Transcriptomic Data Analysis Reveals a Down-Expression of Galectin-8 in Schizophrenia Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Selection and Analysis
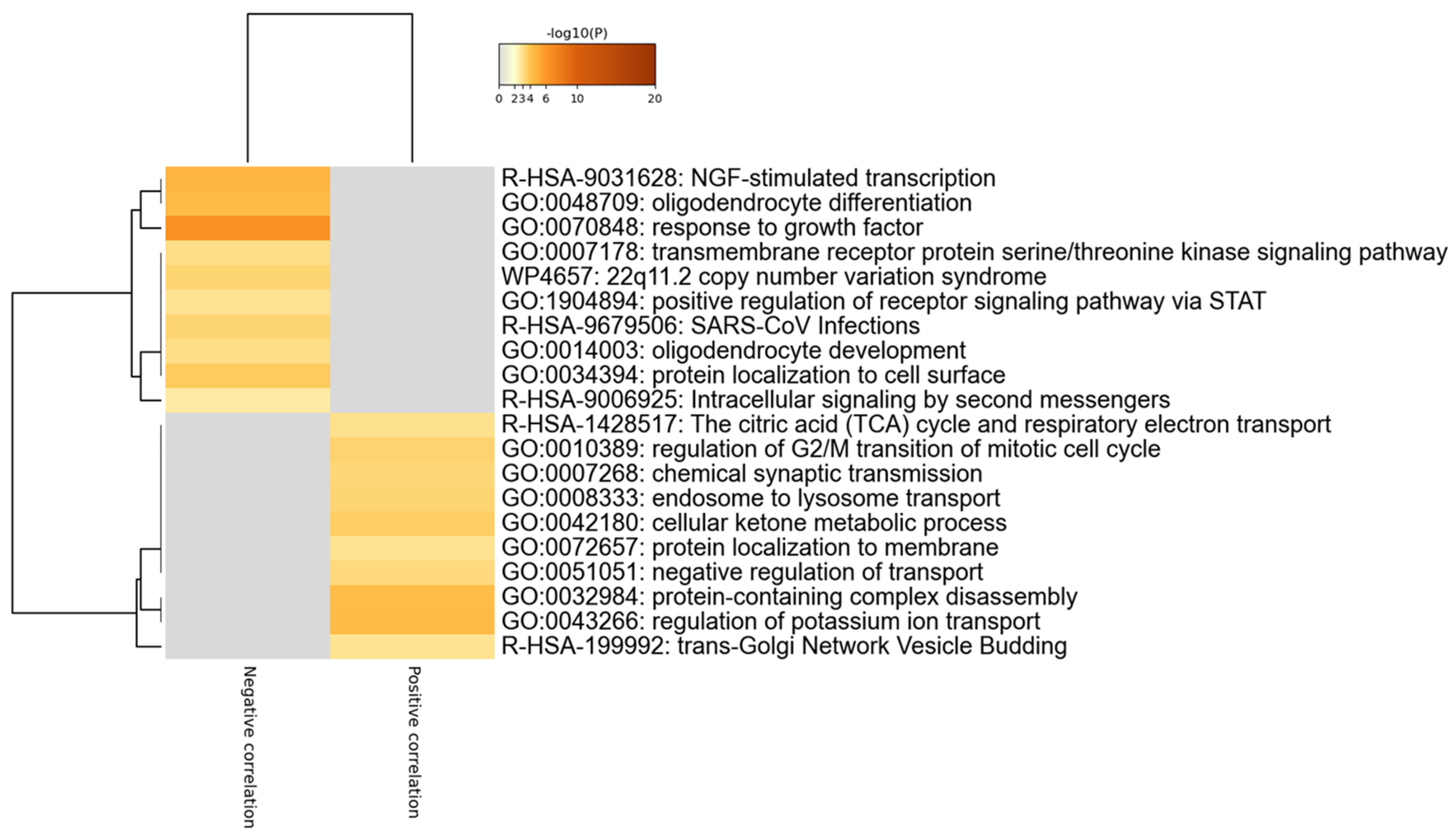
2.2. Gene Ontology Analysis
2.3. Gene Expression Regulation
2.4. Statistical Analysis
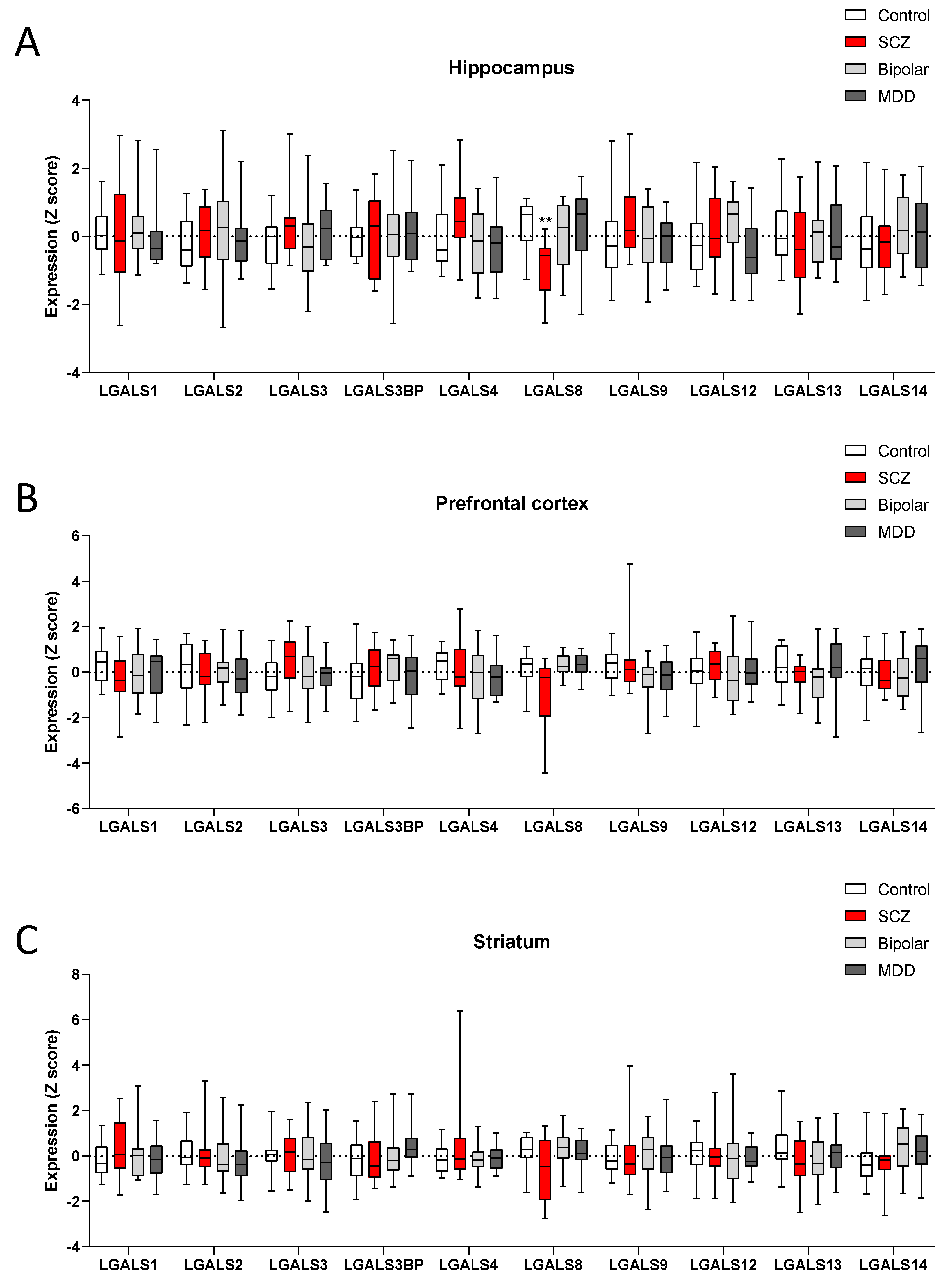
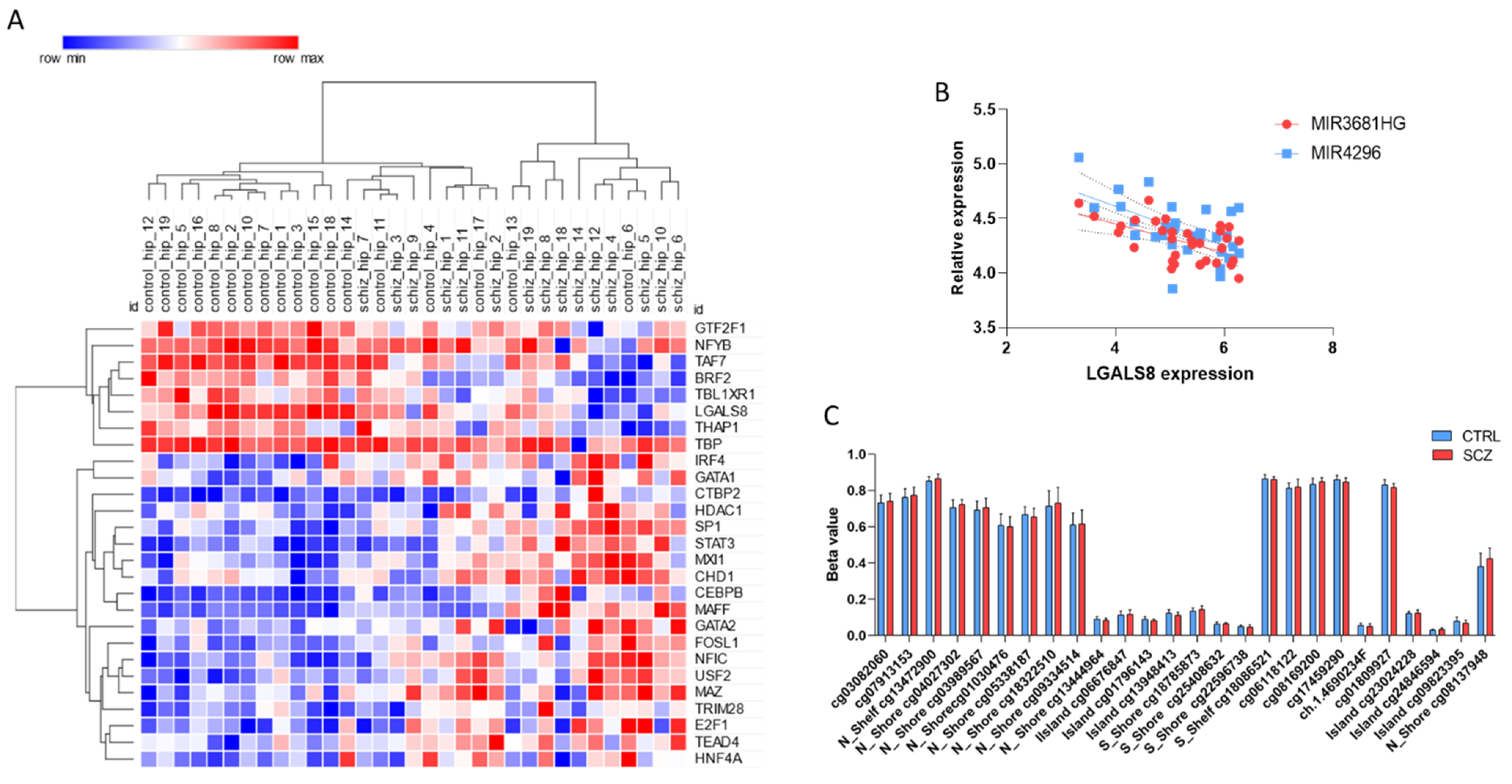
3. Results
Expression Levels of the Galectin Gene Family Members in Different Brain Regions from Control Donors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueser, K.T.; McGurk, S.R. Schizophrenia. Lancet 2004, 363, 2063–2072. [Google Scholar] [CrossRef]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Guillozet-Bongaarts, A.L.; Hyde, T.M.; Dalley, R.A.; Hawrylycz, M.J.; Henry, A.; Hof, P.R.; Hohmann, J.; Jones, A.R.; Kuan, C.L.; Royall, J.; et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry 2014, 19, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Sanders, A.R.; Gejman, P.V. Genome-wide approaches to schizophrenia. Brain Res. Bull. 2010, 83, 93–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleinman, J.E.; Law, A.J.; Lipska, B.K.; Hyde, T.M.; Ellis, J.K.; Harrison, P.J.; Weinberger, D.R. Genetic neuropathology of schizophrenia: New approaches to an old question and new uses for postmortem human brains. Biol. Psychiatry 2011, 69, 140–145. [Google Scholar] [CrossRef]
- Richard, M.D.; Brahm, N.C. Schizophrenia and the immune system: Pathophysiology, prevention, and treatment. Am. J. Heal. Pharm. 2012, 69, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Zuelli, F.; Loureiro, C.M.; Shuhama, R.; Fachim, H.A.; Menezes, P.R.; Louzada-Junior, P.; Mondelli, V.; Del-Ben, C.M. Cytokine profile in first-episode psychosis, unaffected siblings and community-based controls: The effects of familial liability and childhood maltreatment. Psychol. Med. 2020, 50, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Cattaneo, A.; Hepgul, N.; Di Forti, M.; Aitchison, K.J.; Janiri, L.; Murray, R.M.; Dazzan, P.; Pariante, C.M.; Mondelli, V. Serum and gene expression profile of cytokines in first-episode psychosis. Brain. Behav. Immun. 2013, 31, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kalmady, S.V.; Shivakumar, V.; Jose, D.; Ravi, V.; Keshavan, M.S.; Gangadhar, B.N.; Venkatasubramanian, G. Plasma cytokines in minimally treated schizophrenia. Schizophr. Res. 2018, 199, 292–296. [Google Scholar] [CrossRef]
