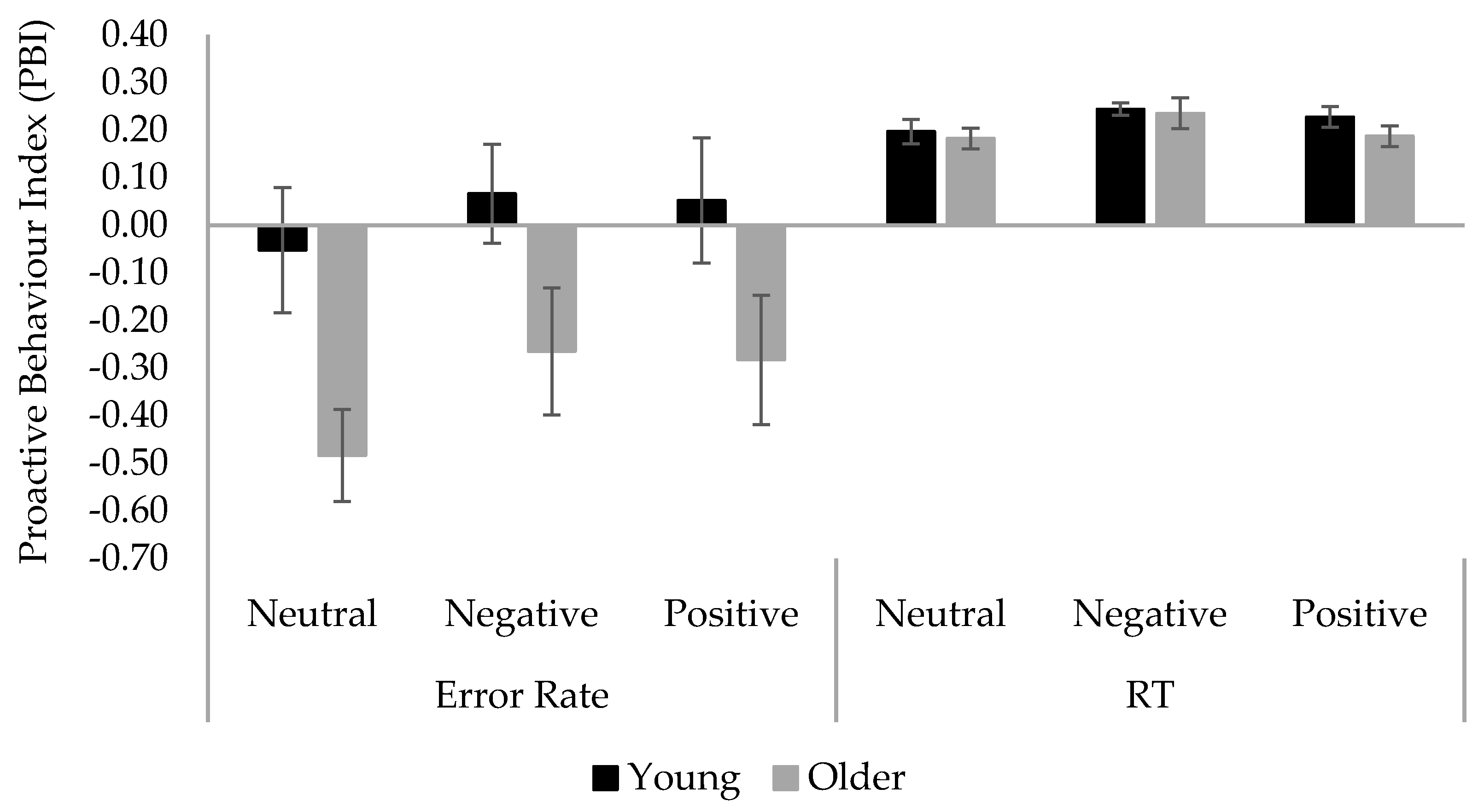
3.2. Results
3.3. Discussion
4. Discussion
4.1. Mood Manipulation
4.2. Age Differences in Cognitive Control
4.3. Mood and Cognitive Control
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braver, T.S.; Barch, D.M.; Keys, B.A.; Carter, C.S.; Cohen, J.D.; Kaye, J.A.; Janowsky, J.S.; Taylor, S.F.; Yesavage, J.A.; Mumenthaler, M.S.; et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. J. Exp. Psychol. Gen. 2001, 130, 746–763. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.D.; Barch, D.M.; Carter, C.; Servan-Schreiber, D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. J. Abnorm. Psychol. 1999, 108, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Paxton, J.L.; Barch, D.M.; Storandt, M.; Braver, T.S. Effects of environmental support and strategy training on older adults’ use of context. Psychol. Aging 2006, 21, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Chiew, K.S.; Braver, T.S. Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn. Affect. Behav. Neurosci. 2014, 14, 509–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreisbach, G.; Goschke, T. How Positive Affect Modulates Cognitive Control: Reduced Perseveration at the Cost of Increased Distractibility. J. Exp. Psychol. Learn. Mem. Cogn. 2004, 30, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröber, K.; Dreisbach, G. The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cogn. Affect. Behav. Neurosci. 2014, 14, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Fröber, K.; Dreisbach, G. How performance (non-)contingent reward modulates cognitive control. Acta Psychol. 2016, 168, 65–77. [Google Scholar] [CrossRef]
- Hefer, C.; Dreisbach, G. Prospect of performance-contingent reward distorts the action relevance of predictive context information. J. Exp. Psychol. Learn. Mem. Cogn. 2020, 46, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S. The variable nature of cognitive control: A dual-mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braver, T.S.; Paxton, J.L.; Locke, H.S.; Barch, D.M. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 7351–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wouwe, N.C.; Band, G.P.H.; Ridderinkhof, K.R. Positive Affect Modulates Flexibility and Evaluative Control. J. Cogn. Neurosci. 2011, 23, 524–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreisbach, G. How positive affect modulates cognitive control: The costs and benefits of reduced maintenance capability. Brain Cogn. 2006, 60, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S.; Barch, D.M. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci. Biobehav. Rev. 2002, 26, 809–817. [Google Scholar] [CrossRef]
