“Stay at Home” in Italy during the COVID-19 Outbreak: A Longitudinal Study on Individual Well-Being among Different Age Groups
Abstract
:1. Introduction
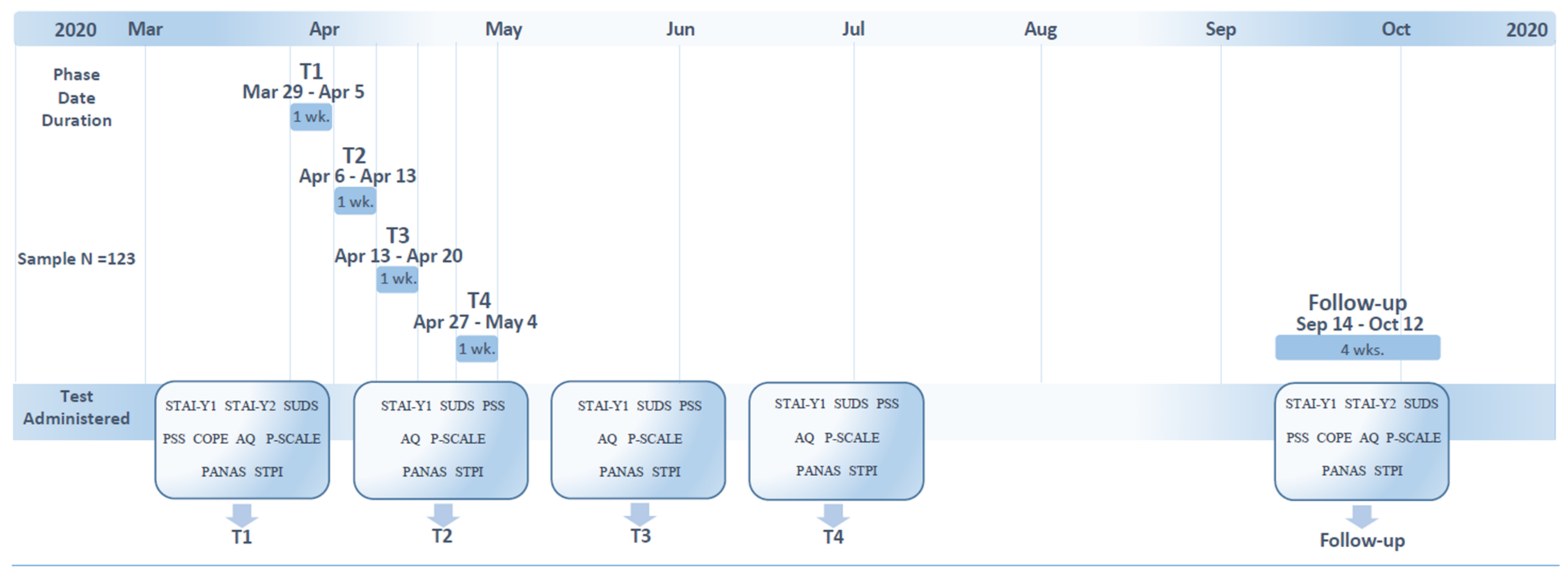
2. Materials and Methods
2.1. Participants
2.2. Procedure and Materials
2.2.1. State-Trait Anxiety Inventory (STAI-Y1; STAI-Y2)
2.2.2. Subjective Units of Distress Scale (SUDS)
2.2.3. Perceived Stress Scale (PSS)
2.2.4. Brief COPE (COPE) Inventory
2.2.5. Aggression Questionnaire (AQ)
2.2.6. Positivity Scale (P-Scale)
2.2.7. Positive and Negative Affect Schedule-Trait (PANAS)
2.2.8. Stanford Time Perspective Inventory (STPI)—Short Form
2.3. Data Analysis
3. Results
3.1. Participants
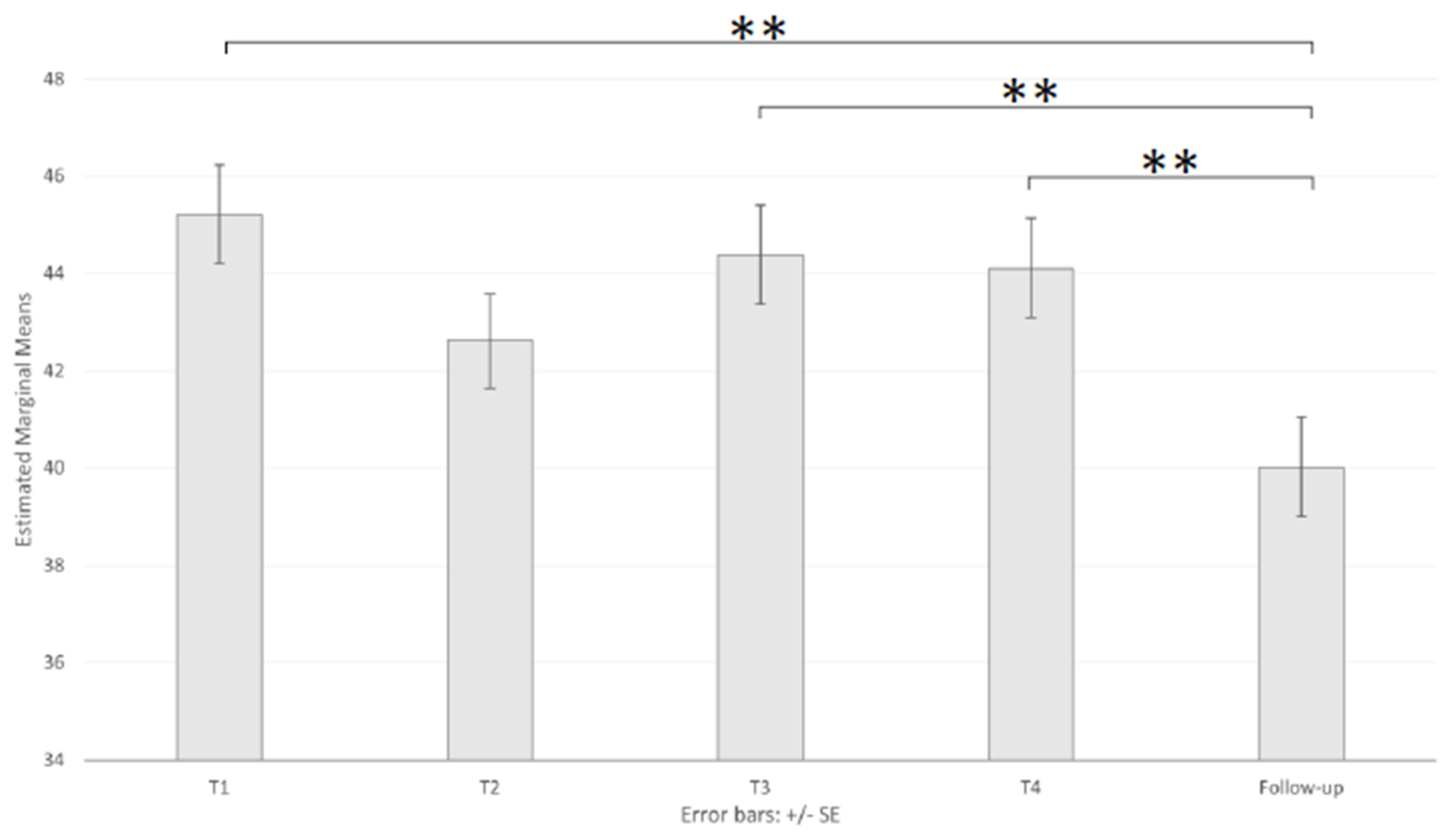
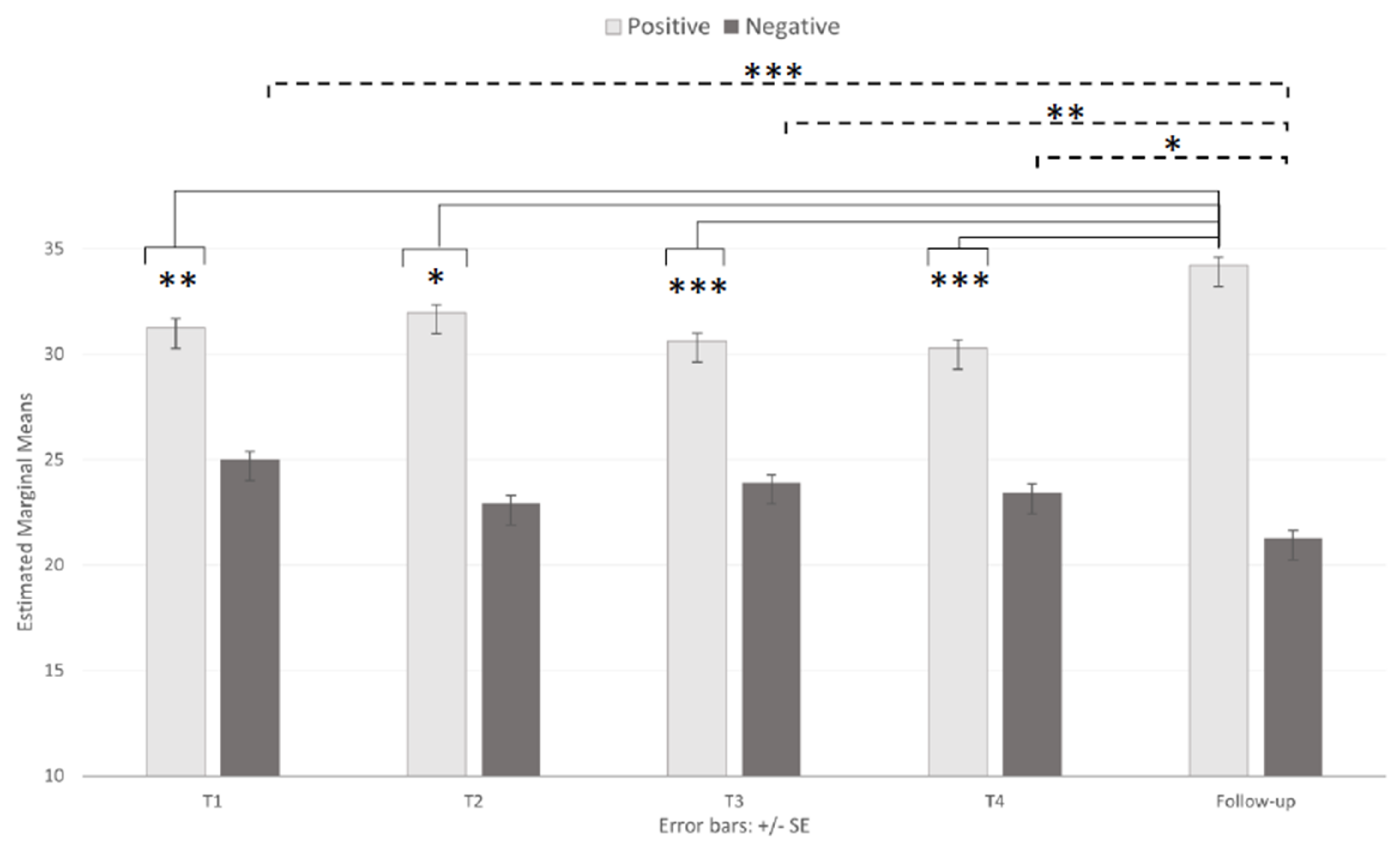
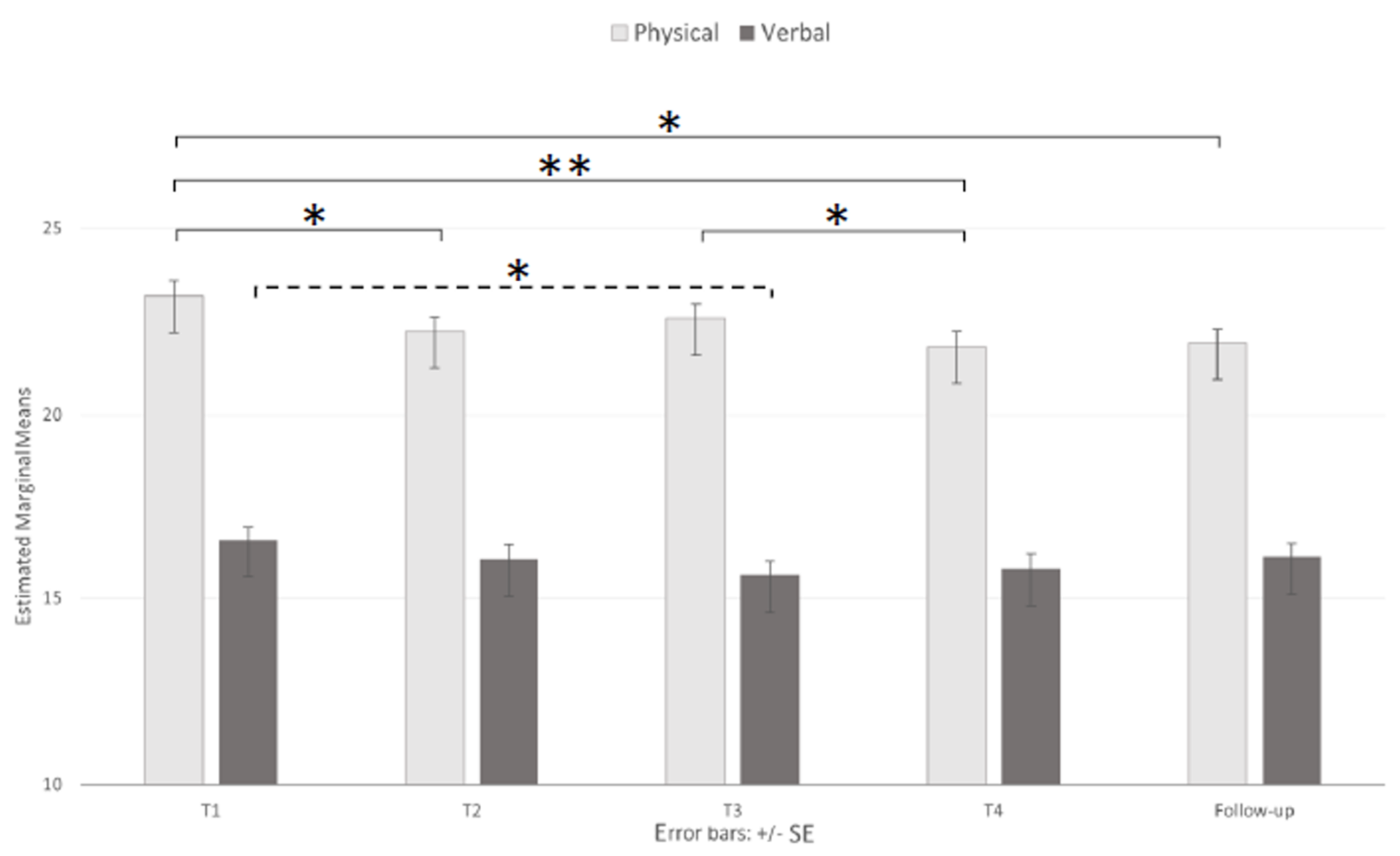
3.2. Emotional State
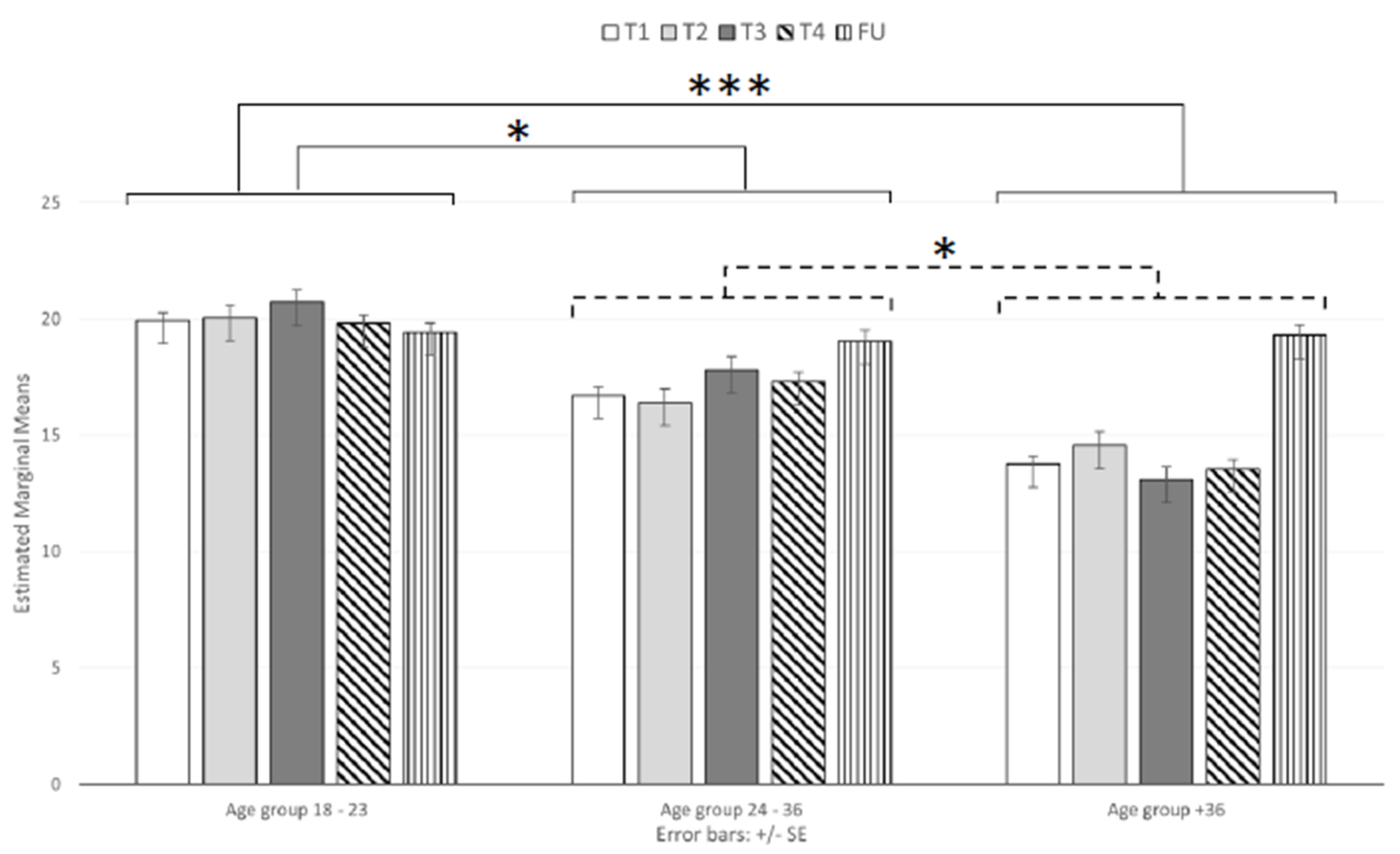
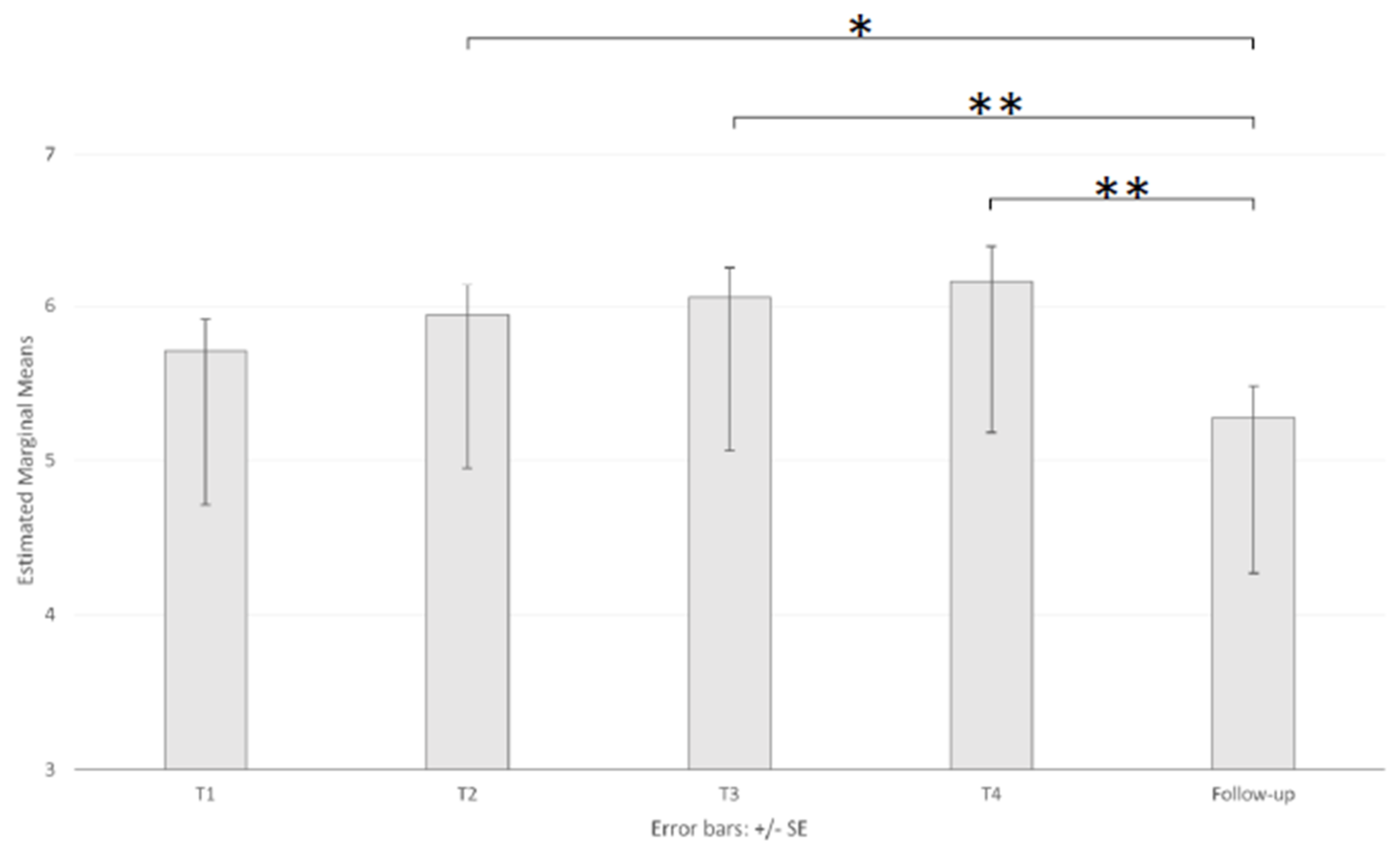
3.3. Perceived Stress
3.4. Future Perspective
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Remarks at The Media Briefing on 2019-nCoV on 11 February 2020. World Health Organization, Geneva. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 15 January 2021).
- Giattino, C.; Ritchie, H.; Roser, M.; Ortiz-Ospina, E.; Hasell, J. Excess Mortality during the Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/excess-mortality-covid (accessed on 13 January 2021).
- Perone, G. The determinants of COVID-19 case fatality rate (CFR) in the Italian regions and provinces: An analysis of environmental, demographic, and healthcare factors. Sci. Total. Environ. 2021, 755, 142523. [Google Scholar] [CrossRef]
- Townsend, E.; Nielsen, E.; Allister, R.; Cassidy, S.A. Key ethical questions for research during the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 381–383. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, L.; Zhang, C.; Xiang, Y.T.; Liu, Z.; Hu, S.; Zhang, B. Online mental health services in China during the COVID-19 outbreak. Lancet Psychiatry 2020, 7, e17–e18. [Google Scholar] [CrossRef]
