Impulsive and Omission Errors: Potential Temporal Processing Endophenotypes in ADHD †
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Clinical Assessment
2.2.1. ADHD Diagnosis
2.2.2. Neuropsychological Assessment
Stroop’s Color and Word Test
Cross-Out-Squares Test
Trail Making Test (TMT)
2.2.3. Reaction Time Tasks Assessment
Conner’s Continuous Performance Test-II (CPT-II)®
Go/No-Go Tasks
2.3. Statistical Analysis
2.4. Heritabilty Estimation
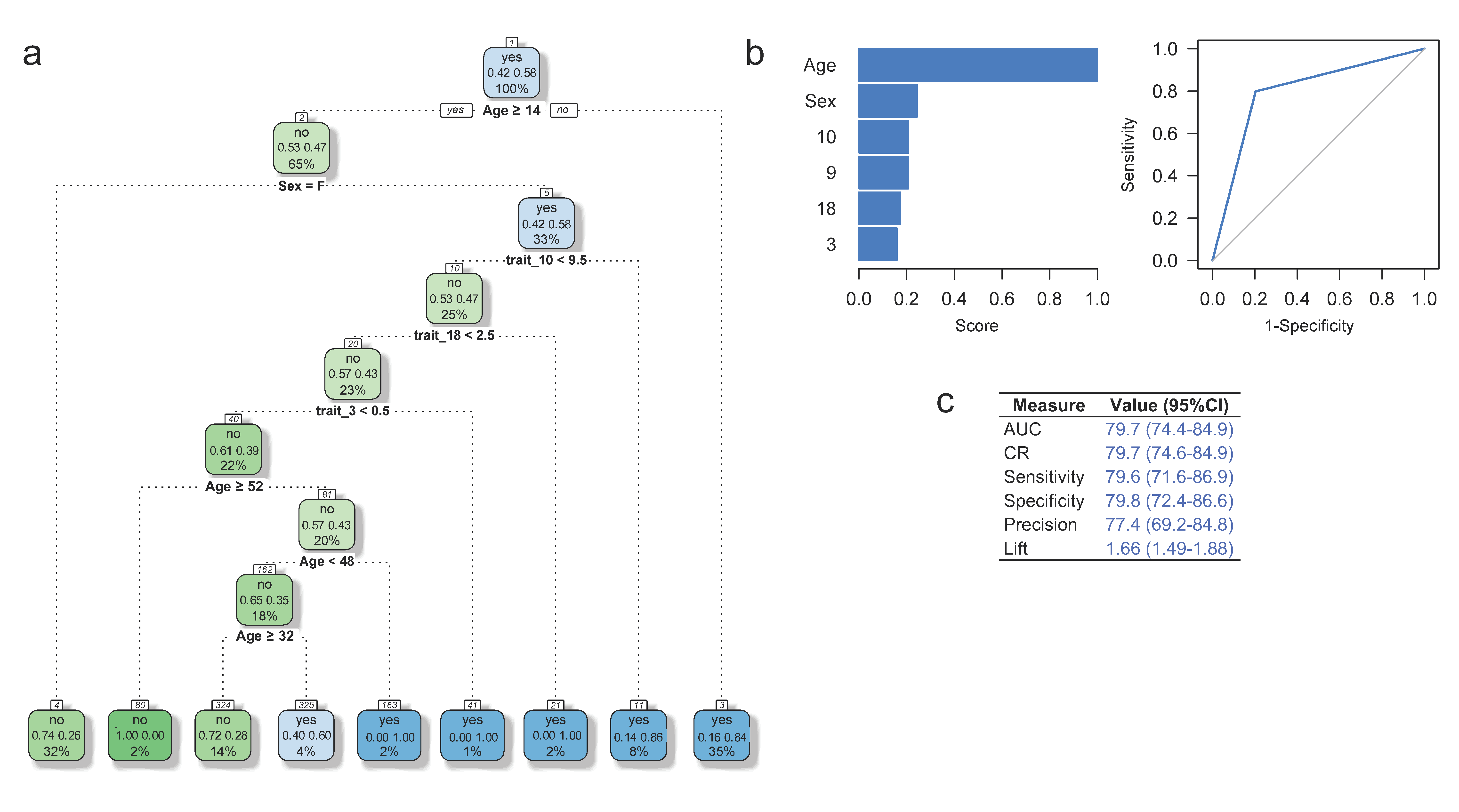
2.5. Predictive Model for ADHD
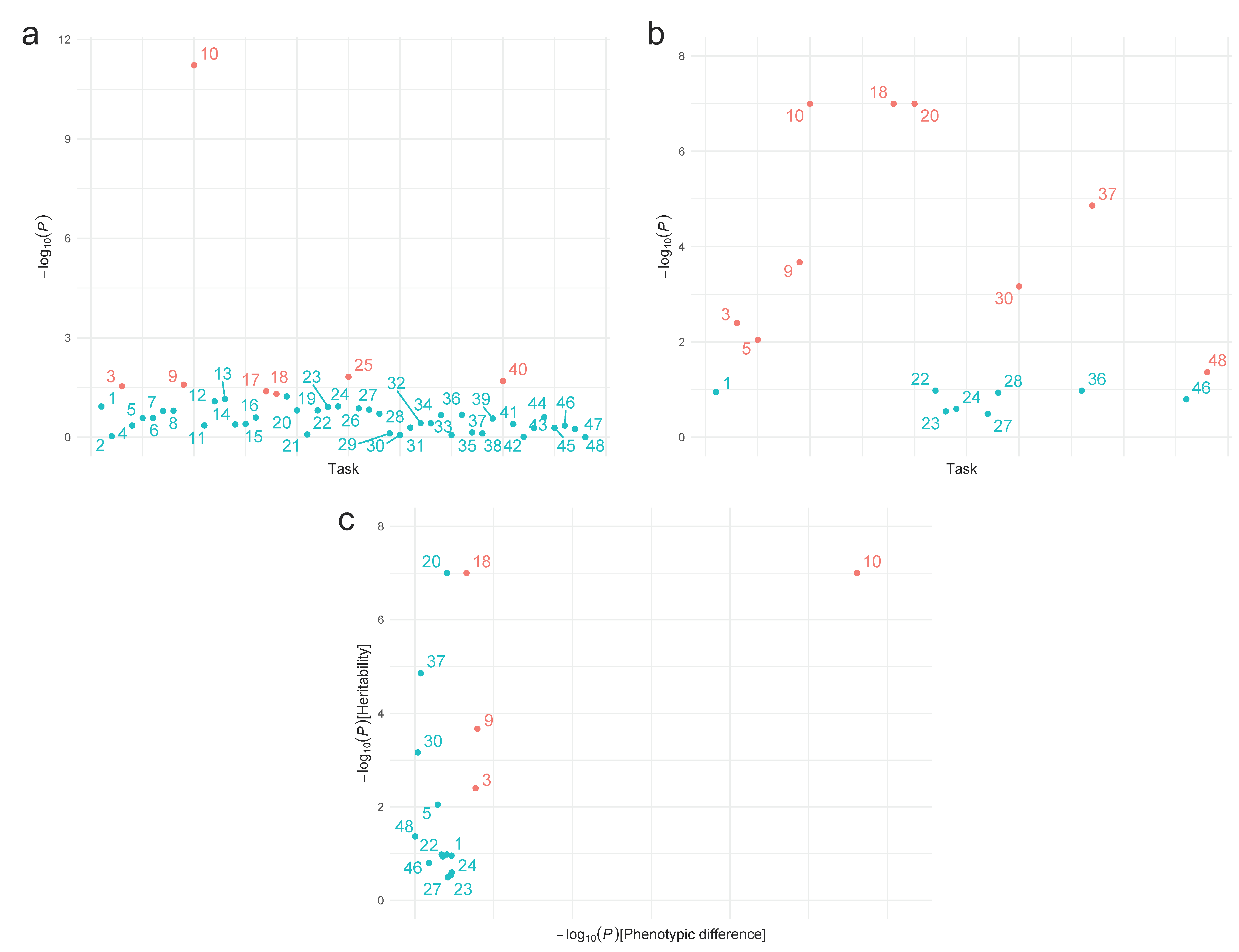
3. Results
3.1. Subjects
3.2. Differences in TP Neuropsychological Tests between Affected and Unaffected Individuals
3.3. Heritability Estimates
3.4. Potential Endophenotypes
| # | Task | Affected (n = 124) | Unaffected (n = 108) | d | p | Heritability | |
|---|---|---|---|---|---|---|---|
| h2 (SD) | p | ||||||
| MOART | |||||||
| Part A (Go) | Mean (SD) | Mean (SD) | |||||
| 1 | RT (ms) | 532.54 (130.41) | 472.22 (87.7) | 0.536 | 0.118 b | 0.188 (0.154) | 0.111 |
| 2 | Commission errors | 0.95 (1.39) | 0.52 (1.09) | 0.340 | 0.929 b | a | a |
| 3 | Omission errors | 0.27 (0.64) | 0.07 (0.38) | 0.362 | 0.029b | 0.349 (0.131) | 0.004 |
| 4 | Early responses | 1.46 (2.64) | 0.59 (1.51) | 0.400 | 0.445 | a | a |
| Part B (No-Go) | |||||||
| 5 | RT (ms) | 513 (119.63) | 463.84 (97.32) | 0.448 | 0.264 b | 0.369 (0.157) | 0.009 |
| 6 | Commission errors | 1.4 (1.93) | 0.69 (1.29) | 0.430 | 0.263 b | a | a |
| 7 | Omission errors | 0.31 (0.77) | 0.11 (0.37) | 0.320 | 0.160 b | a | a |
| 8 | Early responses | 2.48 (4.33) | 0.83 (1.76) | 0.485 | 0.159 b | a | a |
| Cross-out-squares test | |||||||
| 9 | Hits | 41.62 (5.71) | 42.88 (4.48) | 0.244 | 0.026 | 0.626 (0.178) | 2.14 × 10−4 |
| 10 | Omissions | 6.32 (5.71) | 4.77 (4.34) | 0.303 | 6.02 × 10−12b | 0.66 (0.106) | <1 × 10−7 |
| 11 | Commissions | 1.76 (5.6) | 1.82 (5.08) | 0.011 | 0.440 b | a | a |
| 12 | RT (ms) | 209.35 (112.12) | 156.67 (64.32) | 0.567 | 0.082 b | a | a |
| Trail Making Test | |||||||
| 13 | Hits in Part A | 23.62 (0.81) | 23.89 (0.31) | 0.426 | 0.071 | a | a |
| 14 | RT in Part A (ms) | 54.02 (64.61) | 38.33 (21.86) | 0.317 | 0.410 b | a | a |
| 15 | Hits in Part B | 22.43 (3.32) | 22.96 (2.05) | 0.189 | 0.398 | a | a |
| 16 | RT in Part B (ms) | 128.28 (91.06) | 96.4 (64.05) | 0.401 | 0.254 b | a | a |
| Stroop test | |||||||
| Lecture | |||||||
| 17 | RT (ms) | 76.94 (48.01) | 60.43 (20.42) | 0.438 | 0.041b | a | a |
| 18 | Errors | 1.3 (2.01) | 0.6 (1.12) | 0.419 | 0.049b | 0.705 (0.088) | <1 × 10−7 |
| Denomination | |||||||
| 19 | RT (ms) | 124.64 (76.05) | 96.85 (49.74) | 0.427 | 0.059 b | a | a |
| 20 | Errors | 3.77 (4.43) | 2.4 (4.19) | 0.318 | 0.155 | 0.661 (0.086) | <1 × 10−7 |
| Mismatch | |||||||
| 21 | RT (ms) | 112.4 (80.55) | 95.8 (70.05) | 0.219 | 0.827 b | a | a |
| 22 | Errors | 6.32 (6.35) | 3.75 (5.29) | 0.437 | 0.155 b | 0.207 (0.165) | 0.105 |
| CPT | |||||||
| 23 | Omissions | 14.66 (15.88) | 8.09 (9.89) | 0.490 | 0.121 b | 0.1 (0.178) | 0.287 |
| 24 | Omissions (%) | 4.55 (4.92) | 2.5 (3.07) | 0.492 | 0.117 b | 0.119 (0.179) | 0.254 |
| 25 | Punctuation 1 | 62.22 (32.63) | 58.48 (20.17) | 0.136 | 0.015b | a | a |
| 26 | Commission | 20.55 (14.93) | 13.24 (7.84) | 0.602 | 0.134 c | a | a |
| 27 | Commission (%) | 53.74 (22.68) | 36.71 (21.58) | 0.768 | 0.147 c | 0.076 (0.165) | 0.323 |
| 28 | Punctuation 2 | 51.02 (8.36) | 49.41 (8.01) | 0.196 | 0.196 | 0.2 (0.167) | 0.116 |
| 29 | Average hit RT (ms) | 479.32 (83.98) | 477.7 (66.43) | 0.021 | 0.764 | a | a |