- Enache, D.; Nikkheslat, N.; Fathalla, D.; Morgan, B.P.; Lewis, S.; Drake, R.; Deakin, B.; Walters, J.; Lawrie, S.M.; Egerton, A.; et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr. Res. 2021, 230, 1–8. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, R.N.; Göverti, D.; Kahve, A.C.; Çakmak, I.B.; Yücel, Ç.; Göka, E. Galectin-1 and galectin-3 levels in patients with schizophrenia and their unaffected siblings. Psychiatr. Q. 2020, 91, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Stancic, M.; van Horssen, J.; Thijssen, V.L.; Gabius, H.-J.; van der Valk, P.; Hoekstra, D.; Baron, W. Increased expression of distinct galectins in multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 2011, 37, 654–671. [Google Scholar] [CrossRef]
- Siew, J.J.; Chern, Y. Microglial lectins in health and neurological diseases. Front. Mol. Neurosci. 2018, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.A.; Reinhart, V.; Sheehan, M.J.; Rizzo, S.J.S.; Bove, S.E.; James, L.C.; Volfson, D.; Lewis, D.A.; Kleiman, R.J. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: A comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Mishra, R. mRNA transcriptomics of galectins unveils heterogeneous organization in mouse and human brain. Front. Mol. Neurosci. 2016, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.; Hannon, E.; Dempster, E.; Pidsley, R.; Macdonald, R.; Knox, O.; Spiers, H.; Troakes, C.; Al-Saraj, S.; Turecki, G.; et al. Schizophrenia-associated methylomic variation: Molecular signatures of disease and polygenic risk burden across multiple brain regions. Hum. Mol. Genet. 2017, 26, 210–225. [Google Scholar] [CrossRef]
- Petralia, M.C.; Ciurleo, R.; Saraceno, A.; Pennisi, M.; Basile, M.S.; Fagone, P.; Bramanti, P.; Nicoletti, F.; Cavalli, E. Meta-analysis of transcriptomic data of dorsolateral prefrontal cortex and of peripheral blood mononuclear cells identifies altered pathways in schizophrenia. Genes 2020, 11, 390. [Google Scholar] [CrossRef]
- Henseler, I.; Falkai, P.; Gruber, O. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: Relation to performance and clinical symptoms. J. Psychiatr. Res. 2010, 44, 364–372. [Google Scholar] [CrossRef]