- Pieh, C.; Budimir, S.; Humer, E.; Probst, T. Comparing mental health during the COVID-19 lockdown and 6 months after the lockdown in austria: A longitudinal study. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zheng, P.; Jia, Y.; Chen, H.; Mao, Y.; Chen, S.; Wang, Y.; Fu, H.; Dai, J. Mental health problems and social media exposure during COVID-19 outbreak. PLoS ONE 2020, 15, e0231924. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kong, D.; Li, S.; Yang, N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923549-1–e923549-8. [Google Scholar] [CrossRef]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiatry 2020, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrai, J.; Roma, P.; Barchielli, B.; Biondi, S.; Cordellieri, P.; Fraschetti, A.; Pizzimenti, A.; Mazza, C.; Ferracuti, S.; Giannini, A.M. Psychological and Emotional Impact of Patients Living in Psychiatric Treatment Communities during Covid-19 Lockdown in Italy. J. Clin. Med. 2020, 9, 3787. [Google Scholar] [CrossRef]
- Roma, P.; Monaro, M.; Colasanti, M.; Ricci, E.; Biondi, S.; Di Domenico, A.; Cristina Verrocchio, M.; Napoli, C.; Ferracuti, S.; Mazza, C. A 2-month follow-up study of psychological distress among italian people during the COVID-19 lockdown. Int. J. Environ. Res. Public Health 2020, 17, 8180. [Google Scholar] [CrossRef] [PubMed]
- Kooij, D.T.; Kanfer, R.; Betts, M.; Rudolph, C.W. Future time perspective: A systematic review and meta-analysis. J. Appl. Psychol. 2018, 103, 867. [Google Scholar] [CrossRef] [PubMed]
- Shipp, A.J.; Edwards, J.R.; Lambert, L.S. Conceptualization and measurement of temporal focus: The subjective experience of the past, present, and future. Organ. Behav. Hum. Decis. Process. 2009, 110(1), 1–22. [Google Scholar] [CrossRef]
- Jovančević, A.; Milićević, N. Optimism-pessimism, conspiracy theories and general trust as factors contributing to COVID-19 related behavior–A cross-cultural study. Personal. Individ. Differ. 2020, 167, 110216. [Google Scholar] [CrossRef]
- Sobol, M.; Blachnio, A.; Przepiórka, A. Time of pandemic: Temporal perspectives related to compliance with public health regulations concerning the COVID-19 pandemic. Soc. Sci. Med. 2020, 265, 113408. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, X.; Lyu, H. The relationship between the duration of attention to pandemic news and depression during the outbreak of coronavirus disease 2019: The roles of risk perception and future time perspective. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bruine de Bruin, W. Age differences in COVID-19 risk perceptions and mental health: Evidence from a national US survey conducted in March 2020. J. Gerontol. Ser. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Brailovskaia, J.; Margraf, J. Predicting adaptive and maladaptive responses to the Coronavirus (COVID-19) outbreak: A prospective longitudinal study. Int. J. Clin. Health Psychol. 2020, 20, 183–191. [Google Scholar] [CrossRef]
- Canet-Juric, L.; Andrés, M.L.; Del Valle, M.; López-Morales, H.; Poó, F.; Galli, J.I.; Yerro, M.; Urquijo, S. A longitudinal study on the emotional impact cause by the COVID-19 pandemic quarantine on general population. Front. Psychol. 2020, 11, 2431. [Google Scholar] [CrossRef]
- Gopal, A.; Sharma, A.J.; Subramanyam, M.A. Dynamics of psychological responses to COVID-19 in India: A longitudinal study. PLoS ONE 2020, 15, e0240650. [Google Scholar] [CrossRef]
- Pierce, M.; Hope, H.; Ford, T.; Hatch, S.; Hotopf, M.; John, A.; Kontopantelis, E.; Webb, R.; Wessely, S.; McManus, S.; et al. Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020. [Google Scholar] [CrossRef]
- Salfi, F.; Lauriola, M.; Amicucci, G.; Corigliano, D.; Viselli, L.; Tempesta, D.; Ferrara, M. Gender-related time course of sleep disturbances and psychological symptoms during the COVID-19 lockdown: A longitudinal study on the Italian population. Neurobiol. Stress 2020, 13, 100259. [Google Scholar] [CrossRef]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Cunnan, A.J.; DeWeese, B.H.; Wagle, J.P.; Carroll, K.M.; Sausaman, R.; Hornsby, W.G.; Haff, G.G.; Triplett, N.T.; Pierce, K.C.; Stone, M.H. The general adaptation syndrome: A foundation for the concept of periodization. Sports Med. 2018, 48, 787–797. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and health: A review of psychobiological processes. Annu. Rev. Psychol. 2021, 72, 663–688. [Google Scholar] [CrossRef]
- Akkaya-Kalayci, T.; Kothgassner, O.D.; Wenzel, T.; Goreis, A.; Chen, A.; Ceri, V.; Özlü-Erkilic, Z. The impact of the COVID-19 pandemic on mental health and psychological well-being of young people living in austria and turkey: A multicenter study. Int. J. Environ. Res. Public Health 2020, 17, 9111. [Google Scholar] [CrossRef]