- Oleson, E.B.; Gentry, R.N.; Chioma, V.C.; Cheer, J.F. Subsecond Dopamine Release in the Nucleus Accumbens Predicts Conditioned Punishment and Its Successful Avoidance. J. Neurosci. 2012, 32, 14804–14808. [Google Scholar] [CrossRef]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.; Lin, S.J. The Dissociable Effects of Induced Positive and Negative Moods on Cognitive Flexibility. Sci. Rep. 2019, 9, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, A.E.; Carstensen, L.L. The Theory behind the Age-Related Positivity Effect. Front. Psychol. 2012, 3, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, L.; Yang, L. Friend or foe? Decoding the facilitative and disruptive effects of emotion on working memory in younger and older adults. Front. Psychol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldar, E.; Rutledge, R.B.; Dolan, R.J.; Niv, Y. Mood as Representation of Momentum. Trends Cogn. Sci. 2016, 20, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Sauzeon, B.N.; Lespinet, V.; Guillem, F.; Helene, B.C. Age Effect in Recall Performance According to the Levels of Processing, Elaboration, and Retrieval Cues. Exp. Aging Res. 2000, 26, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.; Pachana, N. Ecological validity in neuropsychological assessment: A case for greater consideration in research with neurologically intact populations. Arch. Clin. Neuropsychol. 2006, 21, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, H.; Ferdinand, N.K.; Kray, J. The influence of monetary incentives on context processing in younger and older adults: An event-related potential study. Cogn. Affect. Behav. Neurosci. 2015, 15, 416–434. [Google Scholar] [CrossRef] [Green Version]
- Craik, F.; Routh, D.; Broadbent, D. On the Transfer of Information from Temporary to Permanent Memory [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1983, 302, 341–359. [Google Scholar] [CrossRef]
- Lindenberger, U.; Mayr, U. Cognitive Aging: Is There a Dark Side to Environmental Support? Trends Cogn. Sci. 2014, 18, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. Manual for the Depression Anxiety Stress Scales; The Psychology Foundation of Australia: Sydney, Australia, 1995. [Google Scholar]
- Katzman, R.; Brown, T.; Fuld, P.; Peck, A.; Schechter, R.; Schimmel, H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am. J. Psychiatry 1983, 140, 734–739. [Google Scholar] [CrossRef]
- Emery, L.; Hess, T.M. Viewing instructions impact emotional memory differently in older and young adults. Psychol. Aging 2008, 23, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.R.; Carstensen, L.L. Future Time Perspective Scale; Stanford University: Stanford, CA, USA, 1995. [Google Scholar]
- Biss, R.K.; Weeks, J.C.; Hasher, L. Happily distracted: Mood and a benefit of attention dysregulation in older adults. Front. Psychol. 2012, 3, 399. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings; The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Spaniol, J.; Hasher, L.; Wilkinson, A.J.; Yu, J.; Niu, Y. Aging, Culture, and Memory for Socially Meaningful Item-Context Associations: An East-West Cross-Cultural Comparison Study. PLoS ONE 2013, 8, e60703. [Google Scholar] [CrossRef] [Green Version]
- Northwestern University and the National Institutes of Health. [NIH Toolbox]. [Internet]. 2012. Available online: http://www.nihtoolbox.org (accessed on 11 November 2021).