| 30 | Punctuation 3 | 61.34 (10.92) | 62.77 (9.5) | 0.139 | 0.851 b | 0.506 (0.158) | 6.87 × 10−4 |
| 31 | Standard deviation of hit RT (ms) | 10.84 (7.03) | 8.23 (3.97) | 0.449 | 0.513 b | a | a |
| 32 | Punctuation 4 | 57.17 (12.13) | 58.37 (9.93) | 0.107 | 0.376 b | a | a |
| 33 | Variability | 20.71 (20.55) | 12.95 (10.48) | 0.467 | 0.380 b | a | a |
| 34 | Punctuation 5 | 56.48 (12.27) | 56.75 (9.7) | 0.024 | 0.216 b | a | a |
| 35 | Detectability | 0.5 (0.68) | 0.69 (0.4) | 0.347 | 0.856 b | a | a |
| 36 | Punctuation 6 | 52.09 (8.37) | 50.22 (8.75) | 0.219 | 0.210 | 0.203 (0.162) | 0.105 |
| 37 | PR standard deviation | 0.95 (0.94) | 1.05 (1.17) | 0.095 | 0.714 b | 0.499 (0.119) | 1.38 × 10−5 |
| 38 | Punctuation 7 | 53.76 (12.73) | 53.5 (11.69) | 0.021 | 0.765 | a | a |
| 39 | Perseverations | 7.48 (14.21) | 2.33 (4.66) | 0.475 | 0.272 b | a | a |
| 40 | Punctuation 8 | 60.77 (23.63) | 58.6 (22.16) | 0.094 | 0.020b | a | a |
| 41 | HRTBCH | 0.01 (0.03) | 0 (0.02) | 0.104 | 0.399 b | a | a |
| 42 | Punctuation 9 | 49.77 (9.85) | 49.68 (9.94) | 0.009 | 0.979 | a | a |
| 43 | HSEBCH | 0.06 (0.1) | 0.04 (0.07) | 0.251 | 0.527 b | a | a |
| 44 | Punctuation 10 | 50.56 (12.31) | 53.14 (11.23) | 0.218 | 0.248 | a | a |
| 45 | HRTISICH | 0.07 (0.07) | 0.04 (0.05) | 0.456 | 0.517 b | a | a |
| 46 | Punctuation 11 | 50.49 (12.76) | 47.08 (11.93) | 0.276 | 0.445 b | 0.163 (0.163) | 0.159 |
| 47 | HSEISICH | 0.09 (0.12) | 0.06 (0.09) | 0.300 | 0.566 b | a | a |
| 48 | Punctuation 12 | 49.87 (10.3) | 48.4 (10.97) | 0.139 | 0.992 c | 0.246 (0.144) | 0.043 |
3.5. Predictive Model for ADHD Diagnosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Vélez, J.I.; Acosta, M.T.; Palacio, L.G.; Balog, J.; Roessler, E.; Pineda, D.; Londoño, A.C.; Palacio, J.D.; Arbelaez, A.; et al. A cooperative interaction between LPHN3 and 11q doubles the risk for ADHD. Mol. Psychiatry 2012, 17, 741–747. [Google Scholar] [CrossRef]
- Arcos-Burgos, M.; Jain, M.; Acosta, M.T.; Shively, S.; Stanescu, H.; Wallis, D.; Domené, S.; Vélez, J.I.; Karkera, J.D.; Balog, J.; et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol. Psychiatry 2010, 15, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Bukstein, O.G. Attention deficit hyperactivity disorder and substance use disorders. Behav. Neurosci. Atten. Deficit. Hyperact. Disord. Its Treat. 2011, 145–172. [Google Scholar] [CrossRef]
- Pelham, W.E., Jr.; Wheeler, T.; Chronis, A. Empirically supported psychosocial treatments for attention deficit hyperactivity disorder. J. Clin. Child. Psychol. 1998, 27, 190–205. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM), 4th ed.; APA: Washington, DC, USA, 2000. [Google Scholar]
- Sibley, M.H.; Pelham, W.E.; Molina, B.S.G.; Gnagy, E.M.; Waschbusch, D.A.; Garefino, A.C.; Kuriyan, A.B.; Babinski, D.E.; Karch, K.M. Diagnosing ADHD in adolescence. J. Consult. Clin. Psychol. 2012, 80, 139–150. [Google Scholar] [CrossRef]
- Faraone, S.V.; Mick, E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr. Clin. N. Am. 2010, 33, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Tannock, R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat. Rev. Neurosci. 2002, 3, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A.; Rockstroh, B.S. Progress and prospects for endophenotypes for schizophrenia in the time of genomics, epigenetics, oscillatory brain dynamics, and the Research Domain Criteria. Neurobiol. Schizophr. 2016, 17–38. [Google Scholar] [CrossRef]