- Liang, L.; Ren, H.; Cao, R.; Hu, Y.; Qin, Z.; Li, C.; Mei, S. The effect of COVID-19 on youth mental health. Psychiatr. Q. 2020, 91, 841–852. [Google Scholar] [CrossRef]
- Burns, R. Psychosocial Well-being. Encycl. Geropsychol. 2016, 1–8. [Google Scholar] [CrossRef]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory; Mind Garden: Palo Alto, CA, USA, 1983. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Nuova Versione Italiana Dello STAI–Forma Y [New Italian Version of STAI–Y]; Organizzazioni Speciali: Firenze, Italy, 1989. [Google Scholar]
- Wolpe, J. The Practice of Behavior Therapy; Pergamon Press: New York, NY, USA, 1990. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Mondo, M.; Sechi, C.; Cabras, C. Psychometric evaluation of three versions of the Italian Perceived Stress Scale. Curr. Psychol. 2019, 1–9. [Google Scholar] [CrossRef]
- Carver, C.S. You want to measure coping but your protocol’s too long: Consider the brief cope. Int. J. Behav. Med. 1997, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Sica, C.; Magni, C.; Ghisi, M.; Altoè, G.; Sighinolfi, C.; Chiri, L.R.; Franceschini, S. Coping Orientation to Problems Experienced-Nuova Versione Italiana (COPE-NVI): Uno strumento per la misura degli stili di coping. Psicoter. Cogn. Comport. 2008, 14, 27–53. [Google Scholar]
- Buss, A.H.; Perry, M. The aggression questionnaire. J. Personal. Soc. Psychol. 1992, 63, 452–459. [Google Scholar] [CrossRef]
- Sommantico, M.; Donizzetti, A.R.; DeRosa, B.; Parrello, S.; Osorio, G.M. L’invarianza per età nella validazione italiana dell’Aggression Questionnaire (AQ) di Buss e Perry. Psicol. Della Salut. 2015, 3, 111–125. [Google Scholar]
- Caprara, G.V.; Alessandri, G.; Eisenberg, N.; Kupfer, A.; Steca, P.; Caprara, M.G.; Yamaguchi, S.; Fukuzawa, A.; Abela, J. The positivity scale. Psychol. Assess. 2012, 24, 701. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Terraciano, A.; McCrae, R.R.; Costa Jr, P.T. Factorial and construct validity of the Italian Positive and Negative Affect Schedule (PANAS). Eur. J. Psychol. Assess. 2003, 19, 131. [Google Scholar] [CrossRef]
- Zimbardo, P.G.; Boyd, J.N. Putting time in perspective: A valid, reliable individual-differences metric. J. Personal. Soc. Psychol. 1999, 77, 1271–1288. [Google Scholar] [CrossRef]