- Benetti, S.; Mechelli, A.; Picchioni, M.; Broome, M.; Williams, S.; McGuire, P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain 2009, 132, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Miletich, R.S.; Kohn, P.D.; Esposito, G.; Carson, R.E.; Quarantelli, M.; Weinberger, D.R.; Berman, K.F. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002, 5, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Saetre, P.; Emilsson, L.; Axelsson, E.; Kreuger, J.; Lindholm, E.; Jazin, E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of psychotropic drugs on inflammation: Consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.R.; Kum, L.M.; Wehring, H.J.; Koola, M.M.; Buchanan, R.W.; Kelly, D.L. A review of anti-inflammatory agents for symptoms of schizophrenia. J. Psychopharmacol. 2013, 27, 337–342. [Google Scholar] [CrossRef]
- Macêdo, D.S.; Araújo, D.P.; Sampaio, L.R.L.; Vasconcelos, S.M.M.; Sales, P.M.G.; Sousa, F.C.F.; Hallak, J.E.; Crippa, J.A.; Carvalho, A.F. Animal models of prenatal immune challenge and their contribution to the study of schizophrenia: A systematic review. Braz. J. Med. Biol. Res. 2012, 45, 179–186. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Schedlowski, M.; Yee, B.K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005, 29, 913–947. [Google Scholar] [CrossRef]
- Garay, P.A.; Hsiao, E.Y.; Patterson, P.H.; McAllister, A.K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain. Behav. Immun. 2013, 31, 54–68. [Google Scholar] [CrossRef]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Voget, M.; Willi, R.; Winter, C.; Riva, M.A.; Mortensen, P.B.; Schedlowski, M.; et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013, 339, 1100–1102. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. In Molecular Psychiatry; Nature Publishing Group: London, UK, 2016; Volume 21, pp. 1696–1709. [Google Scholar]
- Çakici, N.; Sutterland, A.L.; Penninx, B.W.J.H.; De Haan, L.; Van Beveren, N.J.M. Changes in peripheral blood compounds following psychopharmacological treatment in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: A meta-analysis. Psychol. Med. 2021, 51, 538–549. [Google Scholar] [CrossRef]