- Ebner, N.C.; Riediger, M.; Lindenberger, U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav. Res. Methods 2010, 42, 351–362. [Google Scholar] [CrossRef]
- Braver, T.S.; Satpute, A.B.; Rush, B.K.; Racine, C.A.; Barch, D.M. Context Processing and Context Maintenance in Healthy Aging and Early Stage Dementia of the Alzheimer’s Type. Psychol. Aging 2005, 20, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Rush, B.K.; Barch, D.M.; Braver, T.S. Accounting for Cognitive Aging: Context Processing, Inhibition or Processing Speed? Aging Neuropsychol. Cogn. 2006, 13, 588–610. [Google Scholar] [CrossRef]
- Larcom, M.J.; Isaacowitz, D.M. Rapid Emotion Regulation after Mood Induction: Age and Individual Differences. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2009, 64B, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Ezekiel, F.; Bosma, R.; Morton, J.B. Dimensional Change Card Sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev. Cogn. Neurosci. 2012, 5, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Redick, T.S. Cognitive control in context: Working memory capacity and proactive control. Acta Psychol. 2014, 145, 1–9. [Google Scholar] [CrossRef]
- Koshino, H.; Minamoto, T.; Ikeda, T.; Osaka, M.; Otsuka, Y.; Osaka, N. Anterior Medial Prefrontal Cortex Exhibits Activation during Task Preparation but Deactivation during Task Execution. PLoS ONE 2011, 6, e22909. [Google Scholar] [CrossRef] [Green Version]
- Jimura, K.; Braver, T.S. Age-Related Shifts in Brain Activity Dynamics during Task Switching. Cereb. Cortex 2010, 20, 1420–1431. [Google Scholar] [CrossRef] [Green Version]
- Ashby, F.G.; Isen, A.M.; Turken, A.U. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999, 106, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.M.; Peters, E.; Västfjäll, D.; Isen, A.M. Positive feelings facilitate working memory and complex decision making among older adults. Cogn. Emot. 2013, 27, 184–192. [Google Scholar] [CrossRef]
- Chu, O. The Effect of Mood on Set-Switching Abilities in Younger and Older Adults; University of Windsor: Windsor, ON, Canada, 2014. [Google Scholar]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-Stage Parkinson’s Patients Show Selective Impairment in Reactive but Not Proactive Inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Young Adults | Older Adults | ||||||
|---|---|---|---|---|---|---|---|---|
| Neutral Mood (n = 26) | Negative Mood (n = 28) | Neutral Mood (n = 28) | Negative Mood (n = 27) | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Age | 19.12 | 2.03 | 20.32 | 3.00 | 71.21 | 4.90 | 70.74 | 5.22 |