- D’Alessio, M.; Guarino, A.; De Pascalis, V.; Zimbardo, P.G. Testing Zimbardo’s Stanford time perspective inventory (STPI)-short form. Time Soc. 2003, 12, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis pro-gram. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Social Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar] [CrossRef]
- Fancourt, D.; Steptoe, A.; Bu, F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: A longitudinal observational study. Lancet Psychiatry 2021, 8, 141–149. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.X.; Li, W.; Yang, Y.; Wang, Y.; Zhang, Q.; Cheung, T.; Wu, X.; Xiang, Y.T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2020, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Lausi, G.; Quaglieri, A.; Burrai, J.; Mari, E.; Giannini, A.M. Development of the DERS-20 among the Italian population: A study for a short form of the difficulties in emotion regulation scale. Mediterr. J. Clin. Psychol. 2020, 8. [Google Scholar] [CrossRef]
- Nordman, J.; Ma, X.; Li, Z. Traumatic stress induces prolonged aggression increase through synaptic potentiation in the medial amygdala circuits. Eneuro 2020, 7. [Google Scholar] [CrossRef]
- Berkowitz, L. Frustration-aggression hypothesis: Examination and reformulation. Psychol. Bull. 1989, 106, 59–73. [Google Scholar] [CrossRef]
- Evans, M.L.; Lindauer, M.; Farrell, M.E. A pandemic within a pandemic—Intimate partner violence during Covid-19. New Engl. J. Med. 2020, 383, 2302–2304. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Facchin, F.; Micci, L.; Rendiniello, M.; Giulini, P.; Cattaneo, C.; Vercellini, P.; Kustermann, A. COVID-19, lockdown, and intimate partner violence: Some data from an Italian service and suggestions for future approaches. J. Women’s Health 2020, 29, 1239–1242. [Google Scholar] [CrossRef]
- Usher, K.; Bhullar, N.; Durkin, J.; Gyamfi, N.; Jackson, D. Family violence and COVID-19: Increased vulnerability and reduced options for support. Int. J. Ment. Health Nurs. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, S.P.; Gardiner, D.; Bausch, J. Youth and COVID-19: Impacts on Jobs, Education, Rights, and Mental Well-Being: Survey Report 2020. Available online: https://www.youthforum.org/sites/default/files/publication-pdfs/Youth-and-COVID-19-Survey-Report_English.pdf (accessed on 1 June 2021).