- Noto, C.; Ota, V.K.; Gouvea, E.S.; Rizzo, L.B.; Spindola, L.M.N.; Honda, P.H.S.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; Gadelha, A.; et al. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int. J. Neuropsychopharmacol. 2015, 18, pyu042. [Google Scholar] [CrossRef]
- Candido, S.; Lupo, G.; Pennisi, M.; Basile, M.; Anfuso, C.; Petralia, M.; Gattuso, G.; Vivarelli, S.; Spandidos, D.; Libra, M.; et al. The analysis of miRNA expression profiling datasets reveals inverse microRNA patterns in glioblastoma and Alzheimer’s disease. Oncol. Rep. 2019, 42, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.D.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Bramanti, P.; Nania, R.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Upregulation of IL-1 receptor antagonist in a mouse model of migraine. Brain Sci. 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.D.; Presti, M.; Mangano, K.; Petralia, M.C.; Basile, M.S.; Libra, M.; Candido, S.; Fagone, P.; Mazzon, E.; Nicoletti, F.; et al. Prediction of PD-L1 expression in neuroblastoma via computational modeling. Brain Sci. 2019, 9, 221. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Falzone, L.; Bramanti, P.; Nicoletti, F.; Basile, M.S. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp. Ther. Med. 2019, 18, 2055–2062. [Google Scholar] [CrossRef]
- Lombardo, S.D.; Mazzon, E.; Mangano, K.; Basile, M.S.; Cavalli, E.; Mammana, S.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Transcriptomic analysis reveals involvement of the macrophage migration inhibitory factor gene network in duchenne muscular dystrophy. Genes 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.D.; Mazzon, E.; Basile, M.S.; Campo, G.; Corsico, F.; Presti, M.; Bramanti, P.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; et al. Modulation of tetraspanin 32 (TSPAN32) expression in T cell-mediated immune responses and in multiple sclerosis. Int. J. Mol. Sci. 2019, 20, 4323. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Basile, M.S.; Cutuli, M.; Di Marco, R.; Scandurra, F.; Saraceno, A.; Fagone, P.; Nicoletti, F.; Mangano, K. Effects of treatment with the hypomethylating agent 5-aza-2′-deoxycytidine in murine type II collagen-induced arthritis. Pharmaceuticals 2019, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mazzon, E.; Mammana, S.; Di Marco, R.; Spinasanta, F.; Basile, M.; Petralia, M.; Bramanti, P.; Nicoletti, F.; Mangano, K. Identification of CD4+ T cell biomarkers for predicting the response of patients with relapsing-remitting multiple sclerosis to natalizumab treatment. Mol. Med. Rep. 2019. [Google Scholar] [CrossRef]
- Nicoletti, F.; Mazzon, E.; Fagone, P.; Mangano, K.; Mammana, S.; Cavalli, E.; Basile, M.S.; Bramanti, P.; Scalabrino, G.; Lange, A.; et al. Prevention of clinical and histological signs of MOG-induced experimental allergic encephalomyelitis by prolonged treatment with recombinant human EGF. J. Neuroimmunol. 2019, 332, 224–232. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Di Marco, R.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Upregulated expression of macrophage migration inhibitory factor, its analogue D-dopachrome tautomerase, and the CD44 receptor in peripheral CD4 T cells from clinically isolated syndrome patients with rapid conversion to clinical defined multiple sclerosis. Medicina 2019, 55, 667. [Google Scholar] [CrossRef]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mammana, S.; Pennisi, M.; Fagone, P.; Kalfin, R.; Martinovic, V.; Ivanovic, J.; Andabaka, M.; et al. In silico and in vivo analysis of IL37 in multiple sclerosis reveals its probable homeostatic role on the clinical activity, disability, and treatment with fingolimod. Molecules 2019, 25, 20. [Google Scholar] [CrossRef]
- Günther, S.; Fagone, P.; Jalce, G.; Atanasov, A.G.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today 2019, 24, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Mangano, K.; Pennisi, M.; Petralia, M.C.; Lombardo, S.D.; Nicoletti, F.; Fagone, P.; Cavalli, E. Impaired expression of tetraspanin 32 (TSPAN32) in memory T cells of patients with multiple sclerosis. Brain Sci. 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mangano, K.; Mammana, S.; Cavalli, E.; Di Marco, R.; Barcellona, M.L.; Salvatorelli, L.; Magro, G.; Nicoletti, F. Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin. Immunol. 2015, 157, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mazzon, E.; Mammana, S.; Basile, M.S.; Lombardo, S.D.