- Walters, J.T.R.; Owen, M.J. Endophenotypes in psychiatric genetics. Mol. Psychiatry 2007, 12, 886–890. [Google Scholar] [CrossRef]
- Lee Gregory, M.; Burton, V.J.; Shapiro, B.K. Developmental Disabilities and Metabolic Disorders. In Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders; Michael, J.Z., Lewis, P.R., Joseph, T.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 18–41. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Longman: Essex, UK, 1996. [Google Scholar]
- Flint, J.; Munafò, M.R. The endophenotype concept in psychiatric genetics. Psychol. Med. 2007, 37, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Brotman, M.A.; Rich, B.A.; Guyer, A.E.; Lunsford, J.R.; Horsey, S.E.; Reising, M.M.; Thmas, L.A.; Fromm, S.J.; TOwbin, K.; Pine, D.S.; et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am. J. Psychiatry 2010, 167, 61–69. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Tsuang, M.T.; Faraone, S.V. The frustrating search for schizophrenia genes. Am. J. Med. Genet. 2000, 97, 1–3. [Google Scholar] [CrossRef]
- Cervantes-Henríquez, M.L.; Acosta-López, J.E.; Martínez-Banfi, M.L.; Vélez, J.I.; Mejía-Segura, E.; Lozano-Gutiérrez, S.G.; Sánchez-Rojas, M.; Zurbarán, M.A.; Zurek, E.E.; Arcos-Burgos, M.; et al. ADHD Endophenotypes in Caribbean Families. J. Atten. Disord. 2018, 2114. [Google Scholar] [CrossRef]
- Mastronardi, C.A.; Pillai, E.; Pineda, D.A.; Martinez, A.F.; Lopera, F.; Velez, J.I.; Palacio, J.D.; Patel, H.; Easteal, S.; Acosta, M.T.; et al. Linkage and association analysis of ADHD endophenotypes in extended and multigenerational pedigrees from a genetic isolate. Mol. Psychiatry 2016, 21, 1434–1440. [Google Scholar] [CrossRef]
- Pineda, D.A.; Lopera, F.; Puerta, I.C.; Trujillo-Orrego, N.; Aguirre-Acevedo, D.C.; Hincapie-Henao, L.; Arango, C.P.; Acosta, M.T.; Holzinger, S.I.; Palacio, J.D.; et al. Potential cognitive endophenotypes in multigenerational families: Segregating ADHD from a genetic isolate. Atten. Defic. Hyperact. Disord. 2011, 3, 291. [Google Scholar] [CrossRef]
- Gau, S.S.; Shang, C.Y. Executive functions as endophenotypes in ADHD: Evidence from the Cambridge Neuropsychological Test Battery (CANTAB). J. Child Psychol. Psychiatry 2010, 51, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Sweet, L.H. N-Back Paradigm. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1718–1719. [Google Scholar]
- Woods, S.P.; Lovejoy, D.W.; Stutts, M.L.; Ball, J.D.; Fals-Stewart, W. Comparative efficiency of a discrepancy analysis for the classification of Attention-Deficit/Hyperactivity Disorder in adults. Arch. Clin. Neuropsychol. 2002, 17, 351–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noreika, V.; Falter, C.M.; Rubia, K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia 2013, 51, 235–266. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Halari, R.; Cubillo, A.; Mohammad, A.-M.; Brammer, M.; Taylor, E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009, 57, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.J.; Rapport, M.D.; Sarver, D.E.; Raiker, J.S.; Orban, S.A.; Friedman, L.M.; Kolomeyer, E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013, 33, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Heitz, R.P. The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Front. Neurosci. 2014, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Rommelse, N.N.J.; Oosterlaan, J.; Buitelaar, J.; Faraone, S.V.; Sergeant, J.A. Time reproduction in children with ADHD and their nonaffected siblings. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 582–590. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Lee, P.P.; Sharp, W.; Jeffries, N.O.; Greenstein, D.K.; Clasen, L.S.; Blumenthal, J.D.; James, R.S.; Ebens, C.L.; Walter, J.M.; et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002, 288, 1740–1748. [Google Scholar] [CrossRef]