- Wang, Y.; Jing, X.; Han, W.; Jing, Y.; Xu, L. Positive and negative affect of university and college students during COVID-19 outbreak: A network-based survey. Int. J. Public Health 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Song, G.; Kim, K.; Yee, J.; Kim, J.H.; Lee, K.E.; Gwak, H.S. Association between anxiety and aggression in adolescents: A cross-sectional study. BMC Pediatrics 2019, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- De Bles, N.J.; Ottenheim, N.R.; van Hemert, A.M.; Pütz, L.E.; van der Does, A.W.; Penninx, B.W.; Giltay, E.J. Trait anger and anger attacks in relation to depressive and anxiety disorders. J. Affect. Disord. 2019, 259, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Person, B.; Sy, F.; Holton, K.; Govert, B.; Liang, A. Fear and stigma: The epidemic within the SARS outbreak. Emerg. Infect. Dis. 2004, 10, 358. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Alias, H.; Wong, L.P. Knowledge, Attitudes, Impact, and Anxiety Regarding COVID-19. Infection among the Public in China. Front. Public Health 2020, 8, 236. [Google Scholar] [CrossRef]
- Smirni, P.; Lavanco, G.; Smirni, D. Anxiety in Older Adolescents at the Time of COVID-19. J. Clin. Med. 2020, 9, 3064. [Google Scholar] [CrossRef]
- Pérez-Fuentes, M.D.C.; Molero Jurado, M.D.M.; Martos Martínez, Á.; Gázquez Linares, J.J. Threat of COVID-19 and emotional state during quarantine: Positive and negative affect as mediators in a cross-sectional study of the Spanish population. PLoS ONE 2020, 15, e0235305. [Google Scholar] [CrossRef]
- Seyahi, L.S.; Ozcan, S.G.; Sut, N.; Mayer, A.; Poyraz, B.C. Social and psychiatric effects of COVID-19 pandemic and distance learning on high school students: A cross-sectional web-based survey comparing turkey and denmark. MedRxiv 2020. [Google Scholar] [CrossRef]
- Whitehead, B.R. COVID-19 as a Stressor: Pandemic Expectations, Perceived Stress, and Negative Affect in Older Adults. J. Gerontol. Ser. B 2020, 76. [Google Scholar] [CrossRef]
- Troy, A.S.; Shallcross, A.J.; Brunner, A.; Friedman, R.; Jones, M.C. Cognitive reappraisal and acceptance: Effects on emotion, physiology, and perceived cognitive costs. Emotion 2018, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Sandal, G.M.; van deVijver, F.J.; Smith, N. Psychological hibernation in Antarctica. Front. Psychol. 2018, 9, 2235. [Google Scholar] [CrossRef]
- Mari, E.; Lausi, G.; Fraschetti, A.; Pizzo, A.; Baldi, M.; Quaglieri, A.; Burrai, J.; Barchielli, B.; Avallone, F.; Giannini, A.M. Teaching during the Pandemic: A Comparison in Psychological Wellbeing among Smart Working Professions. Sustainability 2021, 13, 4850. [Google Scholar] [CrossRef]
- Litz, B.T.; Gray, M.J.; Bryant, R.A.; Adler, A.B. Early intervention for trauma: Current status and future directions. Clin. Psychol. Sci. Pract. 2002, 9, 112–134. [Google Scholar] [CrossRef]
- Kerig, P.K.; Chaplo, S.D.; Bennett, D.C.; Modrowski, C.A. “Harm as harm” gang membership, perpetration trauma, and posttraumatic stress symptoms among youth in the juvenile justice system. Crim. Justice Behav. 2016, 43, 635–652. [Google Scholar] [CrossRef]
- Charles, S.T. Strength and vulnerability integration: A model of emotional wellbeing across adulthood. Psychol. Bull. 2010, 136, 1068–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, S.T.; Luong, G. Emotional experience across adulthood: The theoretical model of strength and vulnerability integration. Curr. Dir. Psychol. Sci. 2013, 22, 443–448. [Google Scholar] [CrossRef]
- Pearman, A.; Hughes, M.L.; Smith, E.L.; Neupert, S.D. Age differences in risk and resilience factors in COVID-19-related stress. J. Gerontol. Ser. B 2021, 76, e38–e44. [Google Scholar] [CrossRef]
- Kimhi, S.; Eshel, Y.; Marciano, H.; Adini, B. Distress and resilience in the days of COVID-19: Comparing two ethnicities. Int. J. Environ. Res. Public Health 2020, 17, 3956. [Google Scholar] [CrossRef]
- Kimhi, S.; Marciano, H.; Eshel, Y.; Adini, B. Recovery from the COVID-19 pandemic: Distress and resilience. Int. J. Disaster Risk Reduct. 2020, 50, 101843. [Google Scholar] [CrossRef] [PubMed]
- Mari, E.; Fraschetti, A.; Lausi, G.; Pizzo, A.; Baldi, M.; Paoli, E.; Giannini, A.M.; Avallone, F. Forced cohabitation during coronavirus lockdown in Italy: A study on coping, stress and emotions among different family patterns. J. Clin. Med. 2020, 9, 3906. [Google Scholar] [CrossRef] [PubMed]
- Justo-Alonso, A.; García-Dantas, A.; González-Vázquez, A.I.; Sánchez-Martín, M.; Del Río-Casanova, L. How did different generations cope with the COVID-19 pandemic? Early stages of the pandemic in Spain. Psicothema 2020, 32, 490–500. [Google Scholar] [CrossRef]
- Chew, Q.H.; Chia, F.L.; Ng, W.K.; Lee, W.; Tan, P.; Wong, C.S.; Puah, S.H.; Shelat, V.G.; Seah, E.D.; Huey, C.; et al. Perceived stress, stigma, traumatic stress levels and coping responses amongst residents in training across multiple specialties during COVID-19 pandemic-a longitudinal study. Int. J. Environ. Res. Public Health 2020, 17, 6572. [Google Scholar] [CrossRef]