| F/M (ratio) | 22/4 | 26/2 | 16/12 | 19/8 | ||||
| Years of formal Education a,b | 12.85 | 1.99 | 14.11 | 1.72 | 15.63 | 3.11 | 17.00 | 4.24 |
| Health rating | 7.90 | 0.80 | 7.73 | 1.14 | 8.21 | 1.37 | 8.13 | 1.67 |
| FTP a | 54.58 | 6.29 | 52.32 | 7.66 | 39.54 | 10.86 | 43.19 | 9.58 |
| PANAS-PA a | 25.15 | 8.18 | 22.96 | 4.91 | 33.93 | 6.72 | 36.48 | 6.47 |
| PANAS-NA a | 16.08 | 4.20 | 14.82 | 4.29 | 11.89 | 1.99 | 12.93 | 3.69 |
| DASS-Dep a | 6.92 | 5.07 | 7.43 | 5.98 | 3.86 | 4.77 | 4.37 | 4.00 |
| DASS-Anx a | 7.69 | 5.71 | 7.14 | 5.97 | 2.50 | 3.38 | 3.04 | 3.70 |
| DASS-Strs a | 13.54 | 7.64 | 11.57 | 6.83 | 7.29 | 6.89 | 6.81 | 5.64 |
| VSWM a | 0.64 | 0.15 | 0.64 | 0.14 | 0.34 | 0.18 | 0.32 | 0.19 |
| Pattern Comparison * a,b | 74.73 | 10.74 | 81.00 | 8.52 | 49.31 | 8.84 | 52.04 | 11.53 |
| DCCST * a | 9.18 | 0.59 | 9.29 | 0.64 | 7.57 | 1.18 | 8.08 | 0.98 |
| Flanker * a | 9.62 | 0.38 | 9.54 | 0.30 | 8.45 | 0.66 | 8.72 | 0.64 |
| Vocabulary * a | 1539.88 | 111.01 | 1582.11 | 155.18 | 2047.00 | 576.31 | 1965.71 | 220.86 |
| SBT | 0.79 | 1.00 | 0.22 | 0.16 | ||||
| Conditions | Young Adults | Older Adults | ||||
|---|---|---|---|---|---|---|
| Probe-Lure | Cue-Lure | PBI | Probe-Lure | Cue-Lure | PBI | |
| Error rate | ||||||
| Neutral | 0.14 (0.21) | 0.08 (0.08) | −0.02 (0.69) | 0.16 (0.22) | 0.04 (0.08) | −0.28 (0.80) |
| Negative | 0.15 (0.18) | 0.10 (0.08) | 0.06 (0.76) | 0.13 (0.23) | 0.05 (0.11) | −0.36 (0.64) |
| RT | ||||||
| Neutral | 0.19 (0.65) | 0.61 (0.43) | 0.05 (0.11) | 0.78 (0.97) | 0.35 (0.30) | −0.05 (0.14) |
| Negative | 0.09 (0.71) | 0.57 (0.50) | 0.06 (0.13) | 0.56 (0.87) | 0.42 (0.43) | −0.02 (0.13) |
| Characteristic | Young Adults | Older Adults | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral Mood (n = 27) | Negative Mood (n = 26) | Positive Mood (n = 27) | Neutral Mood (n = 25) | Negative Mood (n = 27) | Positive Mood (n = 25) | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Age | 20.63 | 3.00 | 20.62 | 2.73 | 21.04 | 3.20 | 74.04 | 6.84 | 72.00 | 4.90 | 73.44 | 7.33 |
| F/M (ratio) | 22/5 | 20/6 | 23/4 | 20/5 | 24/3 | 21/4 | ||||||
| Years of formal education aXb | 14.07 | 1.67 | 14.00 | 2.18 | 14.41 | 1.59 | 16.94 | 3.04 | 14.85 | 2.33 | 15.28 | 3.09 |
| Health rating a | 7.82 | 1.39 | 7.85 | 1.05 | 7.65 | 1.28 | 8.25 | 1.11 | 8.33 | 1.41 | 8.92 | 0.93 |
| FTP a | 51.78 | 8.47 | 50.65 | 7.57 | 51.89 | 9.61 | 38.12 | 7.39 | 38.56 | 11.15 | 40.48 | 12.03 |
| PANAS-PA a | 24.74 | 5.47 | 24.08 | 8.05 | 26.00 | 7.64 | 35.00 | 5.59 | 35.67 | 6.09 | 38.92 | 5.85 |