- Valko, L.; Schneider, G.; Doehnert, M.; Müller, U.; Brandeis, D.; Steinhausen, H.-C.; Drechsler, R. Time processing in children and adults with ADHD. J. Neural Transm. 2010, 117, 1213–1228. [Google Scholar] [CrossRef]
- Prevatt, F.; Proctor, B.; Baker, L.; Garrett, L.; Yelland, S. Time estimation abilities of college students with ADHD. J. Atten. Disord. 2011, 15, 531–538. [Google Scholar] [CrossRef]
- Meck, W.H. Neuropsychology of timing and time perception. Brain Cogn. 2005, 58, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez-García, A.G.; Reyes-Platas, D.I.; Picazo, O. Percepción del tiempo en la neuropsicopatología: Una revisión sistemática. Psiquiatría Biológica 2017, 24, 85–96. [Google Scholar] [CrossRef]
- Mette, C.; Grabemann, M.; Zimmermann, M.; Strunz, L.; Scherbaum, N.; Wiltfang, J.; Kis, B. No clear association between impaired short-term or working memory storage and time reproduction capacity in adult ADHD patients. PLoS ONE 2015, 10, e0133714. [Google Scholar] [CrossRef]
- Barkley, R.A.; Murphy, K.R.; Bush, T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology 2001, 15, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Narbona, N.; Leal-Campanario, R.; Gruart, A. El procesamiento temporal en el Trastorno por Déficit de Atención e Hiperactividad. Rev. Psicol. Clínica Niños Adolesc. 2021, 8, 9–15. [Google Scholar]
- Barkley, R.A.; Koplowitz, S.; Anderson, T.; McMurray, M.B. Sense of time in children with ADHD: Effects of duration, distraction, and stimulant medication. J. Int. Neuropsychol. Soc. 1997, 3, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65. [Google Scholar] [CrossRef]
- Barkley, R.A. Attention-deficit hyperactivity disorder. Sci. Am. 1998, 279, 66–71. [Google Scholar] [CrossRef]
- Barkley, R.A. Attention-deficit/hyperactivity disorder, self-regulation, and time: Toward a more comprehensive theory. J. Dev. Behav. Pediatr. 1997, 18, 271–279. [Google Scholar]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.; Chang, Z.; D’Onofrio, B.M.; Lichtenstein, P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol. Med. 2014, 44, 2223–2229. [Google Scholar] [CrossRef]
- Lopera, F.; Palacio, L.G.; Jimenez, I.; Villegas, P.; Puerta, I.C.; Pineda, D.; Jiménez, M.; Arcos-Burgos, M. Discrimination between genetic factors in attention deficit. Rev. Neurol. 1999, 28, 660–664. [Google Scholar]
- Puentes Rozo Acosta-Lopez, J.E.; Cervantes-Henriquez, M.; Martinez-Banfi, M.; Lozano-Gutierrez, S.; Jimenez-Figueroa, G.; Pineda-Alhucema, W.; Mejia-Segura, E.; Zurbaran, M.A.; Zurek, E.E.; Sanchez-Rojas, M.; et al. Attention Deficit /Hyperactivity Disorder and Comorbidities in 120 Nuclear Families from a Caribbean Community. Unpublished work. 2017. [Google Scholar]
- Villalón, J. Colonias Extranjeras en Barranquilla; Ediciones Uninorte: Barranquilla, Colombia, 2008. [Google Scholar]
- Wabgou, M.; Vargas, D.; Carabali, J.A. Las migraciones internacionales en Colombia. Investig. Desarro. 2012, 20, 142–167. [Google Scholar]
- Barragán-Duarte, J.L. Mapa genético de los colombianos. UN Periódico 2007, 105. Available online: http://historico.unperiodico.unal.edu.co/ediciones/105/15.html (accessed on 15 February 2017).