- Holman, E.A.; Silver, R.C. Future- oriented thinking and adjustment in a nationwide longitudinal study following the September 11th terrorist attacks. Motiv. Emot. 2005, 29, 385–406. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, G.; Caprara, G.V.; Tisak, J. A unified latent curve, latent state- trait analysis of the developmental trajectories and correlates of positive orientation. Multivar. Behav. Res. 2012, 47, 341–368. [Google Scholar] [CrossRef]
- Schiavon, C.C.; Marchetti, E.; Gurgel, L.G.; Busnello, F.M.; Reppold, C.T. Optimism and hope in chronic disease: A systematic review. Front. Psychol. 2017, 7, 2022. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzo, E.; Mari, L.; Pasetto, D.; Miccoli, S.; Casagrandi, R.; Gatto, M.; Rinaldo, A. The geography of COVID-19 spread in Italy and implications for the relaxation of confinement measures. Nat. Commun. 2020, 11, 4264. [Google Scholar] [CrossRef] [PubMed]
- Son, C.; Hegde, S.; Smith, A.; Wang, X.; Sasangohar, F. Effects of COVID-19 on college students’ mental health in the United States: Interview survey study. J. Med. Internet Res. 2020, 22, e21279. [Google Scholar] [CrossRef] [PubMed]
- Aristovnik, A.; Keržič, D.; Ravšelj, D.; Tomaževič, N.; Umek, L. Impacts of the COVID-19 pandemic on life of higher education students: A global perspective. Sustainability 2020, 12, 8438. [Google Scholar] [CrossRef]
| N | % | ||
|---|---|---|---|
| Sex | Male | 32 | 26.0% |
| Female | 91 | 74.0% | |
| Education | Middle school | 10 | 8.1% |
| High school | 52 | 42.3% | |
| Bachelor’s degree | 36 | 29.3% | |
| Second degree | 25 | 20.3% | |
| Marital Status | Single | 88 | 71.5% |
| Married | 30 | 24.4% | |
| Separated | 3 | 2.4% | |
| Divorced | 2 | 1.6% | |
| Occupation | Student | 59 | 48.0% |
| Police Forces | 1 | 0.8% | |
| Healthcare Profession | 4 | 3.3% | |
| Employed | 18 | 14.6% | |
| Unemployed | 1 | 0.8% | |
| Worker | 8 | 6.5% | |
| Freelancer | 12 | 9.8% | |
| Homemaker | 4 | 3.3% | |
| Teacher | 5 | 4.1% | |
| Retiree | 5 | 4.1% | |
| Other | 6 | 4.9% | |
| House Sqm | −40 sqm | 3 | 2.4% |
| from 40 to 80 sqm | 40 | 32.5% | |
| +80 sqm | 80 | 65.0% | |
| Outdoor spaces | No | 5 | 4.1% |
| Balcony | 51 | 41.5% | |
| Rooftop | 15 | 12.2% | |
| Garden | 33 | 26.8% | |
| Courtyard | 19 | 15.4% | |
| Live with other people | Yes | 117 | 95.1% |
| No | 6 | 4.9% | |
| Domestic animal | Yes | 59 | 48.0% |
| No | 64 | 52.0% |
| Variables | Age Group (N) | T1 M (SD) | T2 M (SD) | T3 M (SD) | T4 M (SD) | FU M (SD) |
|---|---|---|---|---|---|---|
| STAI-Y1 | 18–23 (46) | 50.78 (10.92) | 45.85 (10.49) | 47.57 (12.36) | 46.78 (10.04) | 41.70 (13.01) |
| 24–36 (38) | 43.26 (12.46) | 41.00 (8.91) | 43.97 (10.75) | 43.32 (11.58) | 39.58 (8.78) | |
| +36 (39) | 41.59 (10.34) | 41.10 (11.39) | 41.59 (10.59) | 42.21 (12.69) | 38.72 (12.47) | |
| Total (123) | 45.54 (11.89) | 42.85 (10.51) | 44.56 (11.52) | 44.26 (11.49) | 40.10 (11.66) | |
| STAI-Y2 | 18–23 (46) | 50.09 (10.61) | - | - | - | 48.59 (10.73) |
| 24–36 (38) | 44.13 (9.19) | - | - | - | 42.71 (8.19) | |
| +36 (39) | 39.95 (8.68) | - | - | - | 40.49 (10.52) | |
| Total (123) | 45.03 (10.43) | - | - | - | 44.20 (10.47) | |
| PANAS-PA | 18–23 (46) | 29.07 (8.53) | 30.07 (8.17) | 29.30 (8.63) | 30.17 (6.67) | 33.02 (8.26) |
| 24–36 (38) | 30.76 (7.70) | 31.87 (7.19) | 30.79 (7.83) | 30.21 (7.93) | 35.24 (6.63) | |
| +36 (39) | 33.97 (6.20) | 32.95 (5.77) | 31.79 (6.76) | 30.46 (7.98) | 34.41 (8.68) | |
| Total (123) | 31.15 (7.81) | 31.85 (7.25) | 30.55 (7.84) | 30.28 (8.16) | 34.15 (7.93) | |
| PANAS-NA | 18–23 (46) | 26.22 (7.36) | 24.83 (7.53) | 25.30 (7.45) | 23.93 (7.32) | 22.83 (8.16) |
| 24–36 (38) | 24.82 (8.10) | 22.45 (7.77) | 23.97 (7.74) | 23.76 (8.87) | 20.87 (7.16) | |
| +36 (39) | 24.00 (7.05) | 21.46 (7.58) | 22.41 (8.26) | 22.64 (9.05) | 20.10 (7.74) | |
| Total (123) | 25.08 (7.50) | 23.02 (7.70) | 23.98 (7.83) | 23.47 (8.33) | 21.36 (7.76) | |
| AQ-PA | 18–23 (46) | 25.52 (4.05) | 24.54 (4.85) | 24.24 (4.29) | 23.65 (4.37) | 24.30 (4.52) |
| 24–36 (38) | 23.11 (4.76) | 22.13 (4.72) | 22.61 (3.51) | 22.26 (3.70) | 21.16 (4.60 | |
| +36 (39) | 21.03 (4.89) | 20.10 (5.32) | 21.00 (4.99) | 19.64 (5.21) | 20.38 (5.54) | |
| Total (123) | 23.35 (4.89) | 22.39 (5.26) | 22.71 (4.48) | 21.95 (4.74) | 22.09 (5.15) | |
| AQ-VA | 18–23 (46) | 17.46 (4.88) | 17.22 (5.51) | 16.89 (5.41) | 16.65 (5.31) | 17.35 (5.23) |
| 24–36 (38) | 16.61 (4.54) | 16.08 (4.62) | 16.13 (4.72) | 16.84 (4.58) | 16.11 (4.99) | |
| +36 (39) | 15.64 (5.25) | 14.85 (5.41) | 13.85 (4.44) | 13.87 (4.50) | 14.85 (4.56) | |
| Total (123) | 16.62 (4.92) | 16.11 (5.27) | 15.69 (5.04) | 15.89 (4.99) | 16.17 (5.02) | |
| AQ-HO | 18–23 (46) | 21.07 (5.05) | 21.35 (6.21) | 21.52 (6.17) | 21.72 (5.46) | 20.33 (4.87) |
| 24–36 (38) | 19.39 (5.33) | 19.13 (5.85) | 19.63 (6.07) | 19.50 (5.63) | 19.42 (5.33) | |
| +36 (39) | 17.67 (5.88) | 17.21 (6.19) | 17.03 (5.02) | 16.85 (5.82) | 17.13 (5.44) | |
| Total (123) | 19.47 (5.55) | 19.35 (6.28) | 19.51 (6.05) | 19.49 (5.94) | 19.03 (5.33) |