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Overexpression of macrophage migration inhibitory factor and its homologue D-dopachrome tautomerase as negative prognostic factor in neuroblastoma. Brain Sci. 2019, 9, 284. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Russo, A.; Longo, A.; Avitabile, T.; Nicoletti, F.; Reibaldi, M.; Basile, M.S. Characterization of the pathophysiological role of CD47 in uveal melanoma. Molecules 2019, 24, 2450. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; McCubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 2018, 9, 8263–8277. [Google Scholar] [CrossRef]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: Pathogenetic cellular effectors and potential therapeutic targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Bendtzen, K.; Nicoletti, F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020, 263, 15–24. [Google Scholar] [CrossRef]
- Fagone, P.; Mangano, K.; Mammana, S.; Pesce, A.; Pesce, A.; Caltabiano, R.; Giorlandino, A.; Rosanna Portale, T.; Cavalli, E.; Lombardo, G.A.G.; et al. Identification of novel targets for the diagnosis and treatment of liver fibrosis. Int. J. Mol. Med. 2015, 36, 747–752. [Google Scholar] [CrossRef]
- Basile, M.; Mazzon, E.; Krajnovic, T.; Draca, D.; Cavalli, E.; Al-Abed, Y.; Bramanti, P.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Anticancer and differentiation properties of the nitric oxide derivative of lopinavir in human glioblastoma cells. Molecules 2018, 23, 2463. [Google Scholar] [CrossRef]
- Petralia, M.C.; Battaglia, G.; Bruno, V.; Pennisi, M.; Mangano, K.; Lombardo, S.D.; Fagone, P.; Cavalli, E.; Saraceno, A.; Nicoletti, F.; et al. The role of macrophage migration inhibitory factor in Alzheimer’s disease: Conventionally pathogenetic or unconventionally protective? Molecules 2020, 25, 291. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef]
- Schepici, G.; Cavalli, E.; Bramanti, P.; Mazzon, E. Autism spectrum disorder and miRNA: An overview of experimental models. Brain Sci. 2019, 9, 265. [Google Scholar] [CrossRef]
- Pardo, E.; Cárcamo, C.; Martín, R.U.S.; Ciampi, E.; Segovia-Miranda, F.; Curkovic-Peña, C.; Montecino, F.; Holmes, C.; Tichauer, J.E.; Acuña, E.; et al. Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS ONE 2017, 12, e0177472. [Google Scholar] [CrossRef] [PubMed]