- Martinez, B.; Caraballo, L.; Gusmao, L.; Amorim, A.; Carracedo, A. Autosomic STR population data in two Caribbean samples from Colombia. Forensic Sci. Int. 2005, 152, 79–81. [Google Scholar] [CrossRef]
- Usaquén Martinez, W. Validación y Consistencia de Información en Estudios de diversidad Genética Humana a Partir de Marcadores Microsatélites. Tesis de Doctorado en Ciencias-Biología, Universidad Nacional de Colombia, Bogota, Colombia, 2012. [Google Scholar]
- Arcos-Burgos, M.; Muenke, M. Genetics of population isolates. Clin. Genet. 2002, 61, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Burgos, M.; Castellanos, F.X.; Pineda, D.; Lopera, F.; Palacio, J.D.; Palacio, L.G.; Rapoport, J.L.; Berg, K.; Bailey-Wilson, J.; Muenke, M.; et al. Attention-deficit/hyperactivity disorder in a population isolate: Linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am. J. Hum. Genet. 2004, 75, 998–1014. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.L.; Valenzuela, C.Y.; Arcos-Burgos, O.M. Polymorphisms and phyletic relationships of the Paisa community from Antioquia (Colombia). Gene Geogr. 1996, 10, 11–17. [Google Scholar] [PubMed]
- Arcos-Burgos, M.; Castellanos, F.X.; Lopera, F.; Pineda, D.; Palacio, J.D.; Garcia, M.; Henao, G.C.; Palacio, L.G.; Berg, K.; Bailey-Wilson, J.E.; et al. Attention-deficit/hyperactivity disorder (ADHD): Feasibility of linkage analysis in a genetic isolate using extended and multigenerational pedigrees. Clin. Genet. 2002, 61, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Burgos, M.; Castellanos, F.X.; Konecki, D.; Lopera, F.; Pineda, D.; Palacio, J.D.; Rapoport, J.L.; Berg, K.; Bailey-Wilson, J.; Muenke, M. Pedigree disequilibrium test (PDT) replicates association and linkage between DRD4 and ADHD in multigenerational and extended pedigrees from a genetic isolate. Mol. Psychiatry 2004, 9, 252–259. [Google Scholar] [CrossRef]
- Pineda, D.A.; Acosta López, J.; Cervantes-Henríquez, M.L.; Jimenez-Figueroa, G.; Sánchez-Rojas, M.; Pineda-Alhucema, W.; Mejía-Segura, E.; Puentes-Rozo, P.J. Conglomerados de clases latentes en 408 miembros de 120 familias nucleares de Barranquilla con un caso índice afectado de trastorno de atención hiperactividad (TDAH). Acta Neurol. Colomb. 2016, 32, 275–284. [Google Scholar] [CrossRef]
- Cervantes-Henríquez, M.L.; Acosta-López, J.E.; Martinez, A.F.; Arcos-Burgos, M.; Puentes-Rozo, P.J.; Vélez, J.I. Machine Learning Prediction of ADHD Severity: Association and Linkage to ADGRL3, DRD4, and SNAP25. J. Atten. Disord. 2021. [Google Scholar] [CrossRef]
- Puentes-Rozo, P.J.; Acosta-López, J.E.; Cervantes-Henríquez, M.L.; Martínez-Banfi, M.L.; Mejia-Segura, E.; Sánchez-Rojas, M.; Anaya-Romero, M.; Anaya-Romero, M.E.; Acosta-Hoyos, A.; García-Llinás, G.A.; et al. Genetic Variation Underpinning ADHD Risk in a Caribbean Community. Cells. 2019, 8, 907. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.D.; Castellanos, F.X.; Pineda, D.A.; Lopera, F.; Arcos-Burgos, M.; Quiroz, Y.T.; Henao, G.C.; Puerta, I.C.; Ramírez, D.L.; Rapoport, J.L.; et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 1506–1515. [Google Scholar] [CrossRef]
- Llanos Lizcano, L.J.; García Ruiz, D.J.; González Torres, H.J.; Puentes Rozo, P. Trastorno por déficit de atención e hiperactividad (TDAH) en niños escolarizados de 6 a 17 años. Pediatría Atención Primaria 2019, 21, e101–e108. [Google Scholar]
- Reich, W. Diagnostic interview for children and adolescents (DICA). J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, G.; Coppola, M.T.; Musazzi, A.; Altamura, A.C.; Invernizzi, G. Multinational validation of the Composite International Diagnostic Interview (CIDI). Minerva Psichiatr. 1994, 35, 63–80. [Google Scholar] [PubMed]
- Pineda, D.A.; Kamphaus, R.W.; Mora, O.; Restrepo, M.A.; Puerta, I.C.; Palacio, L.G.; Jiménez, I.; Mejía, S.; García, M.; Arango, J.C.; et al. A system of multidimensional behavior assessment. A scale for parents of children from 6 to 11 years of age. Colombian version. Rev. Neurol. 1999, 28, 672–681. [Google Scholar] [PubMed]
- Pichot, P.; López-Ibor Aliño, J.J.; Valdés Miyar, M. DSM-IV: Manual Diagnóstico y Estadístico de los Trastornos Mentales; Masson, S.A.: Milano, Italy, 2001. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM); APA: Washington, DC, USA, 1994; pp. 143–147. [Google Scholar]
- Bekker, E.M.; Kenemans, J.L.; Hoeksma, M.R.; Talsma, D.; Verbaten, M.N. The pure electrophysiology of stopping. Int. J. Psychophysiol. 2005, 55, 191–198. [Google Scholar] [CrossRef]
- Golden, C. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Company: Wood Dale, IL, USA, 1978. [Google Scholar]
- Golden, F.S. A Manual for the Adult Stroop Color and Word Test; Stoelting Company: Wood Dale, IL, USA, 2002; pp. 1–11. [Google Scholar]
- Puentes, P. Neuropsicología de las Funciones Ejecutivas; Ediciones Universidad Simón Bolívar: Barranquilla, Colombia, 2009. [Google Scholar]
- Pineda, D.; Ardila, A.; Rosselli, M.; Arias, B.E.; Henao, G.C.; Gomez, L.F.; Mejía, S.E.; Miranda, M.L. Prevalence of attention-deficit/hyperactivity disorder symptoms in 4- to 17-year-old children in the general population. J. Abnorm. Child Psychol. 1999, 27, 455–462. [Google Scholar] [CrossRef]
- Acosta-López, J.; Cervantes-Henríquez, M.; Sánchez-Rojas, M.; Núñez-Barragán, M.; Puentes Rozo, P.; Aguirre-Acevedo, D.C.; Pineda, D.A. Alteraciones del Control Inhibitorio Conductual en Niños de 6 A 11 Años Con TDAH Familiar de Barranquilla. Psicogente 2010, 13, 274–291. [Google Scholar]
- Reitan, R.M. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955, 19, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R.M. The validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. Category Test and Trail Making Test as measures of frontal lobe functions. Clin. Neuropsychol. 1995, 9, 50–56. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 1st ed.; Neuropsychology Press: Tucson, AZ, USA, 1985. [Google Scholar]
- Reitan, R.M.; Wolfson, D. The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch. Clin. Neuropsychol. 2004, 19, 281–288. [Google Scholar] [CrossRef]
- Conners, C.K.; Epstein, J.N.; Angold, A.; Klaric, J. Continuous performance test performance in a normative epidemiological sample. J. Abnorm. Child Psychol. 2003, 31, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Conners, C.K.; Sitarenios, G. Conners’ Continuous Performance Test (CPT). In Encyclopedia of Clinical Neuropsychology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 681–683. [Google Scholar]