| Variables | Age Group (N) | T1 M (SD) | T2 M (SD) | T3 M (SD) | T4 M (SD) | FU M (SD) |
|---|---|---|---|---|---|---|
| SUDS | 18–23 (46) | 6.33 (1.96) | 6.37 (2.03) | 6.91 (1.86) | 6.84 (2.12) | 5.44 (2.25) |
| 24–36 (38) | 5.71 (2.21) | 5.87 (2.21) | 6.00 (2.27) | 5.90 (2.64) | 5.00 (2.27) | |
| +36 (39) | 5.09 (2.58) | 5.60 (2.43) | 5.27 (2.69) | 5.80 (2.72) | 5.38 (2.28) | |
| Total (123) | 5.75 (2.29) | 5.97 (2.22) | 6.11 (2.36) | 6.22 (2.51) | 5.28 (2.26) | |
| PSS-TOT | 18–23 (46) | 19.93 (4.88) | 20.07 (6.09) | 20.72 (6.29) | 19.83 (5.09) | 19.41 (2.62) |
| 24–36 (38) | 16.71 (5.09) | 16.42 (6.09) | 17.82 (6.60) | 17.32 (5.63) | 19.05 (2.45) | |
| +36 (39) | 13.77 (4.66) | 14.59 (7.44) | 13.10 (5.57) | 13.56 (5.31) | 19.31 (2.41) | |
| Total (123) | 16.98 (5.48) | 17.20 (6.90) | 17.41 (6.90) | 17.07 (5.90) | 19.27 (2.49) | |
| COPE-AV | 18–23 (46) | 23.11 (3.85) | - | - | - | 22.67 (4.72) |
| 24–36 (38) | 21.58 (4.57) | - | - | - | 20.47 (3.85) | |
| +36 (39) | 20.41 (3.40) | - | - | - | 19.67 (3.73) | |
| Total (123) | 21.78 (4.08) | - | - | - | 21.04 (4.33) | |
| COPE-AP | 18–23 (46) | 31.59 (5.39) | - | - | - | 31.70 (5.16) |
| 24–36 (38) | 31.24 (4.35) | - | - | - | 31.24 (4.16) | |
| +36 (39) | 32.05 (4.70) | - | - | - | 31.21 (4.62) | |
| Total (123) | 31.63 (4.84) | - | - | - | 31.40 (4.67) | |
| COPE-HU | 18–23 (46) | 4.52 (1.60) | - | - | - | 4.17 (1.53) |
| 24–36 (38) | 4.66 (1.67) | - | - | - | 4.32 (1.56) | |
| +36 (39) | 4.03 (1.60) | - | - | - | 3.92 (1.51) | |
| Total (123) | 4.41 (1.63) | - | - | - | 4.14 (1.53) | |
| COPE-RE | 18–23 (46) | 2.41 (0.78) | - | - | - | 2.63 (1.06) |
| 24–36 (38) | 2.76 (1.48) | - | - | - | 2.66 (1.17) | |
| +36 (39) | 3.36 (1.77) | - | - | - | 3.18 (1.82) | |
| Total (123) | 2.82 (1.42) | - | - | - | 2.81 (1.39) |
| Variables | Age Group (N) | T1 M (SD) | T2 M (SD) | T3 M (SD) | T4 M (SD) | FU M (SD) |
|---|---|---|---|---|---|---|
| P-SCALE | 18–23 (46) | 27.00 (5.80) | 27.00 (5.52) | 26.35 (5.36) | 26.20 (5.34) | 25.72 (6.30) |
| 24–36 (38) | 28.92 (4.75) | 28.55 (5.16) | 28.05 (5.30) | 27.39 (5.26) | 28.66 (4.66) | |
| +36 (39) | 29.08 (5.55) | 28.79 (5.96) | 28.05 (5.75) | 27.49 (6.83) | 28.74 (6.51) | |
| Total (123) | 28.25 (5.46) | 28.05 (5.57) | 27.41 (5.49) | 26.98 (5.82) | 27.59 (6.04) | |
| STPI-FUT | 18–23 (46) | 32.46 (6.81) | 32.09 (6.37) | 32.24 (6.37) | 31.63 (6.79) | 32.89 (5.89) |
| 24–36 (38) | 31.97 (4.80) | 32.32 (4.74) | 32.63 (4.61) | 31.87 (5.07) | 33.61 (4.76) | |
| +36 (39) | 34.36 (4.83) | 33.79 (7.56) | 34.00 (5.99) | 33.54 (5.55) | 34.18 (6.23) | |
| Total (123) | 32.91 (5.69) | 32.70 (6.34) | 32.92 (5.76) | 32.31 (5.93) | 33.52 (5.66) | |
| STPI-HED | 18–23 (46) | 23.22 (4.03) | 24.24 (3.43) | 23.35 (3.62) | 23.28 (4.11) | 23.67 (3.29) |
| 24–36 (38) | 23.87 (3.52) | 23.82 (3.83) | 23.53 (3.92) | 23.47 (3.76) | 22.87 (3.85) | |
| +36 (39) | 22.59 (3.42) | 22.56 (3.89) | 22.08 (3.22) | 23.31 (3.28) | 22.87 (3.22) | |
| Total (123) | 23.22 (3.69) | 23.58 (3.74) | 23.00 (3.62) | 23.35 (3.72) | 23.17 (3.45) | |
| STPI-FAT | 18–23 (46) | 14.26 (3.85) | 13.89 (3.39) | 14.22 (3.49) | 14.07 (3.39) | 13.91 (3.49) |
| 24–36 (38) | 13.97 (4.04) | 14.45 (4.41) | 14.37 (3.70) | 14.26 (3.16) | 14.13 (3.60) | |
| +36 (39) | 15.16 (3.66) | 14.97 (3.65) | 15.16 (3.37) | 15.03 (3.76) | 15.05 (3.31) | |
| Total (123) | 14.45 (3.85) | 14.40 (3.48) | 14.56 (3.51) | 14.43 (3.44) | 14.34 (3.48) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaglieri, A.; Lausi, G.; Fraschetti, A.; Burrai, J.; Barchielli, B.; Pizzo, A.; Cordellieri, P.; De Gennaro, L.; Gorgoni, M.; Ferlazzo, F.; et al. “Stay at Home” in Italy during the COVID-19 Outbreak: A Longitudinal Study on Individual Well-Being among Different Age Groups. Brain Sci. 2021, 11, 993. https://doi.org/10.3390/brainsci11080993
Quaglieri A, Lausi G, Fraschetti A, Burrai J, Barchielli B, Pizzo A, Cordellieri P, De Gennaro L, Gorgoni M, Ferlazzo F, et al. “Stay at Home” in Italy during the COVID-19 Outbreak: A Longitudinal Study on Individual Well-Being among Different Age Groups. Brain Sciences. 2021; 11(8):993. https://doi.org/10.3390/brainsci11080993
Chicago/Turabian StyleQuaglieri, Alessandro, Giulia Lausi, Angelo Fraschetti, Jessica Burrai, Benedetta Barchielli, Alessandra Pizzo, Pierluigi Cordellieri, Luigi De Gennaro, Maurizio Gorgoni, Fabio Ferlazzo, and et al. 2021. "“Stay at Home” in Italy during the COVID-19 Outbreak: A Longitudinal Study on Individual Well-Being among Different Age Groups" Brain Sciences 11, no. 8: 993. https://doi.org/10.3390/brainsci11080993
APA StyleQuaglieri, A., Lausi, G., Fraschetti, A., Burrai, J., Barchielli, B., Pizzo, A., Cordellieri, P., De Gennaro, L., Gorgoni, M., Ferlazzo, F., Sdoia, S., Zivi, P., Giannini, A. M., & Mari, E. (2021). “Stay at Home” in Italy during the COVID-19 Outbreak: A Longitudinal Study on Individual Well-Being among Different Age Groups. Brain Sciences, 11(8), 993. https://doi.org/10.3390/brainsci11080993