| PANAS-NA a,b | 14.59 | 3.43 | 14.96 | 3.45 | 12.15 | 3.34 | 12.28 | 2.97 | 13.63 | 5.81 | 12.04 | 3.30 |
| DASS-Dep a | 7.00 | 6.48 | 8.38 | 6.50 | 7.26 | 5.44 | 4.58 | 4.66 | 5.19 | 5.69 | 4.64 | 4.99 |
| DASS-Anx a | 8.00 | 5.38 | 7.08 | 4.57 | 6.81 | 5.58 | 2.56 | 3.98 | 3.56 | 4.24 | 2.75 | 2.88 |
| DASS-Strs a | 13.33 | 7.96 | 13.31 | 7.90 | 12.44 | 8.31 | 7.12 | 5.36 | 9.11 | 5.91 | 9.36 | 6.50 |
| VSWM a | 0.50 | 0.14 | 0.61 | 0.15 | 0.58 | 0.29 | 0.25 | 0.14 | 0.27 | 0.15 | 0.29 | 0.21 |
| Pattern Comparison a | 71.56 | 14.38 | 67.81 | 47.44 | 71.19 | 11.29 | 45.32 | 9.53 | 47.44 | 9.92 | 47.74 | 9.62 |
| DCCST a | 8.84 | 1.19 | 9.04 | 0.64 | 9.06 | 0.55 | 7.75 | 0.84 | 7.59 | 1.17 | 7.73 | 1.14 |
| Flanker a | 9.42 | 0.52 | 9.42 | 0.53 | 9.50 | 0.44 | 8.53 | 0.53 | 8.50 | 0.81 | 8.34 | 0.82 |
| Vocabulary a | 1475.22 | 217.17 | 1497.46 | 211.78 | 1551.15 | 139.67 | 1925.56 | 250.78 | 1930.37 | 226.87 | 2082.00 | 612.66 |
| SBT | 20.63 | 0.40 | 0.20 | 0.74 | 1.13 | 0.96 | 1.65 | |||||
| Conditions | Young Adults | Older Adults | ||||
|---|---|---|---|---|---|---|
| Probe-Lure | Cue-Lure | PBI | Probe-Lure | Cue-Lure | PBI | |
| Error rate | ||||||
| Neutral | 0.23 (0.27) | 0.14 (0.11) | −0.05 (0.68) | 0.17 (0.22) | 0.05 (0.08) | −0.48 (0.48) |
| Negative | 0.12 (0.19) | 0.11 (0.12) | 0.07 (0.53) | 0.15 (0.22) | 0.07 (0.09) | −0.26 (0.69) |
| Positive | 0.15 (0.24) | 0.09 (0.09) | 0.05 (0.68) | 0.13 (0.15) | 0.07 (0.08) | −0.26 (0.68) |
| RT | ||||||
| Neutral | −0.56 (0.57) | 0.65(0.41) | 0.20 (0.13) | −0.56 (0.58) | 0.71 (0.40) | 0.18 (0.11) |
| Negative | −0.85 (0.27) | 0.75 (0.28) | 0.24 (0.07) | −0.59 (0.56) | 1.00 (0.71) | 0.24 (0.16) |
| Positive | −0.69 (0.43) | 0.71 (0.36) | 0.23 (0.11) | −0.60 (0.59) | 0.67 (0.37) | 0.19 (0.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, L.; Kandasamy, K.; Yang, L. Cognitive Control in Young and Older Adults: Does Mood Matter? Brain Sci. 2022, 12, 50. https://doi.org/10.3390/brainsci12010050
Truong L, Kandasamy K, Yang L. Cognitive Control in Young and Older Adults: Does Mood Matter? Brain Sciences. 2022; 12(1):50. https://doi.org/10.3390/brainsci12010050
Chicago/Turabian StyleTruong, Linda, Kesaan Kandasamy, and Lixia Yang. 2022. "Cognitive Control in Young and Older Adults: Does Mood Matter?" Brain Sciences 12, no. 1: 50. https://doi.org/10.3390/brainsci12010050
APA StyleTruong, L., Kandasamy, K., & Yang, L. (2022). Cognitive Control in Young and Older Adults: Does Mood Matter? Brain Sciences, 12(1), 50. https://doi.org/10.3390/brainsci12010050