- Conners, C.K.; Staff, M.H.S.; Connelly, V.; Campbell, S.; MacLean, M.; Barnes, J. Conners’ Continuous Performance Test II (CPT II v. 5); Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2000; Volume 29, pp. 175–196. [Google Scholar]
- Cornblatt, B.A.; Risch, N.J.; Faris, G.; Friedman, D.; Erlenmeyer-Kimling, L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988, 26, 223–238. [Google Scholar] [CrossRef]
- Jiménez-Figueroa, G.; Vidarte Claros, J.A.; Restrepo de Mejía, F. Interference Control in Attention Deficit and Hyperactivity Disorder (ADHD). CES Psicol. 2020, 13, 104–124. [Google Scholar] [CrossRef]
- Jimenez-Figueroa, G.; Ardila-Duarte, C.; Pineda, D.A.; Acosta-Lopez, J.E.; Cervantes-Henriquez, M.L.; Pineda-Alhucema, W.; Cervantes-Gutiérrez, J.; Quintero-Ibarra, M.; QSánchez-Rojas, M.; Vélez,, J.I.; et al. Prepotent response inhibition and reaction times in children with attention deficit/hyperactivity disorder from a Caribbean community. Atten. Defic. Hyperact. Disord. 2017, 9, 199–211. [Google Scholar] [CrossRef]
- Brand, A.; Bradley, M.T.; Best, L.A.; Stoica, G. Accuracy of effect size estimates from published psychological research. Percept. Mot. Skills 2008, 106, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum Associates: Hillside, NJ, USA, 1988. [Google Scholar]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era--concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef]
- Elston, R.C.; Gray-McGuire, C. A review of the “Statistical Analysis for Genetic Epidemiology” (S.A.G.E.) software package. Hum. Genom. 2004, 1, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Elston, R.C.; Satagopan, J.M.; Sun, S. Genetic terminology. Methods Mol. Biol. 2012, 850, 1–9. [Google Scholar] [PubMed]
- Elston, R.C.; Satagopan, J.M.; Sun, S. Statistical Human Genetics; Humana Press: New York, NY, USA, 2012. [Google Scholar]
- Bochud, M. Estimating heritability from nuclear family and pedigree data. Stat. Hum. Genet. Methods Protoc. 2012, 171–186. [Google Scholar] [CrossRef]
- Londono, A.C.; Castellanos, F.X.; Arbelaez, A.; Ruiz, A.; Aguirre-Acevedo, D.C.; Richardson, A.M.; Easteal, S.; Lidbury, B.A.; Arcos-Burgos, M.; Lopera, F. An 1H-MRS framework predicts the onset of Alzheimer’s disease symptoms in PSEN1 mutation carriers. Alzheimers Dement. 2014, 10, 552–561. [Google Scholar] [CrossRef]
- Vélez, I.J.; Correa, C.J.; Arcos-Burgos, M. A new method for detecting significant p-values with applications to genetic data. Rev. Colomb. Estadística 2014, 37, 67–76. [Google Scholar] [CrossRef]
- Velez, J.I.; Lopera, F.; Sepulveda-Falla, D.; Patel, H.R.; Johar, A.S.; Chuah, A.; Tobón, C.; Rivera, D.; Villegas, A.; Cai, Y.; et al. APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer’s disease. Mol. Psychiatry 2016, 21, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Dong, C.; Andreev, V.; Arcos-Burgos, M.; Licinio, J. Prediction of susceptibility to major depression by a model of interactions of multiple functional genetic variants and environmental factors. Mol. Psychiatry 2012, 17, 624–633. [Google Scholar] [CrossRef]
- Wong, M.L.; Dong, C.; Flores, D.L.; Ehrhart-Bornstein, M.; Bornstein, S.; Arcos-Burgos, M.; Licinio, J. Clinical outcomes and genome-wide association for a brain methylation site in an antidepressant pharmacogenetics study in Mexican Americans. Am. J. Psychiatry 2014, 171, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Olshen, R.; Breiman, L. A Conversation with Leo Breiman. Stat. Sci. 2001, 16, 184–198. [Google Scholar] [CrossRef]
- Breiman, L. Statistical modeling: The two cultures (with comments and a rejoinder by the author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Friedman, J.H. SMART User Guide; Technical Report; Stanford University: Stanford, CA, USA, 1984; Available online: https://statistics.stanford.edu/research/smart-users-guide (accessed on 8 September 2021).
- Rao, D.C. CAT scans, PET scans, and genomic scans. Genet. Epidemiol. 1998, 15, 1–18. [Google Scholar] [CrossRef]
- Cervantes-Henríquez, M.L.; Acosta-López, J.; Aguirre-Acevedo, D.C.; Pineda-Álvarez, D.; Puentes Rozo, P. Fenotipo comportamental evaluado con una escala multidimensional de la conducta en niños y adolescentes de 30 familias con trastorno de atención-hiperactividad. Acta Neurol. Colomb. 2008, 24, 53–62. [Google Scholar]
- Schachar, R.; Mota, V.L.; Logan, G.D.; Tannock, R.; Klim, P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 2000, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef]
- Stuss, D.T.; Murphy, K.J.; Binns, M.A.; Alexander, M.P. Staying on the job: The frontal lobes control individual performance variability. Brain 2003, 126, 2363–2380. [Google Scholar] [CrossRef]
- Stuss, D.T.; Binns, M.A.; Murphy, K.J.; Alexander, M.P. Dissociations within the anterior attentional system: Effects of task complexity and irrelevant information on reaction time speed and accuracy. Neuropsychology 2002, 16, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.C.; Rijsdijk, F.; Johnson, K.A.; Andreou, P.; Albrecht, B.; Arias-Vasquez, A.; Buitelaar, J.K.; McLoughlin, G.; Rommelse, N.N.; Sergeant, J.A.; et al. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychol. Med. 2011, 41, 861–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, K.A.; Barry, E.; Bellgrove, M.A.; Cox, M.; Kelly, S.P.; Daibhis, A.; Daly, M.; Keavey, M.; Watchorn, A.; Fitzgerald, M.; et al. Dissociation in response to methylphenidate on response variability in a group of medication naive children with ADHD. Neuropsychologia 2008, 46, 1532–1541. [Google Scholar] [CrossRef]
- Johnson, K.A.; Robertson, I.H.; Kelly, S.P.; Silk, T.J.; Barry, E.; Daibhis, A.; Watchorn, A.; Keavey, M.; Fitzgerald, M.; Gallagher, L.; et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia 2007, 45, 2234–2245. [Google Scholar] [CrossRef]
- Gupta, D.S.; Bahmer, A. Increase in Mutual Information During Interaction with the Environment Contributes to Perception. Entropy 2019, 21, 365. [Google Scholar] [CrossRef]
- Corkum, P.V.; Siegel, L.S. Is the Continuous Performance Task a valuable research tool for use with children with Attention-Deficit-Hyperactivity Disorder? J. Child Psychol. Psychiatry 1993, 34, 1217–1239. [Google Scholar] [CrossRef]