- Elola, M.T.; Ferragut, F.; Cárdenas Delgado, V.M.; Nugnes, L.G.; Gentilini, L.; Laderach, D.; Troncoso, M.F.; Compagno, D.; Wolfenstein-Todel, C.; Rabinovich, G.A. Expression, localization and function of galectin-8, a tandem-repeat lectin, in human tumors. Histol. Histopathol. 2014, 29, 1093–1105. [Google Scholar] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; Von Muhlinen, N.; Foeglein, Á.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins control mTOR in response to endomembrane damage. Mol. Cell 2018, 70, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Staring, J.; Von Castelmur, E.; Blomen, V.A.; Van Den Hengel, L.G.; Brockmann, M.; Baggen, J.; Thibaut, H.J.; Nieuwenhuis, J.; Janssen, H.; Van Kuppeveld, F.J.M.; et al. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature 2017, 541, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.; Noad, J.; McMahon, H.; Randow, F.; Goedert, M. Galectin-8-mediated selective autophagy protects against seeded tau aggregation. J. Biol. Chem. 2018, 293, 2438–2451. [Google Scholar] [CrossRef]
- Cárcamo, C.; Pardo, E.; Oyanadel, C.; Bravo-Zehnder, M.; Bull, P.; Cáceres, M.; Martínez, J.; Massardo, L.; Jacobelli, S.; González, A.; et al. Galectin-8 binds specific β1 integrins and induces polarized spreading highlighted by asymmetric lamellipodia in Jurkat T cells. Exp. Cell Res. 2006, 312, 374–386. [Google Scholar] [CrossRef]
- Norambuena, A.; Metz, C.; Vicuña, L.; Silva, A.; Pardo, E.; Oyanadel, C.; Massardo, L.; González, A.; Soza, A. Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported byprotein kinase A down-regulation. J. Biol. Chem. 2009, 284, 12670–12679. [Google Scholar] [CrossRef]
- Levy, Y.; Arbel-Goren, R.; Hadari, Y.R.; Eshhar, S.; Ronen, D.; Elhanany, E.; Geiger, B.; Zick, Y. Galectin-8 functions as a matricellular modulator of cell adhesion. J. Biol. Chem. 2001, 276, 31285–31295. [Google Scholar] [CrossRef]
- Pardo, E.; Barake, F.; Godoy, J.A.; Oyanadel, C.; Espinoza, S.; Metz, C.; Retamal, C.; Massardo, L.; Tapia-Rojas, C.; Inestrosa, N.C.; et al. Galectin-8 is a neuroprotective factor in the brain that can be neutralized by human autoantibodies. Mol. Neurobiol. 2019, 56, 7774–7788. [Google Scholar] [CrossRef] [PubMed]