- Van der Meere, J.; Vreeling, H.J.; Sergeant, J. A motor presetting study in hyperactive, learning disabled and control children. J. Child Psychol. Psychiatry 1992, 33, 1347–1351. [Google Scholar] [CrossRef]
- Gupta, D.S.; Banerjee, A.; Roy, D.; Piras, F. Editorial: Temporal Structure of Neural Processes Coupling Sensory, Motor and Cognitive Functions of the Brain. Front. Comput. Neurosci. 2020, 14, 73. [Google Scholar] [CrossRef]
- Sarkar, I.N. Biomedical informatics and translational medicine. J. Transl. Med. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Capdevila-Brophy, C.; Artigas-Pallarés, J.; Ramírez-Mallafré, A.; López-Rosendo, M.; Real, J.; Obiols-Llandrich, J.E. Fenotipo neuropsicológico del trastorno de déficit atencional/hiperactividad:¿ existen diferencias entre los subtipos. Rev. Neurol. 2005, 40, 17–23. [Google Scholar] [CrossRef]
- Alderson, R.M.; Rapport, M.D.; Kofler, M.J. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. J. Abnorm. Child Psychol. 2007, 35, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Slachevsky, C.A.; Pérez, C.; Silva, J.R.; Ruiz-Tagle, A.; Mayol, R.; Muñoz-Neira, C.; Núñez-Huasaf, J. Descomponiendo el síndrome de déficit atencional en el adulto: Hacia un entendimiento de su heterogeneidad pronóstica. Rev. Med. Chile 2012, 140, 379–385. [Google Scholar] [CrossRef]
- Bluschke, A.; Broschwitz, F.; Kohl, S.; Roessner, V.; Beste, C. The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bluschke, A.; Roessner, V.; Beste, C. Specific cognitive-neurophysiological processes predict impulsivity in the childhood attention-deficit/hyperactivity disorder combined subtype. Psychol. Med. 2016, 46, 1277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waber, D.P.; Weiler, M.D.; Forbes, P.W.; Bernstein, J.H.; Bellinger, D.C.; Rappaport, L. Neurobehavioral factors associated with referral for learning problems in a community sample: Evidence for an adaptational model for learning disorders. J. Learn. Disabil. 2003, 36, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Rosch, K.S.; Hawk, L.W., Jr. The effects of performance-based rewards on neurophysiological correlates of stimulus, error, and feedback processing in children with ADHD. Psychophysiology 2013, 50, 1157–1173. [Google Scholar] [CrossRef]
- Caspersen, I.D.; Petersen, A.; Vangkilde, S.; Plessen, K.J.; Habekost, T. Perceptual and response-dependent profiles of attention in children with ADHD. Neuropsychology 2017, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Téllez, G.; Romero-Romero, H.; Rivera-García, L.; Prieto-Corona, B.; Bernal-Hernández, J.; Marosi-Holczberger, E.; Guerrero-Juárez, V.; Rodríguez-Camacho, M.; Silva-Pereyra, J.F. Cognitive and executive functions in ADHD. Actas Esp. Psiquiatr. 2012, 40, 293–298. [Google Scholar]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Shiels, K.; Hawk, L.W., Jr. Self-regulation in ADHD: The role of error processing. Clin. Psychol. Rev. 2010, 30, 951–961. [Google Scholar] [CrossRef]
- Mirsky, A.F.; Pascualvaca, D.M.; Duncan, C.C.; French, L.M. A model of attention and its relation to ADHD. Ment. Retard. Dev. Disabil. Res. Rev. 1999, 5, 169–176. [Google Scholar] [CrossRef]
- MacDonald, S.W.S.; Li, S.-C.; Bäckman, L. Neural underpinnings of within-person variability in cognitive functioning. Psychol. Aging 2009, 24, 792. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Avitable, N.; Grant, I.; Matthews, C.G. Further crossvalidation of regression-based neuropsychological norms with an update for the Boston Naming Test. J. Clin. Exp. Neuropsychol. 1999, 21, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, D.; Hoekstra, P.J.; Mennes, M.; von Rhein, D.; Thissen, A.J.A.M.; Heslenfeld, D.; Zwiers, M.P.; Faraone, S.V.; Oosterlaan, J.; Franke, B.; et al. Distinguishing adolescents with ADHD from their unaffected siblings and healthy comparison subjects by neural activation patterns during response inhibition. Am. J. Psychiatry 2015, 172, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, J.H.; Cantwell, D.P.; Satterfield, B.T. Pathophysiology of the hyperactive child syndrome. Arch. Gen. Psychiatry 1974, 31, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Morein-Zamir, S.; Dodds, C.; van Hartevelt, T.J.; Schwarzkopf, W.; Sahakian, B.; Müller, U.; Robbins, T. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Hum. Brain Mapp. 2014, 35, 5141–5152. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C.; Henriksen, L.; Bruhn, P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch. Neurol. 1984, 41, 825–829. [Google Scholar] [CrossRef]
- Silberstein, R.B.; Pipingas, A.; Farrow, M.; Levy, F.; Stough, C.K. Dopaminergic modulation of default mode network brain functional connectivity in attention deficit hyperactivity disorder. Brain Behav. 2016, 6, e00582. [Google Scholar] [CrossRef]
- Bellgrove, M.A.; Hawi, Z.; Kirley, A.; Fitzgerald, M.; Gill, M.; Robertson, I.H. Association between dopamine transporter (DAT1) genotype, left-sided inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology 2005, 30, 2290–2297. [Google Scholar] [CrossRef]
- Doyle, A.E.; Faraone, S.V.; Seidman, L.J.; Willcutt, E.G.; Nigg, J.T.; Waldman, I.D.; Pennington, B.F.; Peart, J.; Biederman, J. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J. Child Psychol. Psychiatry 2005, 46, 774–803. [Google Scholar] [CrossRef]
- Nigg, J.T. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biol. Psychiatry 2005, 57, 1424–1435. [Google Scholar] [CrossRef]
- Kuntsi, J.; Wood, A.C.; Rijsdijk, F.; Johnson, K.A.; Andreou, P.; Albrecht, B.; Arias-Vasquez, A.; Buitelaar, J.K.; McLoughlin, G.; Rommelse, N.N.; et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch. Gen. Psychiatry 2010, 67, 1159–1167. [Google Scholar] [CrossRef]
- Kuntsi, J.; Stevenson, J. Psychological mechanisms in hyperactivity: II The role of genetic factors. J. Child. Psychol. Psychiatry 2001, 42, 211–219. [Google Scholar] [CrossRef]
- Andreou, P.; Neale, B.M.; Chen, W.; Christiansen, H.; Gabriels, I.; Heise, A.; Meidad, S.; Muller, U.C.; Uebel, H.; Banaschewski, T.; et al. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychol. Med. 2007, 37, 1703–1715. [Google Scholar] [CrossRef]
- Pineda, D.A.; Palacio, L.G.; Puerta, I.C.; Merchan, V.; Arango, C.P.; Galvis, A.Y.; Gómez, M.; Aguirre, D.C.; Lopera, F.; Arcos-Burgos, M. Environmental influences that affect attention deficit/hyperactivity disorder: Study of a genetic isolate. Eur. Child Adolesc. Psychiatry 2007, 16, 337–346. [Google Scholar] [CrossRef]
- Rommelse, N.N.; Altink, M.E.; Arias-Vasquez, A.; Buschgens, C.J.; Fliers, E.; Faraone, S.V.; Buitelaar, J.K.; Sergeant, J.A.; Oosterlaan, J.; Franke, B. Differential association between MAOA, ADHD and neuropsychological functioning in boys and girls. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.N.; Hwang, M.E.; Antonini, T.; Langberg, J.M.; Altaye, M.; Arnold, L.E. Examining predictors of reaction times in children with ADHD and normal controls. J. Int. Neuropsychol. Soc. 2010, 16, 138–147. [Google Scholar] [CrossRef]
- Epstein, J.N.; Langberg, J.M.; Rosen, P.J.; Graham, A.; Narad, M.E.; Antonini, T.N.; Brinkman, W.B.; Froehlich, T.; Simon, J.O.; Altaye, M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology 2011, 25, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Henriquez, M.P.; Billeke, P.; Henriquez, H.; Zamorano, F.J.; Rothhammer, F.; Aboitiz, F. Intra-Individual Response Variability Assessed by Ex-Gaussian Analysis may be a New Endophenotype for Attention-Deficit/Hyperactivity Disorder. Front. Psychiatry 2014, 5, 197. [Google Scholar] [CrossRef]
- Hervey, A.S.; Epstein, J.N.; Curry, J.F.; Tonev, S.; Eugene Arnold, L.; Keith Conners, C.; Hinshaw, S.P.; Swanson, J.M.; Hechtman, L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006, 12, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Leth-Steensen, C.; Elbaz, Z.K.; Douglas, V.I. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychol. 2000, 104, 167–190. [Google Scholar] [CrossRef]
- Hongsermeier, T.; Kashyap, V. A Knowledge Management platform for Translational Medicine. AMIA Ann. Symp. Proc 2005, 2005, 984. [Google Scholar]
- Salamanca-Ortíz, D.N.; Vergara-Vergara, J.Y.; Escobar-Córdoba, F.; Rodríguez-Gama, Á.; Caminos-Pinzón, J.E. Genetic and molecular advances in the study of mental disorders. Rev. Fac. Med. 2014, 62, 319–324. [Google Scholar]
- Gómez Sánchez, C.I. Genética y Farmacogenética del Trastorno por Déficit de Atención e Hiperactividad en Niños de la Población Española. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Ribases, M.; Ramos-Quiroga, J.A.; Sanchez-Mora, C.; Bosch, R.; Richarte, V.; Palomar, G.; Gastaminza, X.; Bielsa, A.; Arcos-Burgos, M.; Muenke, M.; et al. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: A replication study. Genes Brain Behav. 2011, 10, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hwang-Gu, S.L.; Gau, S.S. Interval timing deficits assessed by time reproduction dual tasks as cognitive endophenotypes for attention-deficit/hyperactivity disorder. PLoS ONE 2015, 10, e0127157. [Google Scholar] [CrossRef] [PubMed]
- Bruxel, E.M.; Salatino-Oliveira, A.; Akutagava-Martins, G.C.; Tovo-Rodrigues, L.; Genro, J.P.; Zeni, C.P.; Polanczyk, G.V.; Chazan, R.; Schmitz, M.; Arcos-Burgos, M.; et al. LPHN3 and attention-deficit/hyperactivity disorder: A susceptibility and pharmacogenetic study. Genes Brain Behav. 2015, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Burgos, M.; Muenke, M. Toward a better understanding of ADHD: LPHN3 gene variants and the susceptibility to develop ADHD. Atten. Defic. Hyperact. Disord. 2010, 2, 139–147. [Google Scholar] [CrossRef]
- Acosta, M.T.; Vélez, J.I.; Bustamante, M.L.; Balog, J.Z.; Arcos-Burgos, M.; Muenke, M. A two-locus genetic interaction between LPHN3 and 11q predicts ADHD severity and long-term outcome. Transl. Psychiatry 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Payne, P.R.; Embi, P.J.; Sen, C.K. Translational informatics: Enabling high-throughput research paradigms. Physiol. Genom. 2009, 39, 131–140. [Google Scholar] [CrossRef]
- Payne, P.R.; Johnson, S.B.; Starren, J.B.; Tilson, H.H.; Dowdy, D. Breaking the translational barriers: The value of integrating biomedical informatics and translational research. J. Investig. Med. 2005, 53, 192–200. [Google Scholar] [CrossRef]
- Vélez, J.I. Machine Learning based Psychology: Advocating for A Data-Driven Approach. Int. J. Psychol. Res. 2021, 14, 6–11. [Google Scholar] [CrossRef]
| Unaffected | Affected | Statistic Index | p | Cohen’s Effect Size | |
|---|---|---|---|---|---|
| (n = 108) | (n = 124) | ||||
| Sex | Frequency (%) | Frequency (%) | χ2 | ||
| Female | 64 (59.25) | 40 (32.26) | 15.942 | <0.00001 | - |
| Male | 44 (40.75) | 84 (67.74) | |||
| Mean (SD) | Mean (SD) | Mann–Whitney’s U | |||
| Age | 34.11 (13.06) | 23.14 (15.60) | 9239.5 | <0.0001 | 0.758 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-López, J.E.; Suárez, I.; Pineda, D.A.; Cervantes-Henríquez, M.L.; Martínez-Banfi, M.L.; Lozano-Gutiérrez, S.G.; Ahmad, M.; Pineda-Alhucema, W.; Noguera-Machacón, L.M.; Hoz, M.D.L.; et al. Impulsive and Omission Errors: Potential Temporal Processing Endophenotypes in ADHD. Brain Sci. 2021, 11, 1218. https://doi.org/10.3390/brainsci11091218
Acosta-López JE, Suárez I, Pineda DA, Cervantes-Henríquez ML, Martínez-Banfi ML, Lozano-Gutiérrez SG, Ahmad M, Pineda-Alhucema W, Noguera-Machacón LM, Hoz MDL, et al. Impulsive and Omission Errors: Potential Temporal Processing Endophenotypes in ADHD. Brain Sciences. 2021; 11(9):1218. https://doi.org/10.3390/brainsci11091218
Chicago/Turabian StyleAcosta-López, Johan E., Isabel Suárez, David A. Pineda, Martha L. Cervantes-Henríquez, Martha L. Martínez-Banfi, Semiramis G. Lozano-Gutiérrez, Mostapha Ahmad, Wilmar Pineda-Alhucema, Luz M. Noguera-Machacón, Moisés De La Hoz, and et al. 2021. "Impulsive and Omission Errors: Potential Temporal Processing Endophenotypes in ADHD" Brain Sciences 11, no. 9: 1218. https://doi.org/10.3390/brainsci11091218
APA StyleAcosta-López, J. E., Suárez, I., Pineda, D. A., Cervantes-Henríquez, M. L., Martínez-Banfi, M. L., Lozano-Gutiérrez, S. G., Ahmad, M., Pineda-Alhucema, W., Noguera-Machacón, L. M., Hoz, M. D. L., Mejía-Segura, E., Jiménez-Figueroa, G., Sánchez-Rojas, M., Mastronardi, C. A., Arcos-Burgos, M., Vélez, J. I., & Puentes-Rozo, P. J. (2021). Impulsive and Omission Errors: Potential Temporal Processing Endophenotypes in ADHD. Brain Sciences, 11(9), 1218. https://doi.org/10.3390/brainsci11091218