- Katsel, P.; Davis, K.L.; Gorman, J.M.; Haroutunian, V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr. Res. 2005, 77, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Haroutunian, V.; Katsel, P.; Dracheva, S.; Stewart, D.G.; Davis, K.L. Variations in oligodendrocyte-related gene expression across multiple cortical regions: Implications for the pathophysiology of schizophrenia. Int. J. Neuropsychopharmacol. 2007, 10, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pareek, V.; Singh, H.N.; Faiq, M.A.; Narayan, R.K.; Raza, K.; Kumar, P. Altered expression of a unique set of genes reveals complex etiology of schizophrenia. Front. Psychiatry 2019, 10, 906. [Google Scholar] [CrossRef]


| Control | Bipolar | Major Depressive Disorder | Schizophrenia | |
|---|---|---|---|---|
| Sex (M/F) | 10/9 | 10/9 | 10/9 | 10/9 |
| Race (Caucasian/Afro-American) | 18/1 | 19/0 | 18/1 | 13/6 |
| Age (years) | 48.1 ± 10.6 | 46.3 ± 9.5 | 45.2 ± 10.1 | 45.1 ± 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petralia, M.C.; Ciurleo, R.; Bramanti, A.; Bramanti, P.; Saraceno, A.; Mangano, K.; Quattropani, M.C.; Nicoletti, F.; Fagone, P. Transcriptomic Data Analysis Reveals a Down-Expression of Galectin-8 in Schizophrenia Hippocampus. Brain Sci. 2021, 11, 973. https://doi.org/10.3390/brainsci11080973
Petralia MC, Ciurleo R, Bramanti A, Bramanti P, Saraceno A, Mangano K, Quattropani MC, Nicoletti F, Fagone P. Transcriptomic Data Analysis Reveals a Down-Expression of Galectin-8 in Schizophrenia Hippocampus. Brain Sciences. 2021; 11(8):973. https://doi.org/10.3390/brainsci11080973
Chicago/Turabian StylePetralia, Maria Cristina, Rosella Ciurleo, Alessia Bramanti, Placido Bramanti, Andrea Saraceno, Katia Mangano, Maria Catena Quattropani, Ferdinando Nicoletti, and Paolo Fagone. 2021. "Transcriptomic Data Analysis Reveals a Down-Expression of Galectin-8 in Schizophrenia Hippocampus" Brain Sciences 11, no. 8: 973. https://doi.org/10.3390/brainsci11080973
APA StylePetralia, M. C., Ciurleo, R., Bramanti, A., Bramanti, P., Saraceno, A., Mangano, K., Quattropani, M. C., Nicoletti, F., & Fagone, P. (2021). Transcriptomic Data Analysis Reveals a Down-Expression of Galectin-8 in Schizophrenia Hippocampus. Brain Sciences, 11(8), 973. https://doi.org/10.3390/brainsci11080973







